Early Dementia Screening
Abstract
:1. Introduction
2. Definition: Early Dementia
| Criteria | MCI | Mild AD |
|---|---|---|
| Evidence of performance | Objective evidence of poorer performance in one or more cognitive domains greater than expected for the patients age and educational background | Objective evidence of poorer performance in more than one cognitive domain such as memory, language, visuospatial or executive function |
| Interference with daily activities | Limited interference with daily activity; however, complex functional tasks may be completed less efficiently, e.g., preparing meals, shopping alone for clothes and groceries, planning a day’s activity, remembering appointments or paying bills | Significant interference in being able to function effectively at work or during usual activity; however, still able to carry out less complex activity, e.g., ADLs—bathing, dressing and grooming and IADLs—completing chores or attending social functions |
3. Diagnostic Criteria
- Memory complaint, usually corroborated by an informant
- Objective memory impairment for age
- Essentially preserved general cognitive function
- Largely intact functional activities
- Not demented
4. Subtypes
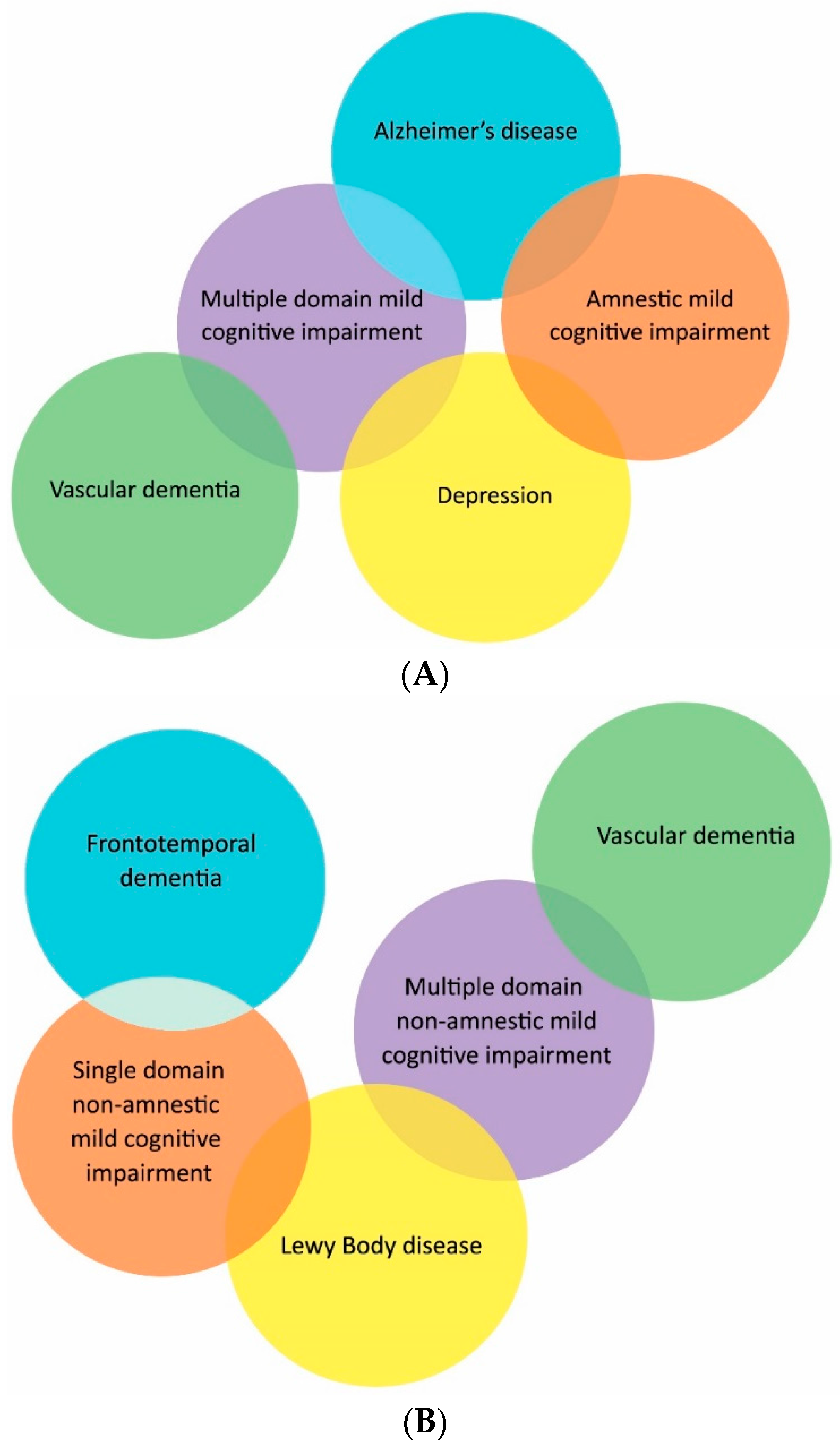
5. Epidemiology
6. Clinical Evaluation
6.1. Cognitive Testing
| Disease | Initial Symptoms | Cognitive Impairment | Mental State Examination | Neurological Examination | Imaging Findings |
|---|---|---|---|---|---|
| AD | Episodic memory loss | Predominance of memory loss with later involvement of all cognitive domains | Initially normal | Initially normal | Entorhinal, cortex and hippocampal atrophy |
| VD | Sudden onset with stepwise deterioration, falls, apathy, focal weakness | Frontal and executive function, generalized slowing, memory may be spared | Apathy, Delusions, Anxiety | Weakness, spasticity, focal neurological deficits | Cortical and/or subcortical infarctions and white matter disease |
| LBD | Visual hallucinations, REM sleep disorder, delirium, Parkinsonism | Drawing and frontal/executive function Spares memory | Delirium, Visual hallucinations, Depression, Delusions | Parkinsonism | Posterior parietal atrophy, larger hippocampi than AD |
| FTD | Apathy, Behavioral and personality change, Poor judgement, Poor speech and language | Frontal/executive, Language, Spares memory and drawing | Apathy, Disinhibition, Hyperorality | May be normal If overlap with PSP/CBD; vertical gaze palsy, axial rigidity, dystonia | Frontal and or temporal atrophy, Spares posterior parietal lobe |
6.2. Functional Status
6.3. Review of Medications
6.4. Neurological Evaluation
6.5. Psychiatric Evaluation
6.6. Social History
6.7. Additional Testing
6.8. Final Assessment
7. Possible Treatments
7.1. Anticholinesterase Inhibitors
7.2. Monoclonal Antibody Treatment
Solanezumab
7.3. Tau
7.4. Non-Pharmacological Treatments
7.4.1. Exercise
7.4.2. Cognitive Training
8. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Milne, A. Dementia screening and early diagnosis: The case for and against. Health Risk Soc. 2010, 12, 65–76. [Google Scholar] [CrossRef]
- Iliffe, S.; Manthorpe, J. The hazards of early recognition of dementia: A risk assessment. Aging Ment. Health 2004, 8, 99–105. [Google Scholar]
- Erlangsen, A.; Zarit, S.; Conwell, Y. Hospital-diagnosed dementia and suicide: A longitudinal study using prospective, nationwide register data. Am. J. Geriatr. Psychiatry 2008, 16, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Hughs, C.P.; Berg, L.; Danziger, W.L.; Coben, L.A.; Martin, R.L. A new clinical scale for the staging of dementia. Am. J. Psychiatry 1982, 140, 566–572. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; de Leon, M.J.; Crook, T. The global deterioration scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [PubMed]
- Rockwood, K.; Strang, D.; MacKnight, C.; Downer, R.; Morris, J.C. Interrater reliability of the Clinical Dementia Rating in a multicenter trial. J. Am. Geriatr. Soc. 2000, 48, 558–559. [Google Scholar] [CrossRef] [PubMed]
- Schafer, K.A.; Tractenberg, R.E.; Sano, M.; Mackell, J.A.; Thomas, R.G.; Gamst, A.; Thal, L.J.; Morris, J.C.; Alzheimer’s Disease Cooperative Study. Reliability of monitoring the clinical dementia rating in multicentre clinical trials. Alzheimer Dis. Assoc. Disord. 2004, 18, 219–222. [Google Scholar] [PubMed]
- McKhann, G.M.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute of Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Demen. 2011, 7, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Tay, L.; Lim, W.S.; Chan, M.; Ali, N.; Mahanum, S.; Chew, P.; Lim, J.; Chong, M.S. New DSM-V neurocognitive disorders criteria and their impact on diagnostic classifications of mild cognitive impairment and dementia in a memory clinic setting. Am. J. Geriatr. Psychiatry 2015, 23, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Smith, G.E.; Waring, S.C.; Ivnik, R.J.; Tangalos, E.G.; Kokmen, E. Mild cognitive impairment: Clinical characterization and outcome. Arch. Neurol. 1999, 56, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.O.; Nordberg, A.; Bäckman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment: Beyond the controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Hughes, T.F.; Snitz, B.E.; Ganguli, M. Should mild cognitive impairment be subtyped? Curr. Opin. Psychiatry 2011, 24, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Norlund, A.; Rolstad, S.; Klang, O.; Edman, A.; Hansen, S.; Wallin, A. Two year outcome of MCI subtypes and aetiologies in the Goteborg MCI study. J. Neurol. Neurosurg. Psychiatry 2010, 81, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Farias, S.T.; Mungas, D.; Reed, B.R.; Harvey, D.; DeCarli, C. Progression of mild cognitive impairment to dementia in clinic-vs community-based cohorts. Arch. Neurol. 2009, 66, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Petersen, R.C.; Knopman, D.S.; Boeve, B.F.; Boeve, B.F.; Geda, Y.E.; Ivnik, R.J.; Smith, G.E.; Jack, C.R., Jr. Mild Cognitive Impairment: 10 years later. Arch. Neurol. 2009, 66, 1447–1455. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, K. Mild cognitive impaiment: An epidemiological perspective. Dialogues Clin. Neurosci. 2004, 6, 401–408. [Google Scholar] [PubMed]
- Ganguli, M.; Dodge, H.H.; Shen, C.; DeKosky, S.T. Mild cognitive impairment, amnestic type: An epidemiologic study. Neurology 2004, 63, 115–121. [Google Scholar] [CrossRef] [PubMed]
- McCarten, J.R. Clinical Evaluaiton of Early Cognitive Symptoms. Clin. Geriatr. Med. 2013, 29, 791–807. [Google Scholar] [CrossRef] [PubMed]
- Dubois, B.; Feldman, H.H.; Jacova, C.; DeKosky, S.T.; Barberger-Gateau, P.; Cummings, J.; Delacourte, A.; Galasko, D.; Gauthier, S.; Jicha, G.; et al. Research criteria for the diagnosis of Alzheimer’s disease: Revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007, 6, 734–746. [Google Scholar] [CrossRef]
- McGlone, J.; Gupta, S.; Humphrey, D.; Oppenheimer, S.; Mirsen, T.; Evans, D.R. Screening for early dementia using memory complaints from patients and relatives. Arch. Neurol. 1990, 47, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Tierney, M.C.; Szalai, J.P.; Snow, W.; Fisher, R.H. The prediction of Alzheimer disease: The role of patient and informant perceptions of cognitive deficits. Arch. Neurol. 1996, 53, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Galvin, J.E.; Fagan, A.M.; Holtzman, D.M.; Mintun, M.A.; Morris, J.C. Relationship of dementia screening tests with biomarkers of Alzheimer’s disease. Brain 2010, 133, 3290–3300. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.; Folstein, S.; McHugh, P. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Lin, J.S.; O’Connor, E.; Rossom, R.; Perdue, L.A.; Burda, B.U.; Thompson, M.; Eckstrom, E. Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventative Services Task Force; Agency for Healthcare Research and Quality (US): Rockville, MD, USA, 2013. [Google Scholar]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V; Charbonneau, S.; Whitehead, V.; Colline, I.; Cummings , L.j.; Chertkow, H. The clinical evaluaiton of early cognitive symptoms. Clin. Geriatr. Med. 2013, 29, 791–807. [Google Scholar]
- Milne, A.; Culverwell, A.; Guss, R.; Tuppen, J.; Whelton, R. Screening for dementia in primary care: A review of the use, efficacy and quality of measures. Int. Psychogeriatr. 2008, 20, 911–926. [Google Scholar] [CrossRef] [PubMed]
- Teng, E.; Becker, B.W.; Woo, E.; Knopman, D.S.; Cummings, J.L.; Lu, P.H. Utility of the functional activities questionaire for distinguishing mild cogntiive impairment from very mild Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2010, 24, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Atkins, E.R.; Bulsara, M.K.; Panegyres, P.K. The natural history of early-onset dementia: The Artemis Project. BMJ Open 2012, 2, e001764. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vossel, K.A.; Beagle, B.A.; Rabinovici, G.D.; Shu, H.; Lee, S.E.; Naasan, G.; Hegde, M.; Cornes, S.B.; Henry, M.L.; Nelson, A.B.; et al. Seizures and epileptiform activity in the early stages of Alzheimer disease. JAMA Neurol. 2013, 70, 1158–1166. [Google Scholar] [CrossRef] [PubMed]
- Yesavage, J.A.; Brink, T.L.; Rose, T.L.; Lum, O.; Huang, V.; Adey, M.; Leirer, V.O. Development and validation of a geriatric depression screening scale: A preliminary report. J. Psychiatr. Res. 1983, 17, 37–49. [Google Scholar] [CrossRef]
- Knopman, D.S.; DeKosky, S.T.; Cummings, J.L.; Chui, H.; Corey-Bloom, J.; Relkin, N.; Small, G.W.; Miller, B.; Stevens, J.C. Practice parameter: Diagnosis of dementia (an evidenced-based review). Report of the Quality of Standards Subcommitee of the American Academy of Neurology. Neurology 2001, 9, 1143–1153. [Google Scholar] [CrossRef]
- Harper, L.; Barkhof, F.; Schltens, P.; Schott, J.M.; Fox, N.C. An algorithmic approach to structural imaging in dementia. J. Neurol. Neurosurg. Psychiatry 2014, 85, 692–698. [Google Scholar] [CrossRef] [PubMed]
- Bloudek, L.M.; Spackman, D.E.; Blankenburg, M.; Sullivan, S.D. Review and meta-analysis of biomarkers and diagnostic imaging in Alzheimer’s disease. J. Alzheimer Dis. 2011, 4, 627–645. [Google Scholar]
- Beynon, R.; Sterne, J.A.; Wilcock, G.; Likeman, M.; Harbord, R.M.; Astin, M.; Burke, M.; Bessell, A.; Ben-Shlomo, Y.; Hawkins, J.; et al. Is MRI better than CT for detecting a vascular component to dementia? A systematic review and meta-analysis. BMC Neurol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Health Quality Ontario. The appropriate use of neuromaging in the diagnostic work-up of dementia. Ont. Health Technol. Assess. Ser. 2014, 14, 1–64. [Google Scholar]
- Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 2001, 69, 89–95. [Google Scholar]
- Davidson, C.M.; O’Brien, J.T. A comparison of FDG-PET and blood flow SPECT in the diagnosis of neurodegenerative dementias: A systematic review. Int. J. Geriatr. Psychiatry 2014, 29, 551–561. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.M.; Lim, X.; Khan, Z.; Pal, S. Systematic review of the diagnostic utility of SPECT imaging in dementia. Eur. Arch. Psychiatry Clin. Neurosci. 2013, 7, 539–552. [Google Scholar] [CrossRef] [PubMed]
- Okello, A.; Koivunen, J.; Edison, P.; Archer, H.A.; Turkheimer, F.E.; Någren, K.; Bullock, R.; Walker, Z.; Kennedy, A.; Fox, N.C.; et al. Conversion of amyloid positive and negative MCI to AD over 3 years: An 11C-PIB PET study. Neurology 2009, 10, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.; Han, D.; Tan, X.; Feng, J.; Guo, Y.; Ding, Y. Diagnostic accuracy of 18 FDG and 11 C-PiB-PET for prediction of short term conversion to Alzheimer’s disease in subjects with mild cognitive impairment International. J. Clin. Pract. 2012, 66, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, J.T.; Berger, E.P.; Lansbury, P.T., Jr. The carboxy terminus of the β-amyloid protein is critical for the seeding of amyloid formation: Implications for the pathogenesis of Alzheimer’s disease. Biochemistry 1993, 32, 4693–4697. [Google Scholar] [CrossRef] [PubMed]
- Faull, M.; Ching, S.; Jarmolowicz, A.; Beilby, J.; Panegyres, P.K. A comparison of two methods for the analysis of CSF Aβ and tau in the diagnosis of Alzheimer’s disease. Am. J. Neurodegener. Dis. 2014, 3, 143–151. [Google Scholar] [PubMed]
- Cooper, C.; Li, R.; Lyketsos, C.; Livingstonn, G. Treatment for mild cognitive impairment: Systematic review. Br. J. Psychiatr. 2013, 203, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, P.M.; Krishnan, K.R.R.; Anand, R.; Danyluk, J.; Hartman, R.D.; Veach, J. Long-term effects of rivastigmine in moderately severe Alzheimer’s disease: Does early initiation of therapy offer sustained benefits? Prog. Neuropsychopharmacol. Biol. Psychiatry 2002, 26, 705–712. [Google Scholar] [CrossRef]
- Panza, F.; Solfrizzi, V.; Imbimbo, B.P.; Logroscino, G. Amyloid-directed monoclonal antibodies for the treatment of Alzheimer’s disease: The point of no return? Expert Opin. Biol. Ther. 2014, 14, 1465–1476. [Google Scholar] [CrossRef] [PubMed]
- Siemers, E.R.; Friedrich, S.; Dean, R.A.; Gonzales, C.R.; Farlow, M.R.; Paul, S.M.; Demattos, R.B. Safety and changes in plasma and cerebrospinal fluid amyloid-β after a single administration of an amyloid-β monoclonal antibody in subjects with Alzheimer disease. Clin. Neuropharmacol. 2010, 33, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Farlow, M.; Arnold, S.E.; van Dyck, C.H.; Aisen, P.S.; Snider, B.J.; Porsteinsson, A.P.; Friedrich, S.; Dean, R.A.; Gonzales, C.; Sethuraman, G.; et al. Safety and biomarker effects of solanezumab in patients with Alzheimer’s disease. Alzheimers Dement. 2012, 8, 261–271. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.; et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A. What’s next for Alzheimer treatment? Ann. Neurol. 2013, 73, A7–A9. [Google Scholar] [CrossRef] [PubMed]
- Brunden, K.R.; Zhang, B.; Carroll, J.; Yao, Y.M.; Potuzak, J.S.; Hogan, A.M.L.; Iba, M.; James, M.J.; Xie, S.X.; Ballatore, C.; et al. Epothilone D improves microtubule density, axonal integrity, and cognition in a transgenic mouse model of tauopathy. J. Neurosci. 2010, 30, 13861–13866. [Google Scholar] [CrossRef] [PubMed]
- Small, G.N.; Rabins, P.C.; Barry, P.P.; Buckholtz, N.S.; DeKosky, S.T.; Ferris, S.H.; Finkel, S.I.; Gwyther, L.P.; Khachaturian, Z.S.; Lebowitz, B.D.; et al. Diagnosis and treatment of Alzheimer disease and related disorders: Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997, 278, 1363–1371. [Google Scholar] [CrossRef] [PubMed]
- Buchner, D.M.; Larson, E.B. Falls and fractures in patients with Alzheimer-type dementia. JAMA 1987, 257, 1492–1495. [Google Scholar] [CrossRef] [PubMed]
- Oleske, D.M.; Wilson, R.S.; Bernard, B.A.; Evans, D.A.; Terman, E.W. Epidemiology of injury in people with Alzheimer’s disease. J. Am. Geriatr. Soc. 1995, 43, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Mather, A.S.; Rodriguez, C.; Guthrie, M.F.; McHarg, A.M.; Reid, I.C.; McMurdo, M.E. Effects of exercise on depressive symptoms in older adults with poorly responsive depressive disorder: Randomized controlled trial. Br. J. Psychiatry 2002, 180, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Bach-y-Rita, P. Theoretical basis for brain plasticity after TBI. Brain Inj. 2003, 17, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Sitzer, D.I.; Twamley, E.W.; Jeste, D.V. Cognitive training in Alzheimer’s disease: A meta-analysis of the literature. Acta Psychiatr. Scand. 2006, 114, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Requena, C.; López Ibor, M.I.; Maestú, F.; Campo, P.; López Ibor, J.J.; Ortiz, T. Effects of cholinergic drugs and cognitive training on dementia. Dement. Geriatr. Cogn. Disord. 2004, 18, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Rocca, M.A.; Filippi, M. Functional MRI to study brain plasticity in clinical neurology. Neurol. Sci. 2006, 27, S24–S26. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panegyres, P.K.; Berry, R.; Burchell, J. Early Dementia Screening. Diagnostics 2016, 6, 6. https://doi.org/10.3390/diagnostics6010006
Panegyres PK, Berry R, Burchell J. Early Dementia Screening. Diagnostics. 2016; 6(1):6. https://doi.org/10.3390/diagnostics6010006
Chicago/Turabian StylePanegyres, Peter K., Renee Berry, and Jennifer Burchell. 2016. "Early Dementia Screening" Diagnostics 6, no. 1: 6. https://doi.org/10.3390/diagnostics6010006
APA StylePanegyres, P. K., Berry, R., & Burchell, J. (2016). Early Dementia Screening. Diagnostics, 6(1), 6. https://doi.org/10.3390/diagnostics6010006





