Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer—Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT
Abstract
:1. Introduction
2. Methods and Patients
| Patient Age | Diagnosis Gleason Score, TNM (Year) | Distribution (before Ra-223) | Dx/modality * | Cycles * Cumulative Activity | Initial S-PSA | Other Treatments before Xofigo |
|---|---|---|---|---|---|---|
| 1 m/63 | GS 9, T4N1M1 (-13) | Prostate Bone lnn | NaF, FCH, MRI, CT | 6 30.86 MBq | 410 | Degarelix, Bicalutamide, Docetaxel, Abiraterone, Enzalutamide, Denosumab |
| 2 m/77 | GS 7, pT3N0M0 (-01) | Bone | NaF, FCH, FACBC, MRI, CT | 6 24.80 MBq | 5.5 | RRP, EBRT (PO), Bicalutamide, Docetaxel, Abiraterone, Enzalutamide, Denosumab, Goserelin |
| 3 m/68 | GS 8, T4N1M1 (-13) | Bone | NaF, FCH, MRI, CT | 6 27.08 MBq | 15,500 | Degarelix,Bicalutamide, Denosumab, EBRT (B), Docetaxel, Abiraterone, EBRT (P), Enzalutamide, Denosumab, Goserelin |
| 4 m/49 | GS 9, T4N1M1 (-13) | Bone | NaF, FCH, MRI, CT | 6 31.57 MBq | 700 | Cyproterone, Degarelix, Bicalutamide, Zoledronate, Docetaxel, Sm-153-EDTMP, Mitoxantrone, EBRT (P), Docetaxel, Abiraterone |
| 5 m/67 | GS 9, T3N1M1 (-13) | Bone | NaF, FCH, MRI, CT | 6 25.87 MBq | 430 | Degarelix, Bicalutamide, Denosumab, Docetaxel, Goserelin, Enzalutamide |
| 6 m/69 | GS 8, T4N1M1 (-11) | Bone | NaF, FCH, MRI, CT | 7 24.13 MBq | 16.2 | Bicalutamide, Goserelin, EBRT (P), Abiraterone, Denosumab, Docetaxel, Enzalutamide |
| 7 m/56 | GS 10, T4NxM1 (-13) | Bone | NaF, FCH, MRI, CT | 6 22.78 MBq | 790 | Bicalutamide, Lupron, Denosumab, EBRT (P), Sm-153-EDTMP, Mitoxantrone, Docetaxel, Abiraterone |
| 8 m/58 | GS 9, T3N0M1 (-12) | Bone | NaF, FCH, MRI, CT | 6 23.71MBq | 6.5 | Denosumab, Degarelix, Bicalutamide, EBRT (P), Goserelin, Docetaxel |
| 9 m/60 | GS 9, T4M1N1 (-12) | Bone | BS, NaF, FCH, MRI, CT | 6 16.45 MBq | 18 | Bicalutamide, Buserelin, TURP, Docetaxel, EBRT (B), Abiraterone, Denosumab, Cabazitaxel, Enzalutamide |
| 10 m/83 | GS 6, T3NxM0 (-01) | Bone Liver lnn | NaF, FCH | 6 24.06 MBq | 18 | Leuprolin, EBRT (P), Docetaxel, Abiraterone, Mitoxantrone, Zoledronate, Denosumab |
| Patient Age | Bone Distribution # (before Ra-223) | NaF-% Change after 1st/ 6th Cycle (2nd/7th) | Number of Cycles Cumulative Activity | S-PSA Response | Treatments during Xofigo |
|---|---|---|---|---|---|
| 1 m/63 | Bone 3/3 | +0.8/−13.8 | 6 30.86 MBq | PD | Degarelix, Enzalutamide, Denosumab |
| 2 m/77 | Bone 2/3 | +6.2/−6.9 | 6 24.80 MBq | PR | Enzalutamide, Denosumab, Goserelin |
| 3 m/68 | Bone 3/3 | −10.8/−9.2 | 6 27.08 MBq | CR | Enzalutamide, Denosumab, Goserelin |
| 4 m/49 | Bone 3/3 | nd/−13.0 | 6 31.57 MBq | PD/SD | Lupron,Denosumab, Abiraterone |
| 5 m/67 | Bone 1/3 | −6.5/−7.8 | 6 25.87 MBq | PR | Denosumab, Goserelin, Enzalutamide |
| 6 m/69 | Bone 3/3 | −10.4/−11.7 | 7 24.13 MBq | PR | Goserelin, Denosumab, Enzalutamide |
| 7 m/56 | Bone 3/3 | nd/−11.4 | 6 22.78 MBq | PR | Lupron, Denosumab, Abiraterone |
| 8 m/58 | Bone 3/3 | nd/−43.0 | 6 23.71MBq | SD/PD | Denosumab, Degarelix, Abiraterone |
| 9 m/60 | Bone 2/3 | nd/−68.4 | 6 16.45 MBq | SD | Buserelin, Denosumab, Enzalutamide |
| 10 m/83 | Bone 1/3 | +6.3/−63.5 | 6 24.06 MBq | PR | Denosumab, Degarelix, Enzalutamide |
3. Results

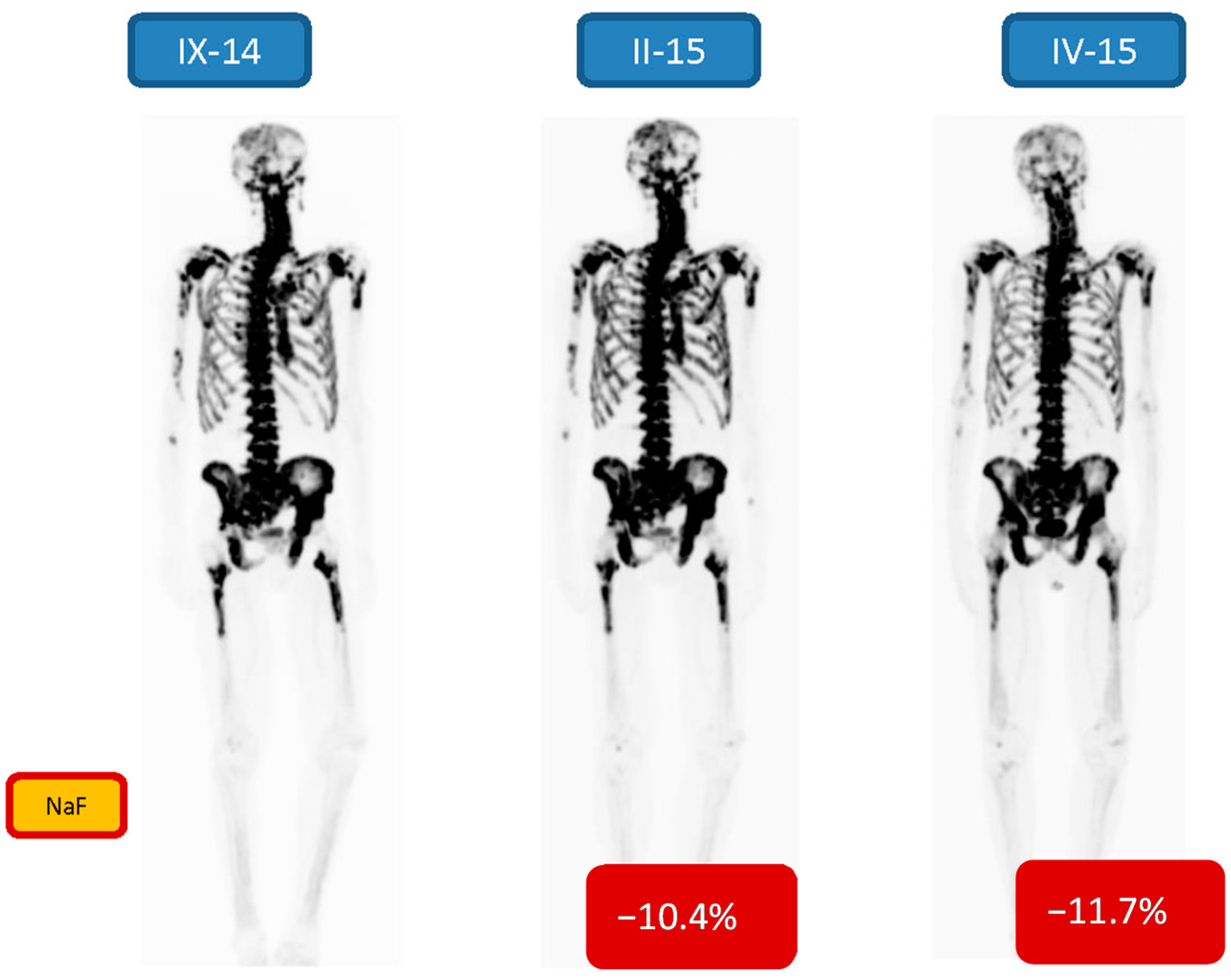
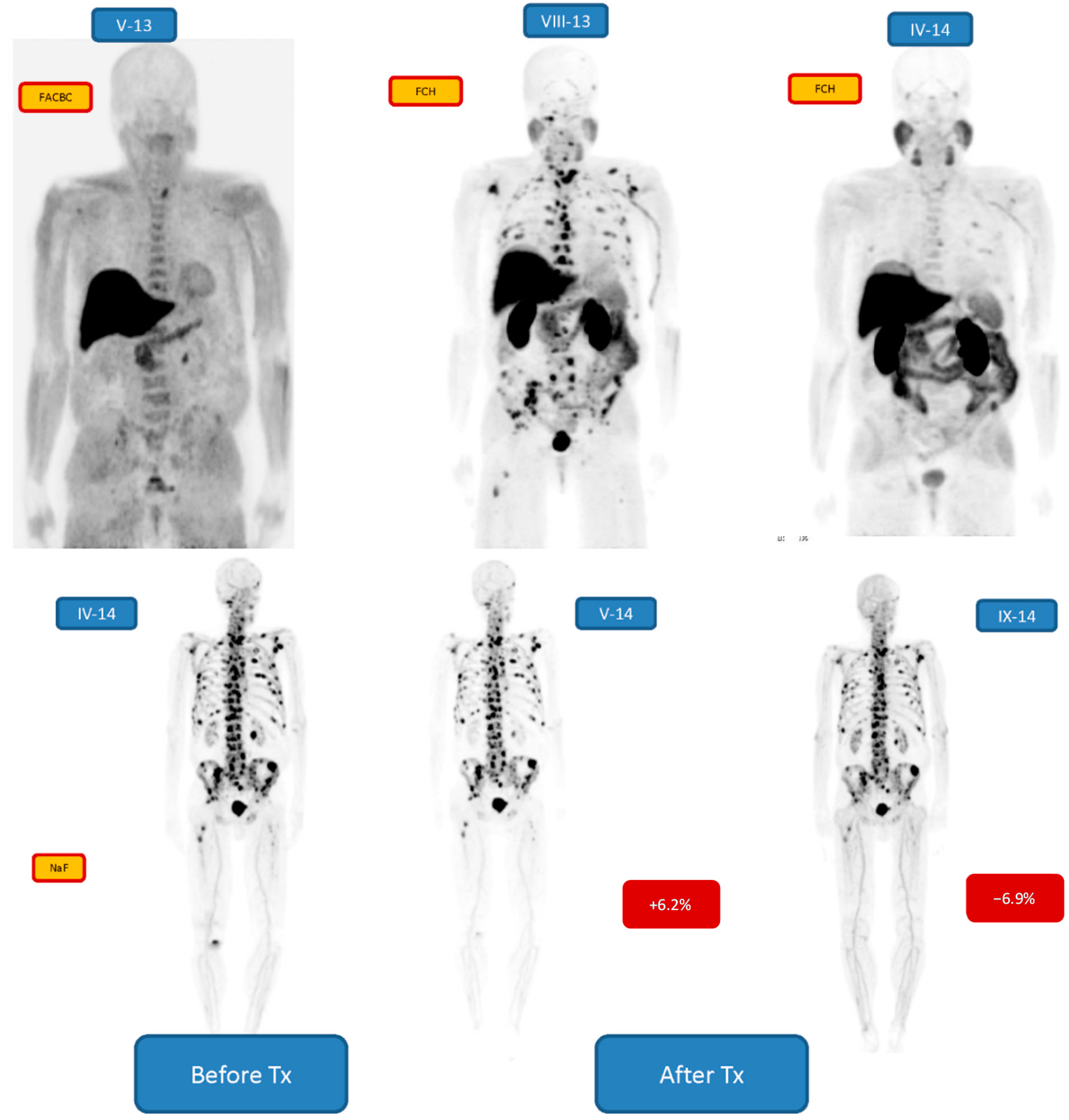
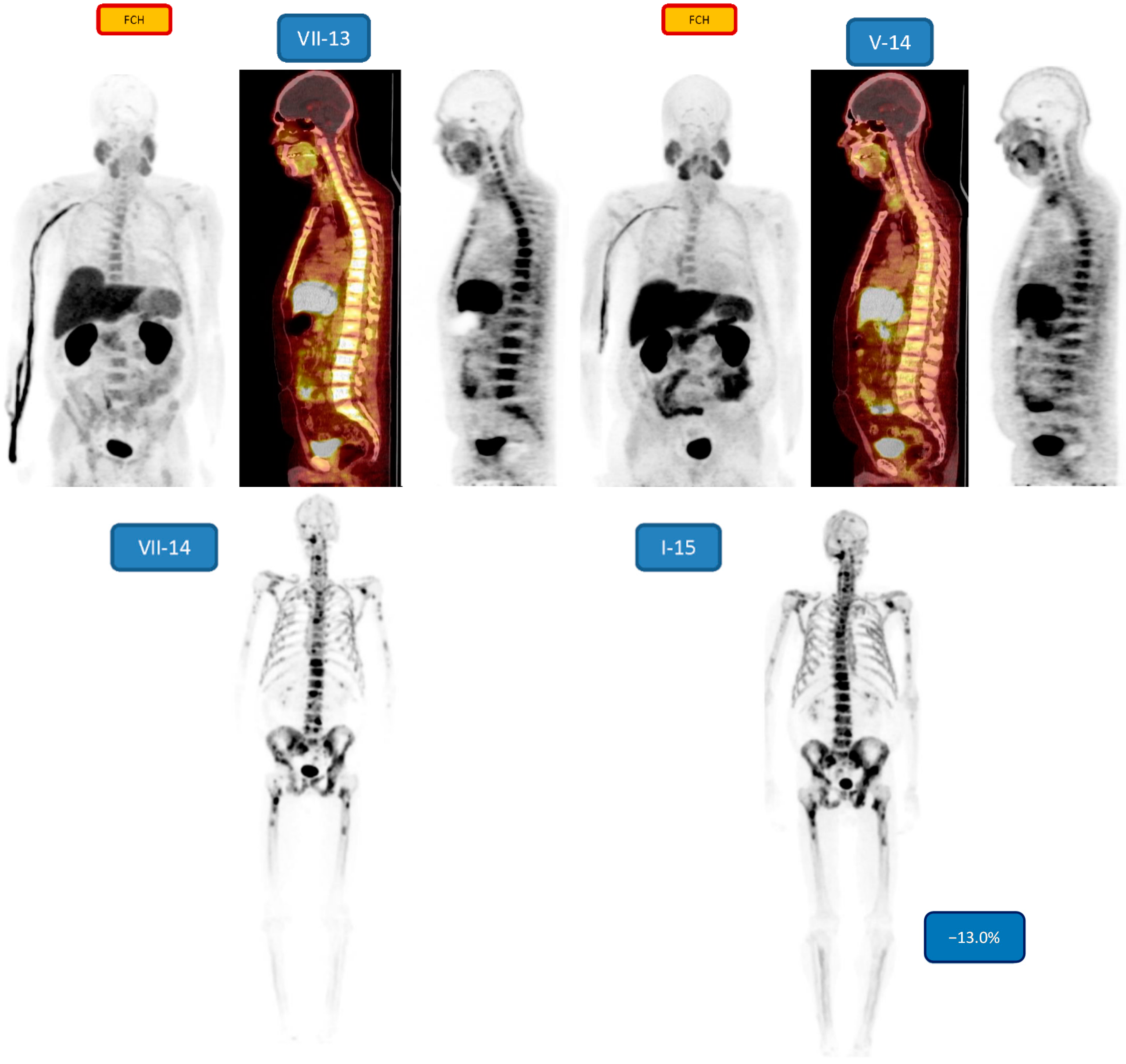

4. Discussion
5. Conclusion
Author Contributions
Conflicts of Interest
References
- Wirth, M.; Horninger, W. How I treat metastatic prostate cancer. J. OncoPathol. 2014, 2, 13–26. [Google Scholar] [CrossRef]
- Petrylak, D.P.; Tangen, C.M.; Hussain, M.H.; Lara, P.N., Jr.; Jones, J.A.; Taplin, M.E.; Burch, P.A.; Berry, D.; Moinpour, C.; Kohli, M.; et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 2004, 351, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Hotte, S.J. Guidelines for the management of castrate-resistant prostate cancer. Can. Urol. Assoc. J. 2010, 4, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Sathiakumar, N.; Delzell, E.; Morrisey, M.A.; Falkson, C.; Yong, M.; Chia, V.; Blackburn, J.; Arora, T.; Kilgore, M.L. Mortality following bone metastasis and skeletal-related events among men with prostate cancer: A population-based analysis of US Medicare beneficiaries, 1999–2006. Prostate Cancer Prostatic Dis. 2011, 14, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Lange, P.H.; Vessella, R.L. Mechanisms, hypotheses and questions regarding prostate cancer micrometastases to bone. Cancer Metastasis Rev. 1998, 17, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Bodei, L.; Lam, M.; Chiesa, C.; Flux, G.; Brans, B.; Chiti, A.; Giammarile, F. European Association of Nuclear Medicine (EANM). EANM procedure guideline for treatment of refractory metastatic bone pain. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Pandit-Taskar, N.; Larson, S.M.; Carrasquillo, J.A. Bone-Seeking radiopharmaceuticals for treatment of osseous metastases, part 1: α Therapy with 223Ra-dichloride. J. Nucl. Med. 2014, 55, 268–274. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, I. Strontium-89 for prostate cancer with bone metastases: The potential of cancer control and improvement of overall survival. Ann. Nucl. Med. 2014, 28, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Heron, D.E.; Brufsky, A.; Beriwal, S.; Kurman, M. Myelotoxicity of samarium Sm 153 lexidronam in patients receiving prior treatment with chemotherapy or radiotherapy. Ann. Oncol. 2008, 19, 1639–1643. [Google Scholar] [CrossRef] [PubMed]
- Biersack, H.J.; Palmedo, H.; Andris, A.; Rogenhofer, S.; Knapp, F.F.; Guhlke, S.; Ezziddin, S.; Bucerius, J.; von Mallek, D. Palliation and survival after repeated 188Re-HEDP therapy of hormone-refractory bone metastases of prostate cancer: A retrospective analysis. J. Nucl. Med. 2011, 52, 1721–1716. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Johnson, M.M.; Mathew, P.; Pagliaro, L.C.; Araujo, J.C.; Aparicio, A.; Corn, P.G.; Tannir, N.M.; Wong, F.C.; Fisch, M.J.; et al. Randomized phase 2 study of bone-targeted therapy containing strontium-89 in advanced castrate-sensitive prostate cancer. Cancer 2015, 121, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.R.; Wong, T.Z.; Armstrong, A.J.; George, D.J. Radium-223 chloride: A potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag. Res. 2013, 5, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sartor, O.; Coleman, R.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: Results from a phase 3, double-blind, randomised trial. Lancet Oncol. 2014, 15, 738–746. [Google Scholar] [CrossRef]
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, G.; Fisher, D.R.; Roeske, J.C.; Bruland, O.S.; Larsen, R.H. Targeting of osseous sites with α-emitting 223Ra: Comparison with β-emitter 89Sr in mice. J. Nucl. Med. 2003, 42, 252–259. [Google Scholar]
- Nilsson, S.; Larsen, R.H.; Fosså, S.D.; Balteskard, L.; Borch, K.W.; Westlin, J.E.; Salberg, G.; Bruland, O.S. First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin. Cancer Res. 2005, 11, 4451–4459. [Google Scholar] [CrossRef] [PubMed]
- Segall, G.; Delbeke, D.; Stabin, M.G.; Even-Sapir, E.; Fair, J.; Sajdak, R.; Smith, G.T. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans. J. Nucl. Med. 2010, 51, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Schirrmeister, H.; Guhlmann, A.; Elsner, K.; Kotzerke, J.; Glatting, G.; Rentschler, M.; Neumaier, B.; Trager, H.; Nussle, K.; Reske, S.N. Sensitivity in detecting osseous lesions depends on anatomic localization: Planar bone scintigraphy versus 18F PET. J. Nucl. Med. 1999, 40, 1623–1629. [Google Scholar] [PubMed]
- Even-Sapir, E.; Metser, U.; Mishani, E.; Lievshitz, G.; Lerman, H.; Leibovitch, I. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP Planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J. Nucl. Med. 2006, 47, 287–297. [Google Scholar] [PubMed]
- Beheshti, M.; Vali, R.; Waldenberger, P.; Fitz, F.; Nader, M.; Loidl, W.; Broinger, G.; Stoiber, F.; Foglman, I.; Langsteger, W. Detection of bone metastases in patients with prostate cancer by 18F fluorocholine and 18F fluoride PET-CT: A comparative study. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 1766–1774. [Google Scholar] [CrossRef] [PubMed]
- Withofs, N.; Grayet, B.; Tancredi, T.; Rorive, A.; Mella, C.; Giacomelli, F.; Mievis, F.; Aerts, J.; Waltregny, D.; Jerusalem, G.; Hustinx, R. 18F-fluoride PET/CT for assessing bone involvement in prostate and breast cancers. Nucl. Med. Commun. 2011, 32, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Jadvar, H.; Desai, B.; Ji, L.; Conti, P.S.; Dorff, T.B.; Groshen, S.G.; Gross, M.E.; Pinski, J.K.; Quinn, D.I. Prospective evaluation of 18F-NaF and 18F-FDG PET/CT in detection of occult metastatic disease in biochemical recurrence of prostate cancer. Clin. Nucl. Med. 2012, 37, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Kjolhede, H.; Ahlgren, G.; Almquist, H.; Liedberg, F.; Lyttkens, K.; Ohlsson, T.; Bratt, O. Combined 18F-fluorocholine and 18F-fluoride positron emission tomography/computed tomography imaging for staging of high-risk prostate cancer. BJU Int. 2012, 110, 1501–1506. [Google Scholar] [CrossRef] [PubMed]
- Mosavi, F.; Johansson, S.; Sandberg, D.T.; Turesson, I.; Sorensen, J.; Ahlstrom, H. Whole-Body diffusion-weighted MRI compared with 18F-NaF PET/CT for detection of bone metastases in patients with high-risk prostate carcinoma. AJR Am. J. Roentgenol. 2012, 199, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Damle, N.A.; Bal, C.; Bandopadhyaya, G.P.; Kumar, L.; Kumar, P.; Malhotra, A.; Lata, S. The role of 18F-fluoride PET-CT in the detection of bone metastases in patients with breast, lung and prostate carcinoma: A comparison with FDG PET/CT and 99mTc-MDP bone scan. Jpn. J. Radiol. 2013, 31, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Hillner, B.E.; Siegel, B.A.; Hanna, L.; Duan, F.; Shields, A.F.; Coleman, R.E. Impact of 18F-fluoride PET in patients with known prostate cancer: Initial results from the National Oncologic PET Registry. J. Nucl. Med. 2014, 55, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Langsteger, W.; Balogova, S.; Huchet, V.; Beheshti, M.; Paycha, F.; Egrot, C.; Janetschek, G.; Loidl, W.; Nataf, V.; Kerrou, K.; et al. Fluorocholine (18F) and sodium fluoride (18F) PET/CT in the detection of prostate cancer: Prospective comparison of diagnostic performance determined by masked reading. Q. J. Nucl. Med. Mol. Imaging 2011, 55, 448–457. [Google Scholar] [PubMed]
- Von Eyben, F.E.; Kairemo, K.; Kiljunen, T.; Joensuu, T. Planning of external beam radiotherapy guided by PET/CT. Curr. Radiopharm. 2015, 8, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Xofigo® 1000 kBq/mL solution for injection. Summary of Product Characteristics. Bayer Pharma AG, Berlin, Germany. Revision dated November 2013. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002653/WC500156172.pdf (accessed on 6 November 2014).
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50, 122S–150S. [Google Scholar] [CrossRef] [PubMed]
- Kairemo, K.; Rasulova, N.; Partanen, K.; Joensuu, T. Preliminary clinical experience of trans-1-Amino-3-(18)F-fluorocyclobutanecarboxylic acid (anti-(18)F-FACBC) PET/CT imaging in prostate cancer catients. Biomed. Res. Int. 2014, 2014, 305182. [Google Scholar] [CrossRef] [PubMed]
- Logothetis, C.J.; Basch, E.; Molina, A.; Fizazi, K.; North, S.A.; Chi, K.N.; Jones, R.J.; Goodman, O.B.; Mainwaring, P.N.; Sternberg, C.N.; et al. Effect of abiraterone acetate and prednisone compared with placebo and prednisone on pain control and skeletal-related events in patients with metastatic castration resistant prostate cancer: Exploratory analysis of data from the COU-AA 301 randomised trial. Lancet Oncol. 2012, 13, 1210–1217. [Google Scholar] [PubMed]
- Scher, H.I.; Fizazi, K.; Saad, F.; Taplin, M.E.; Sternberg, C.N.; Miller, K.; de Wit, R.; Mulders, P.; Chi, K.N.; Shore, N.D.; et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N. Engl. J. Med. 2012, 367, 1187–1197. [Google Scholar] [PubMed]
- Nilsson, S.; Strang, P.; Aksnes, AK.; Franzèn, L.; Olivier, P.; Pecking, A.; Staffurth, J.; Vasanthan, S.; Andersson, C.; Bruland, Ø.S. A randomized, dose-response, multicenter phase II study of radium-223 chloride for the palliation of painful bone metastases in patients with castration-resistant prostate cancer. Eur. J. Cancer 2012, 48, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, S.; Franzén, L.; Parker, C.; Tyrrell, C.; Blom, R.; Tennvall, J.; Lennernäs, B.; Petersson, U.; Johannessen, D.C.; Sokal, M.; et al. Two-Year survival follow-up of the randomized, double-blind, placebo-controlled phase II study of radium-223 chloride in patients with castration-resistant prostate cancer and bone metastases. Clin. Genitourin. Cancer 2013, 11, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Humm, J.L.; Sartor, O.; Parker, C.; Bruland, O.S.; Macklis, R. Radium-223 in the treatment of osteoblastic metastases: A critical clinical review. Int. J. Radiat. Oncol. Biol. Phys. 2015, 91, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Oyen, W.; Sundram, F.; Haug, A.R.; Kairemo, K.; Lewington, V.; Mäenpää, H.; Mortensen, J.; Mottaghy, F.; Virgolini, I.; O’Sullivan, J.M.; et al. Radium-223 dichloride (Ra-223) for the treatment of metastatic castration-resistant prostate cancer: Optimizing clinical practice in nuclear medicine center. J. OncoPathol. 2015, 3, 1–25. [Google Scholar] [CrossRef]
- Basch, E.; Loblaw, D.A.; Oliver, T.K.; Carducci, M.; Chen, R.C.; Frame, J.N.; Garrels, K.; Hotte, S.; Kattan, M.W.; Raghavan, D.; et al. Systemic therapy in men with metastatic castration-resistant prostate cancer: American Society of Clinical Oncology and Cancer Care Ontario clinical practice guideline. J. Clin. Oncol. 2014, 32, 3436–3448. [Google Scholar] [CrossRef] [PubMed]
- Etchebehere, E.C.; Araujo, J.C.; Fox, P.S.; Swanston, N.M.; Macapinlac, H.A.; Rohren, E.M. Prognostic factors in patients treated with Ra-223: The role of skeletal tumor burden on baseline 18F-fluoride-PET/CT in predicting overall survival. J. Nucl. Med. 2015, 56, 1177–1184. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kairemo, K.; Joensuu, T. Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer—Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics 2015, 5, 413-427. https://doi.org/10.3390/diagnostics5040413
Kairemo K, Joensuu T. Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer—Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics. 2015; 5(4):413-427. https://doi.org/10.3390/diagnostics5040413
Chicago/Turabian StyleKairemo, Kalevi, and Timo Joensuu. 2015. "Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer—Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT" Diagnostics 5, no. 4: 413-427. https://doi.org/10.3390/diagnostics5040413
APA StyleKairemo, K., & Joensuu, T. (2015). Radium-223-Dichloride in Castration Resistant Metastatic Prostate Cancer—Preliminary Results of the Response Evaluation Using F-18-Fluoride PET/CT. Diagnostics, 5(4), 413-427. https://doi.org/10.3390/diagnostics5040413





