Osteoprotegerin as an Emerging Biomarker of Carotid Artery Stenosis? A Scoping Review with Meta-Analysis
Abstract
1. Introduction
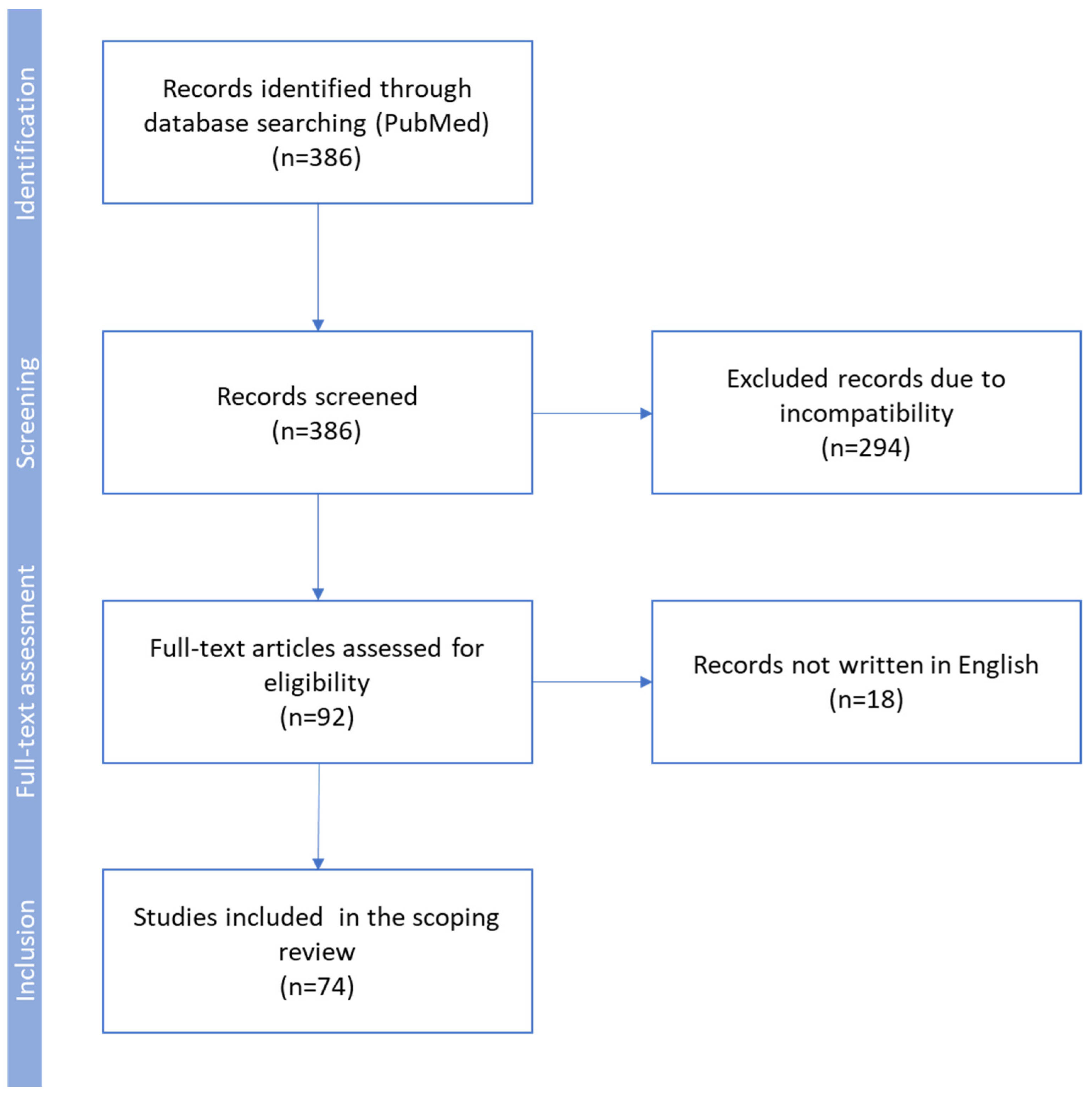
2. Methodology
Statistical Analysis
3. Results and Discussion
3.1. Occurrence of Asymptomatic Carotid Artery Stenosis
3.2. The Physiological Role of Osteoprotegerin
3.3. The Osteoprotegerin Role in Atherosclerosis
3.4. Osteoprotegerin as a Potential Biomarker of Cardiovascular Disorders
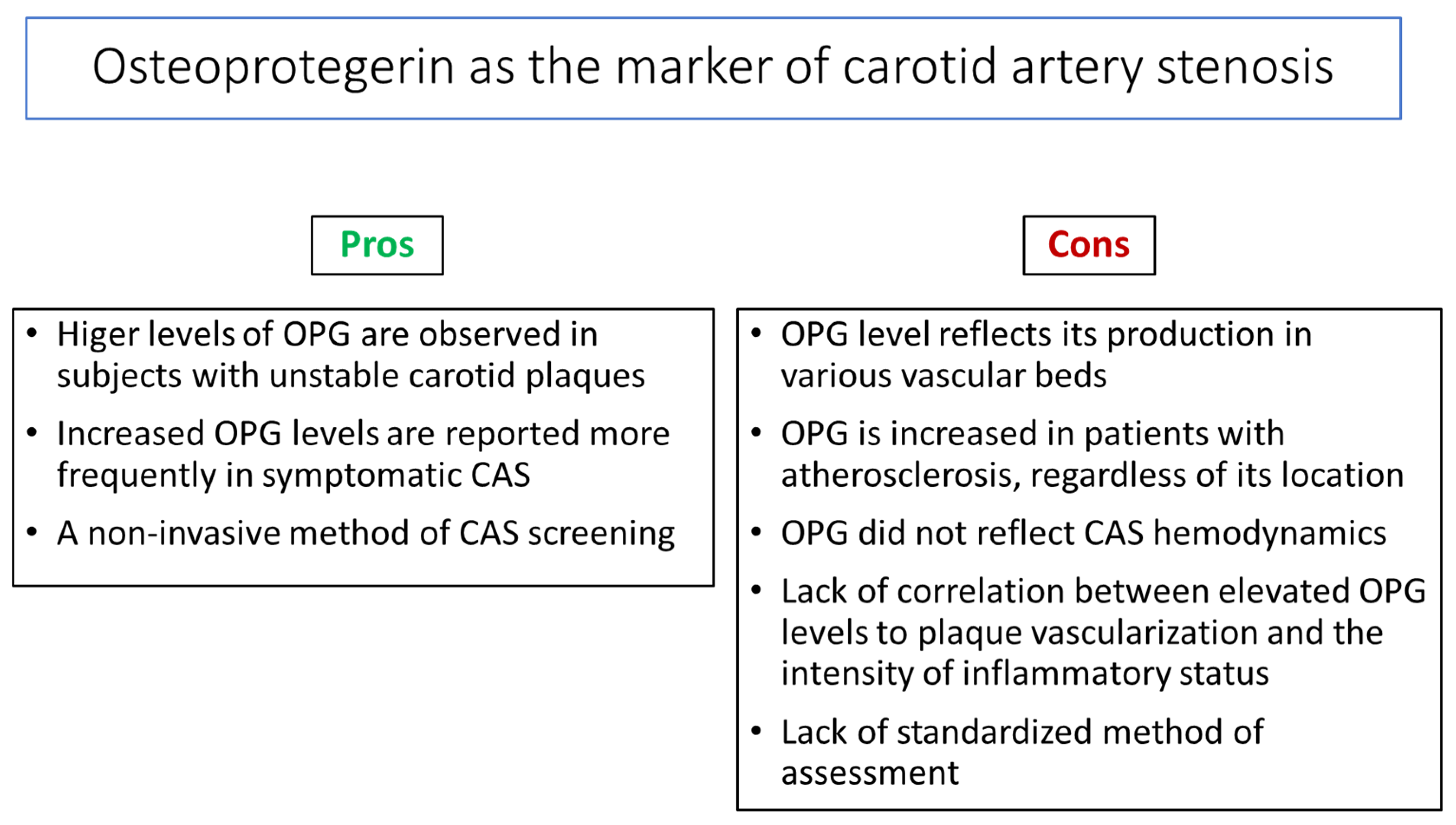
3.5. Osteoprotegerin as a Biomarker in Subjects with Carotid Artery Stenosis
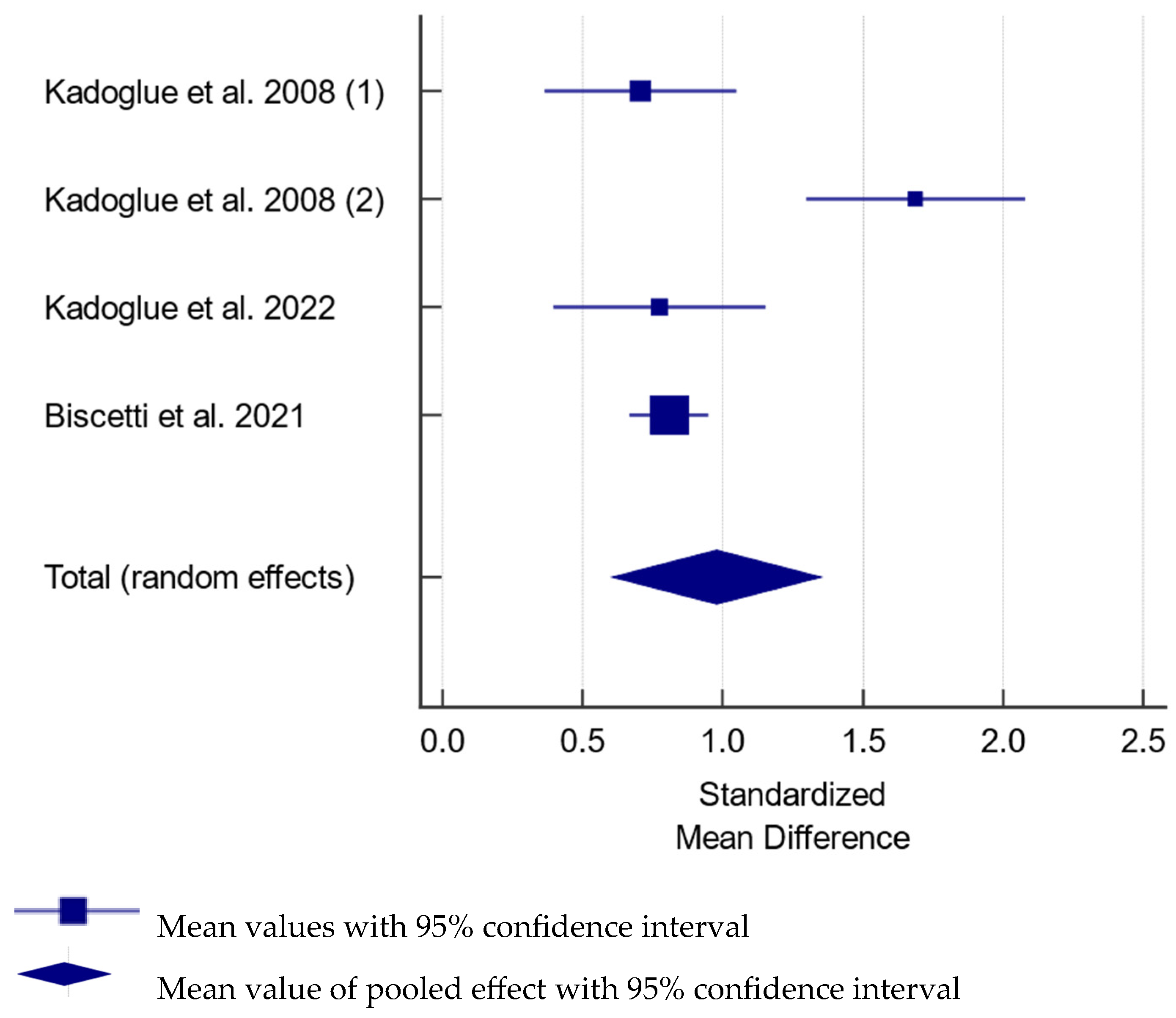
3.6. Meta Analysis of Studies Assessing Serum Osteoprotegerin in Carotid Artery Stenosis
3.7. Osteoprotegerin and Carotid Plaque Vulnerability
4. Summary
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hansson, G.K. Inflammatory mechanisms in atherosclerosis. J. Thromb. Haemost. 2006, 1, 328–331. [Google Scholar] [CrossRef]
- Bonora, E. The metabolic syndrome and cardiovascular disease. Ann. Med. 2006, 38, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Mallika, V.; Goswami, B.; Rajappa, M. Atherosclerosis pathophysiology and the role of novel risk factors: A clinical biochemical perspective. Angiology 2007, 58, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Niessner, A.; Goronzy, J.J.; Weyand, C.M. Immune-mediated mechanisms in atherosclerosis: Prevention and treatment of clinical manifestations. Curr. Pharm. Des. 2007, 13, 3701–3710. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef]
- Spagnoli, L.G.; Bonnano, E.; Sangiorgi, G.; Mauriello, A. Role of inflammation in atherosclerosis. J. Nucl. Med. 2007, 48, 1800–1815. [Google Scholar] [CrossRef]
- Saba, L.; Nardi, V.; Cau, R.; Gupta, A.; Kamel, H.; Suri, J.S.; Balestrieri, A.; Congiu, T.; Butler, A.P.; Gieseg, S.; et al. Carotid Artery Plaque Calcifications: Lessons From Histopathology to Diagnostic Imaging. Stroke 2022, 53, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C.; et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Goldstein, L.B.; Bushnell, C.D.; Adams, R.J.; Appel, L.J.; Braun, L.T.; Chaturvedi, S.; Creager, M.A.; Culebras, A.; Eckel, R.H.; Hart, R.G.; et al. Guidelines for the primary prevention of stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011, 42, 517–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.T.; Yoo, S.H.; Kwon, J.H.; Kwon, S.U.; Kim, J.S. Subtyping of ischemic stroke based on vascular imaging: Analysis of 1167 acute, consecutive patients. J. Clin. Neurol. 2006, 2, 225–230. [Google Scholar] [CrossRef]
- Halliday, A.; Harrison, M.; Hayter, E.; Kong, X.; Mansfield, A.; Marro, J.; Pan, H.; Peto, R.; Potter, J.; Rahimi, K.; et al. 10-year stroke prevention after successful carotid endarterectomy for asymptomatic stenosis (ACST-1): A multicentre randomised trial. Lancet 2010, 376, 1074–1084. [Google Scholar] [CrossRef] [PubMed]
- Vlachopoulos, C.; Xaplanteris, P.; Aboyans, V.; Brodmann, M.; Cífková, R.; Cosentino, F.; De Carlo, M.; Gallino, A.; Landmesser, U.; Laurent, S.; et al. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation. Atherosclerosis 2015, 241, 507–532. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Khuseyinova, N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 15–26. [Google Scholar] [CrossRef]
- Alvarez Garcia, B.; Ruiz, C.; Chacon, P.; Sabin, J.A.; Matas, M. High-sensitivity C-reactive protein in high-grade carotid stenosis: Risk marker for unstable carotid plaque. J. Vasc. Surg. 2003, 38, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.H.; Cho, C.H.; Kim, H.O.; Jo, Y.H.; Yoon, K.S.; Lee, J.H.; Park, J.C.; Park, K.C.; Ahn, T.B.; Chung, K.C.; et al. Plaque rupture is a determinant of vascular events in carotid artery atherosclerotic disease: Involvement of matrix metalloproteinases 2 and 9. J. Clin. Neurol. 2011, 7, 69–76. [Google Scholar] [CrossRef]
- Dahl, T.B.; Yndestad, A.; Skjelland, M.; Øie, E.; Dahl, A.; Michelsen, A.; Damås, J.K.; Tunheim, S.H.; Ueland, T.; Smith, C.; et al. Increased expression of visfatin in macrophages of human unstable carotid and coronary atherosclerosis: Possible role in inflammation and plaque destabilisation. Circulation 2007, 115, 972–980. [Google Scholar] [CrossRef] [PubMed]
- Mannheim, D.; Herrmann, J.; Versari, D.; Gössl, M.; Meyer, F.B.; McConnell, J.P.; Lerman, L.O.; Lerman, A. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008, 39, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- Urbonaviciene, G.; Frystyk, J.; Flyvbjerg, A.; Henneberg, E.W.; Lindholt, J.S. Association of serum adiponectin with risk for cardiovascular events in patients with peripheral arterial disease. Atherosclerosis 2010, 210, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Golledge, J.; McCann, M.; Mangan, S.; Lam, A.; Karan, M. Osteoprotegerin and osteopontin are expressed at high concentrations within symptomatic carotid atherosclerosis. Stroke 2004, 35, 1636–1641. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, N.; Korczyn, A. Asymptomatic carotid artery stenosis (ACAS). J. Neural Transm. 2011, 118, 629. [Google Scholar] [PubMed]
- Cola, C.; Clementi, E.; Biondi-Zoccai, G.; Sangiorgi, G. From carotid plaque biology to serologic markers of vulnerability to predict the risk of cerebrovascular events. Acta Chir. Belg. 2007, 107, 129–142. [Google Scholar] [CrossRef]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.C.; Chang, M.S.; Lüthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar] [CrossRef]
- Kakkos, S.K.; Nicolaides, A.N.; Charalambous, I.; Thomas, D.; Giannopoulos, A.; Naylor, A.R.; Geroulakos, G.; Abbott, A.L. Predictors and clinical significance of progression or regression of asymptomatic carotid stenosis. J. Vasc. Surg. 2014, 59, 956–967.e951. [Google Scholar] [CrossRef] [PubMed]
- Sushrut, D.; Pratik, B.; Seemant, C. Carotid Artery Stenosis: Medical Therapy, Surgery, and Stenting. Curr. Neurol. Neurosci. Rep. 2017, 17, 77. [Google Scholar]
- Fine-Edelstein, J.S.; Wolf, P.A.; O’leary, D.H.; Poehlman, H.; Belanger, A.J.; Kase, C.S.; D’Agostino, R.B. Precursors of extracranial carotid atherosclerosis in the Framingham Study. Neurology 1994, 44, 1046–1050. [Google Scholar] [CrossRef] [PubMed]
- de Weerd, M.; Greving, J.P.; Hedblad, B.; Lorenz, M.W.; Mathiesen, E.B.; O’Leary, D.H.; Rosvall, M.; Sitzer, M.; Buskens, E.; Bots, M.L. Prevalence of asymptomatic carotid artery stenosis in the general population: An individual participant data meta-analysis. Stroke 2010, 41, 1294–1297. [Google Scholar] [CrossRef]
- De Angelis, M.; Scrucca, L.; Leandri, M.; Mincigrucci, S.; Bistoni, S.; Bovi, M.; Calabrese, G.; Pippi, R.; Parretti, D.; Grilli, P.; et al. Prevalence of carotid stenosis in type 2 diabetic patients asymptomatic for cerebrovascular disease. Diabetes Nutr. Metab. 2003, 16, 48–55. [Google Scholar] [PubMed]
- Luedemann, J.; Schminke, U.; Berger, K.; Piek, M.; Willich, S.N.; Döring, A.; John, U.; Kessler, C. Association between behavior-dependent cardiovascular risk factors and asymptomatic carotid atherosclerosis in a general population. Stroke 2002, 33, 2929–2935. [Google Scholar] [CrossRef]
- Mannami, T.; Baba, S.; Konishi, M.; Terao, A.; Kitamura, A.; Iida, M.; Shimamoto, T. Comparison of the prevalence of asymptomatic carotid atherosclerosis detected by highresolution ultrasonography in rural and urban middle-aged Japanese men. J. Stroke Cerebrovasc. Dis. 2000, 9, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Santo Signorelli, S.; Di Pino, L.; Fichera, G.; Celotta, G.; Pennisi, G.; Marchese, G.; Costa, M.P.; Fallico, R.; Torrisi, B.; Virgilio, V. Ultrasound diagnosis of carotid artery lesions in a population of asymptomatic subjects presenting atherosclerosis risk factors. J. Stroke Cerebrovasc. Dis. 2004, 13, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Bal, S.S.; Khurana, D.; Sharma, A.; Lal, V.; Bhansali, A.; Prabhakar, S. Association of metabolic syndrome with carotid atherosclerosis in the young North Indian population. Diabetes Metab. Syndr. 2011, 5, 153–157. [Google Scholar] [CrossRef]
- Simons, P.C.; Algra, A.; Eikelboom, B.C.; Grobbee, D.E.; van der Graaf, Y.; SMART Study Group. Carotid artery stenosis in patients with peripheral arterial disease: The SMART study. SMART study group. J. Vasc. Surg. 1999, 30, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, B.; Al-Khaffaf, H. Prevalence of significant asymptomatic carotid artery disease in patients with peripheral vascular disease: A meta-analysis. Eur. J. Vasc. Endovasc. Surg. 2009, 37, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Bertges, D.J.; Muluk, V.; Whittle, J.; Kelley, M.; MacPherson, D.S.; Muluk, S.C. Relevance of carotid stenosis progression as a predictor of ischemic neurological outcomes. Arch. InternMed. 2003, 163, 2285–2289. [Google Scholar] [CrossRef] [PubMed]
- Muluk, S.C.; Muluk, V.S.; Sugimoto, H.; Rhee, R.Y.; Trachtenberg, J.; Steed, D.L.; Jarrett, F.; Webster, M.W.; Makaroun, M.S. Progression of asymptomatic carotid stenosis: A natural history study in 1004 patients. J. Vasc. Surg. 1999, 29, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.; Makaroun, M.S.; Muluk, V.S.; Webster, M.W.; Muluk, S.C. Etiologic factors in progression of carotid stenosis: A 10-year study in 905 patients. J. Vasc. Surg. 2000, 31, 31–38. [Google Scholar] [CrossRef]
- Hermus, L.; Lefrandt, J.D.; Tio, R.A.; Breek, J.C.; Zeebregts, C.J. Carotid plaque formation and serum biomarkers. Atherosclerosis 2010, 213, 21–29, Erratum in Atherosclerosis 2011, 216, 249. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Kinosaki, M.; Goto, M.; Kobayashi, F.; Tsuda, E.; Morinaga, T.; Higashio, K. Characterisation of structural domains of human Osteoclastogenesis inhibitory factor. J. Biol. Chem. 1998, 273, 5117–5123. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.G.; McDonnell, P.; Burke, M.B.; Deen, K.C.; Lyn, S.; Silverman, C.; Dul, E.; Appelbaum, E.R.; Eichman, C.; DiPrinzio, R.; et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J. Biol. Chem. 1998, 273, 14363–14367. [Google Scholar] [CrossRef] [PubMed]
- Pan, G. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 1997, 277, 815–818. [Google Scholar] [CrossRef]
- Pérez de Ciriza, C.; Lawrie, A.; Varo, N. Osteoprotegerin in cardiometabolic disorders. Int. J. Endocrinol. 2015, 2015, 564934. [Google Scholar] [CrossRef] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Schoppet, M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA 2004, 292, 490–495. [Google Scholar] [CrossRef]
- Kong, Y.Y.; Yoshida, H.; Sarosi, I.; Tan, H.L.; Timms, E.; Capparelli, C.; Morony, S.; Oliveira-dos-Santos, A.J.; Van, G.; Itie, A.; et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 1999, 397, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Bucay, N.; Sarosi, I.; Dunstan, C.R.; Morony, S.; Tarpley, J.; Capparelli, C.; Scully, S.; Tan, H.L.; Xu, W.; Lacey, D.L.; et al. Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev. 1998, 12, 1260–1268. [Google Scholar] [CrossRef] [PubMed]
- Schoppet, M.; Preissner, K.T.; Hofbauer, L.C. RANK ligand and osteoprotegerin. Paracrine regulators of bone metabolism and vascular function. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 549–553. [Google Scholar] [CrossRef]
- Hofbauer, L.C.; Shui, C.; Riggs, B.L.; Dunstan, C.R.; Spelsberg, T.C.; O’Brien, T.; Khosla, S. Effects of immunosuppressants on receptor activator of NF-kappaB ligand and osteoprotegerin production by human osteoblastic and coronary artery smooth muscle cells. Biochem. Biophys. Res. Commun. 2001, 280, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Malyankar, U.M.; Scantena, M.; Suchland, K.L.; Yun, T.J.; Clark, E.A.; Giachelli, C.M. Osteoprotegerin is an alpha vbeta 3-induced, NF-kappa B-dependent survival factor for endothelial cells. J. Biol. Chem. 2000, 275, 20959–20962. [Google Scholar] [CrossRef] [PubMed]
- Hofbauer, L.C.; Heufelder, A.E. Role of receptor activator of nuclear factor-kappaB ligand and osteoprotegerin in bone cell biology. J. Mol. Med. 2001, 79, 24353. [Google Scholar] [CrossRef]
- Yun, T.J.; Chaudhary, P.M.; Shu, G.L.; Frazer, J.K.; Ewings, M.K.; Schwartz, S.M.; Pascual, V.; Hood, L.E.; Clark, E.A. OPG/ FDCR-1, a TNF receptor family member, is expressed in lymphoid cells and is upregulated by ligating CD40. J. Immunol. 1998, 161, 6113–6121. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, M.; Myles, D.; Zhu, X.; Du, J.; Cao, X.; Chen, Y.E. PDGF induces osteoprotegerin expression in vascular smooth muscle cells by multiple signal pathways. FEBS Lett. 2002, 521, 180–184. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Zhang, J.; Lin Yg, Y.G.; Zhu, X.; Wilson, T.M.; Chen, Y.E. Activation of peroxisome proliferator-activated receptor gamma inhibits osteoprotegerin gene expression in human aortic smooth muscle cells. Biochem. Biophys. Res. Commun. 2002, 294, 597601. [Google Scholar] [CrossRef] [PubMed]
- Ishida, A.; Fujita, N.; Kitazava, R.; Tsuruo, T. Transforming growth factor-beta induces expression of receptor activator of NF-kappa B ligand in vascular endothelial cells derived from bone. J. Biol. Chem. 2002, 277, 26217–26224. [Google Scholar] [CrossRef] [PubMed]
- Collin-Osdoby, P. Regulation of vascular calcification by osteoclast regulatory factors RANK-L and osteoprotegerin. Circ. Res. 2004, 95, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Steffens, S.; Mach, F. The immune response is involved in atherosclerotic plaque calcification: Could the RANKL/RANK/OPG system be a marker of plaque instability? Clin. Dev. Immunol. 2007, 2007, 75805. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Mathiesen, E.B.; Brox, J.; Wilsgaard, T.; Njølstad, I.; Jørgensen, L.; Hansen, J.B. Relation between serum osteoprotegerin and carotid intima media thickness in a general population—The Tromsø Study. J. Thromb. Haemost. 2010, 8, 2133–2139. [Google Scholar] [CrossRef]
- Montagnana, M.; Lippi, G.; Danese, E.; Guidi, G.C. The role of osteoprotegerin in cardiovascular disease. Ann. Med. 2013, 45, 254–264. [Google Scholar] [CrossRef]
- Rochette, L.; Meloux, A.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. The Role of Osteoprotegerin and Its Ligands in Vascular Function. Int. J. Mol. Sci. 2019, 20, 705. [Google Scholar] [CrossRef] [PubMed]
- Musialik, K.; Szulińska, M.; Hen, K.; Skrypnik, D.; Bogdański, P. The relation between osteoprotegerin, inflammatory processes, and atherosclerosis in patients with metabolic syndrome. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4379–4385. [Google Scholar]
- Majerczyk, M.; Wajda, J.; Holecki, M.; Chudek, J. Osteoprotegeryna jako wskaźnik nasilenia miażdżycy i czynnik prognostyczny w udarze mózgu [Osteoprotegerin as a marker of atherosclerosis and a prognostic factor in stroke]. Postepy Hig. Med. Dosw. (Online) 2015, 69, 1505–1511. [Google Scholar] [PubMed]
- Bennett, B.J.; Scatena, M.; Kirk, E.A.; Rattazzi, M.; Varon, R.M.; Averill, M.; Schwartz, S.M.; Giachelli, C.M.; Rosenfeld, M.E. Osteoprotegerin inactivation accelerates advanced atherosclerotic lesion progression and calcification in older ApoE-/- mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2117–2124. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, O.; Gylfe, A.; Bailey, L.; Nordström, A.; Rudling, M.; Jung, C.; Bergström, S.; Waldenström, A.; Hansson, G.K.; Nordström, P. Osteoprotegerin promotes fibrous cap formation in atherosclerotic lesions of ApoE-deficient mice-brief report. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1478–1480, Erratum in Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1066–1068. [Google Scholar] [CrossRef]
- Albu, A.; Bondor, C.I.; Crăciun, A.M.; Fodor, D. Circulating osteoprotegerin and asymptomatic carotid atherosclerosis in postmenopausal non diabetic women. Adv. Med. Sci. 2014, 59, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Mathiesen, E.B.; Johnsen, S.H.; Brox, J.; Wilsgaard, T.; Njølstad, I.; Hansen, J.B. Serum osteoprotegerin, sRANKL and carotid plaque formation and growth in a general population-the Tromsø study. J. Thromb. Haemost. 2010, 8, 898–905. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Siasos, G.; Maniatis, K.; Oikonomou, E.; Kioufis, S.; Zaromitidou, M.; Paraskevopoulos, T.; Michalea, S.; Kollia, C.; Miliou, A.; et al. Serum osteoprotegerin and osteopontin levels are associated with arterial stiffness and the presence and severity of coronary artery disease. Int. J. Cardiol. 2013, 167, 1924–1928. [Google Scholar] [CrossRef]
- Del Toro, R.; Cavallari, I.; Tramontana, F.; Park, K.; Strollo, R.; Valente, L.; De Pascalis, M.; Grigioni, F.; Pozzilli, P.; Buzzetti, R.; et al. Association of bone biomarkers with advanced atherosclerotic disease in people with overweight/obesity. Endocrine 2021, 73, 339–346. [Google Scholar] [CrossRef]
- Lieb, W.; Gona, P.; Larson, M.G.; Massaro, J.M.; Lipinska, I.; Keaney, J.F., Jr.; Rong, J.; Corey, D.; Hoffmann, U.; Fox, C.S.; et al. Biomarkers of the osteoprotegerin pathway: Clinical correlates, subclinical disease, incident cardiovascular disease, and mortality. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- Abedin, M.; Omland, T.; Ueland, T.; Khera, A.; Aukrust, P.; Murphy, S.A.; Jain, T.; Gruntmanis, U.; McGuire, D.K.; de Lemos, J.A. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study). Am. J. Cardiol. 2007, 99, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Kiechl, S.; Schett, G.; Wenning, G.; Redlich, K.; Oberhollenzer, M.; Mayr, A.; Santer, P.; Smolen, J.; Poewe, W.; Willeit, J. Osteoprotegerin is a risk factor for progressive atherosclerosis and cardiovascular disease. Circulation 2004, 109, 2175–2180. [Google Scholar] [CrossRef]
- Noheria, A.; Mosley, T.H., Jr.; Kullo, I.J. Association of serum osteoprotegerin with left ventricular mass in African-American adults with hypertension. Am. J. Hypertens. 2010, 23, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Ueland, T.; Dahl, C.P.; Kjekshus, J.; Huthe, J.P.; Mach, F.; Goudev, A.; Linberg, M.; Wikstrand, J.; Aukrust, P.; Gullestad, L. Osteoprotegerin predicts progression of chronic heart failure: Results from CORONA. Circ. Heart Fail. 2011, 4, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Omland, T.; Drazner, M.H.; Ueland, T.; Abedin, M.; Murphy, S.A.; Aukrust, P.; de Lemos, J.A. Plasma osteoprotegerin levels in the general population: Relation to indices of left ventricular structure and function. Hypertension 2007, 49, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Bate, K.A.; Genetzakis, E.; Vescovi, J.; Gray, M.P.; Celermajer, D.S.; McGuire, H.M.; Grieve, S.M.; Vernon, S.T.; Cartland, S.P.; Yang, J.Y.; et al. Vascular Cytokines and Atherosclerosis: Differential Serum Levels of TRAIL, IL-18, and OPG in Obstructive Coronary Artery Disease. Biomolecules 2024, 4, 1119. [Google Scholar] [CrossRef]
- Wajda, J.; Świat, M.; Owczarek, J.A.; Holecki, M.; Duława, J.; Brzozowska, A.; Olszanecka-Glinianowicz, M.; Chudek, J. Osteoprotegerin assessment improves prediction of mortality in stroke patients. J. Stroke Cerebrovasc. Dis. 2019, 28, 1160–1167. [Google Scholar] [CrossRef] [PubMed]
- Özkalaycı, F.; Gülmez, Ö.; Uğur-Altun, B.; Pandi-Perumal, S.R.; Altun, A. The Role of Osteoprotegerin as a Cardioprotective Versus Reactive Inflammatory Marker: The Chicken or the Egg Paradox. Balkan Med. J. 2018, 35, 225–232. [Google Scholar] [CrossRef]
- Ziegler, S.; Kudlacek, S.; Luger, A.; Minar, E. Osteoprotegerin plasma concentrations correlate with severity of peripheral artery disease. Atherosclerosis 2005, 182, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Zagura, M.; Serg, M.; Kampus, P.; Zilmer, M.; Zilmer, K.; Eha, J.; Unt, E.; Lieberg, J.; Kals, J. Association of osteoprotegerin with aortic stiffness in patients with symptomatic peripheral artery disease and in healthy subjects. Am. J. Hypertens. 2010, 23, 586–591. [Google Scholar] [CrossRef]
- Scandale, G.; Dimitrov, G.; Recchia, M.; Carzaniga, G.; Perilli, E.; Carotta, M.; Catalano, M. Arterial stiffness and 5-year mortality in patients with peripheral arterial disease. J. Hum. Hypertens. 2020, 34, 505–511. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Segers, P. Noninvasive evaluation of left ventricular afterload: Part 2: Arterial pressure-flow and pressure-volume relations in humans. Hypertension 2010, 56, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Fehérvári, L.; Frigy, A.; Kocsis, L.; Szabó, I.A.; Szabo, T.M.; Urkon, M.; Jakó, Z.; Nagy, E.E. Serum Osteoprotegerin and Carotid Intima-Media Thickness Are Related to High Arterial Stiffness in Heart Failure with Reduced Ejection Fraction. Diagnostics 2021, 11, 764. [Google Scholar] [CrossRef] [PubMed]
- Browner, W.S.; Lui, L.Y.; Cummings, S.R. Associations of serum osteoprotegerin levels with diabetes, stroke, bone density, fractures, and mortality in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Rhee, E.J.; Oh, K.W.; Lee, W.Y.; Baek, K.H.; Yoon, K.H.; Kang, M.I.; Yun, E.J.; Park, C.Y.; Choi, M.G.; et al. Circulating osteoprotegerin levels are associated with age, waist-to-hip ratio, serum total cholesterol, and low-density lipoprotein cholesterol levels in healthy Korean women. Metabolism 2005, 54, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Toffoli, B.; Bossi, F.; Candido, R.; Stenner, E.; Carretta, R.; Barbone, F.; Fabris, B. Circulating osteoprotegerin is associated with chronic kidney disease in hypertensive patients. BMC Nephrol. 2017, 18, 219. [Google Scholar] [CrossRef]
- Skrzypczyk, P.; Stelmaszczyk-Emmel, A.; Szyszka, M.; Ofiara, A.; Pańczyk-Tomaszewska, M. Circulating calcification inhibitors are associated with arterial damage in pediatric patients with primary hypertension. Pediatr. Nephrol. 2021, 36, 2371–2382. [Google Scholar] [CrossRef]
- Blázquez-Medela, A.M.; García-Ortiz, L.; Gómez-Marcos, M.A.; Recio-Rodriguez, J.I.; Sánchez-Rodríguez, A.; López-Novoa, J.M.; Martínez-Salgado, C. Osteoprotegerin is associated with cardiovascular risk in hypertension and/or diabetes. Eur. J. Clin. Invest. 2012, 42, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Hur, S.; Cho, S.H.; Song, B.K.; Cho, B.J. Effect of Resistance Exercise on Serum Osteoprotegerin Levels and Insulin Resistance in Middle-Aged Women with Metabolic Syndrome. Med. Sci. Monit. 2018, 24, 9385–9391. [Google Scholar] [CrossRef]
- Holecki, M.; Zahorska-Markiewicz, B.; Janowska, J.; Nieszporek, T.; Wojaczyńska-Stanek, K.; Żak-Gołąb, A.; Więcek, A. The influence of weight loss on serum osteoprotegerin concentration in obese perimenopausal women. Obesity 2007, 15, 1925–1929. [Google Scholar] [CrossRef]
- Bergström, I.; Parini, P.; Gustafsson, S.A.; Andersson, G.; Brinck, J. Physical training increases osteoprotegerin in postmenopausal women. J. Bone Miner. Metab. 2012, 30, 202–207. [Google Scholar] [CrossRef]
- Guarner, V.; Rubio-Ruiz, M.E. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip. Top. Gerontol. 2015, 40, 99–106. [Google Scholar]
- Dallmeier, D.; Larson, M.G.; Vasan, R.S.; Keaney Jr, J.F.; Fontes, J.D.; Meigs, J.B.; Fox, C.S.; Benjamin, E.J. Metabolic syndrome and inflammatory biomarkers: A community-based cross-sectional study at the Framingham Heart Study. Diabetol. Metab. Syndr. 2012, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Schoppet, M.; Sattler, A.M.; Schaefer, J.R.; Herzum, M.; Maisch, B.; Hofbauer, L.C. Increased osteoprotegerin serum levels in men with coronary artery disease. J. Clin. Endocrinol. Metab. 2003, 88, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Szulc, P.; Hofbauer, L.C.; Heufelder, A.E.; Roth, S.; Delmas, P.D. Osteoprotegerin serum levels in men: Correlation with age, estrogen, and testosterone status. J. Clin. Endocrinol. Metab. 2001, 86, 3162–3165. [Google Scholar] [CrossRef]
- Knudsen, S.T.; Foss, C.H.; Poulsen, P.L.; Anderson, N.H.; Morgensen, C.E.; Rasmussen, L.M. Increased plasma concentrations of osteoprotegerin in type 2 diabetic patients with microvascular complications. Eur. J. Endocrinol. 2003, 149, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Kazama, J.J.; Shigematsu, T.; Yano, K.; Tsuda, E.; Miura, M.; Iwasaki, Y.; Kawaguchi, Y.; Gejyo, F.; Kurokawa, K.; Fukagawa, M. Increased circulating levels of osteoclastogenesis-inhibitory factor (osteoprotegerin) in patients with chronic renal failure. Am. J. Kidney Dis. 2002, 39, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Khosla, S.; Arrighi, H.M.; Melton, I.I.I.L.J.; Atkinson, E.J.; O’fallon, W.M.; Dunstan, C.; Riggs, B.L. Correlates of osteoprotegerin levels in women and men. Osteoporos. Int. 2002, 13, 3949. [Google Scholar] [CrossRef] [PubMed]
- Kazama, J.J.; Kato, H.; Sato, T.; Shigematsu, T.; Fukagawa, M.; Iwasaki, Y.; Gejyo, F. Circulating osteoprotegerin is not removed through haemodialysis membrane. Nephrol. Dial. Transplant. 2002, 17, 1860–1861. [Google Scholar] [CrossRef][Green Version]
- Boström, K.; Watson, K.E.; Horn, S.; Wortham, C.; Herman, I.M.; Demer, L.L. Bone morphogenetic protein expression in human atherosclerotic lesions. J. Clin. Invest. 1993, 91, 1800–1809. [Google Scholar] [CrossRef] [PubMed]
- Hirota, S.; Imakita, M.; Kohri, K.; Ito, A.; Morii, E.; Adachi, S.; Kim, H.M.; Kitamura, Y.; Yutani, C.H.; Nomura, S. Expression of osteopontin messenger RNA by macrophages in atherosclerotic plaques. A possible association with calcification. Am. J. Pathol. 1993, 143, 1003–1008. [Google Scholar] [PubMed]
- Shanahan, C.M.; Cary, N.R.; Metcalfe, J.C.; Weissberg, P.L. High expression of genes for calcification-regulating proteins in human atherosclerotic plaques. J. Clin. Invest. 1994, 93, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.R.; Garvin, M.R.; Stewart, D.K.; Hinohara, T.; Simpson, J.B.; Schwartz, S.M.; Giachelli, C.M. Osteopontin is synthesised by macrophage, smooth muscle, and endothelial cells in primary and restenotic human coronary atherosclerotic plaques. Arterioscler. Thromb. 1994, 14, 1648–1656. [Google Scholar] [CrossRef]
- Dhore, C.R.; Cleutjens, J.P.; Lutgens, E.; Cleutjens, K.B.; Geusens, P.P.; Kitslaar, P.J.; Tordoir, J.H.; Spronk, H.M.; Vermeer, C.; Daemen, M.J. Differential expression of bone matrix regulatory proteins in human atherosclerotic plaques. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1998–2003. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Gerasimidis, T.; Moumtzouoglou, A.; Kapelouzou, A.; Sailer, N.; Fotiadis, G.; Vitta, I.; Katinios, A.; Kougias, P.; Bandios, S.; et al. Intensive Lipid-lowering Therapy Ameliorates Novel Calcification Markers and GSM Score in Patients with Carotid Stenosis. Eur. J. Vasc. Endovasc. Surg. 2008, 35, 661–668. [Google Scholar] [CrossRef] [PubMed]
- Min, H.; Morony, S.; Sarosi, I.; Dunstan, C.R.; Capparelli, C.; Scully, S.; Van, G.; Kaufman, S.; Kostenuik, P.J.; Lacey, D.L.; et al. Osteoprotegerin reverses osteoporosis by inhibiting endosteal osteoclasts and prevents vascular calcification by blocking a process resembling osteoclastogenesis. J. Exp. Med. 2000, 192, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Candido, R.; Toffoli, B.; Corallini, F.; Bernardi, S.; Zella, D.; Voltan, R.; Grill, V.; Celeghini, C.; Fabris, B. Human full-length osteoprotegerin induces the proliferation of rodent vascular smooth muscle cells both in vitro and in vivo. J. Vasc. Res. 2010, 47, 252–261. [Google Scholar] [CrossRef]
- Kadoglou, N.P.; Gerasimidis, T.; Golemati, S.; Kapelouzou, A.; Karayannacos, P.E.; Liapis, C.D. The relationship between serum levels of vascular calcification inhibitors and carotid plaque vulnerability. J. Vasc. Surg. 2008, 47, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Sailer, N.; Moumtzouoglou, A.; Kapelouzou, A.; Gerasimidis, T.; Liapis, C.D. Aggressive lipid-lowering is more effective than moderate lipid-lowering treatment in carotid plaque stabilization. J. Vasc. Surg. 2010, 51, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.; Gerasimidis, T.; Kapelouzou, A.; Moumtzouoglou, A.; Avgerinos, E.D.; Kakisis, J.D.; Karayannacos, P.E.; Liapis, C.D. Beneficial changes of serum calcification markers and contralateral carotid plaques echogenicity after combined carotid artery stenting plus intensive lipid-lowering therapy in patients with bilateral carotid stenosis. Eur. J. Vasc. Endovasc. Surg. 2010, 39, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Kadoglou, N.P.E.; Moulakakis, K.G.; Mantas, G.; Kakisis, J.D.; Mylonas, S.N.; Valsami, G.; Liapis, C.D. The Association of Arterial Stiffness With Significant Carotid Atherosclerosis and Carotid Plaque Vulnerability. Angiology 2022, 73, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Tinelli, G.; Rando, M.M.; Nardella, E.; Cecchini, A.L.; Angelini, F.; Straface, G.; Filipponi, M.; Arena, V.; Pitocco, D.; et al. Association between carotid plaque vulnerability and high mobility group box-1 serum levels in a diabetic population. Cardiovasc. Diabetol. 2021, 20, 114, Erratum in Cardiovasc. Diabetol. 2021, 20, 184. [Google Scholar] [CrossRef] [PubMed]
- Kwon, A.; Choi, Y.S.; Choi, Y.W.; Chung, W.B.; Park, C.S.; Chung, W.S.; Lee, M.Y.; Youn, H.J. Serum Osteoprotegerin Is Associated With Calcified Carotid Plaque: A Strobe-Compliant Observational Study. Medicine 2016, 95, e3381. [Google Scholar] [CrossRef] [PubMed]
- Davaine, J.M.; Quillard, T.; Brion, R.; Lapérine, O.; Guyomarch, B.; Merlini, T.; Chatelais, M.; Guilbaud, F.; Brennan, M.Á.; Charrier, C.; et al. Osteoprotegerin, pericytes and bone-like vascular calcification are associated with carotid plaque stability. PLoS ONE 2014, 9, e107642. [Google Scholar] [CrossRef] [PubMed]
- Vik, A.; Mathiesen, E.B.; Notø, A.T.; Sveinbjørnsson, B.; Brox, J.; Hansen, J.B. Serum osteoprotegerin is inversely associated with carotid plaque echogenicity in humans. Atherosclerosis 2007, 191, 128–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pelisek, J.; Hahntow, I.N.; Eckstein, H.H.; Ockert, S.; Reeps, C.; Heider, P.; Luppa, P.B.; Frank, H. Impact of chronic kidney disease on carotid plaque vulnerability. J. Vasc. Surg. 2011, 54, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Lenglet, S.; Quercioli, A.; Fabre, M.; Galan, K.; Pelli, G.; Nencioni, A.; Bauer, I.; Pende, A.; Python, M.; Bertolotto, M.; et al. Statin treatment is associated with reduction in serum levels of receptor activator of NF-κB ligand and neutrophil activation in patients with severe carotid stenosis. Mediat. Inflamm. 2014, 2014, 720987. [Google Scholar] [CrossRef]
- Nighoghossian, N.; Derex, L.; Douek, P. The vulnerable carotid artery plaque: Current imaging methods and new perspectives. Stroke 2005, 36, 2764–2772. [Google Scholar] [CrossRef]
- Hunt, J.L.; Fairman, R.; Mitchell, M.E.; Carpenter, J.P.; Golden, M.; Khalapyan, T.; Wolfe, M.; Neschis, D.; Milner, R.; Scoll, B.; et al. Bone formation in carotid plaques: A clinicopathological study. Stroke 2002, 33, 1214–1219. [Google Scholar] [CrossRef] [PubMed]
- Raggi, P.; Cooil, B.; Shaw, L.J.; Aboulhson, J.; Takasu, J.; Budoff, M.; Callister, T.Q. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. Am. J. Cardiol. 2003, 92, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Ehara, S.; Kobayashi, Y.; Yoshiyama, M.; Shimada, K.; Shimada, Y.; Fukuda, D.; Nakamura, Y.; Yamashita, H.; Yamagishi, H.; Takeuchi, K.; et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation 2004, 110, 3424–3429. [Google Scholar] [CrossRef]
- Virmani, R.; Ladich, E.R.; Burke, A.P.; Kolodgie, F.D. Histopathology of carotid atherosclerotic disease. Neurosurgery 2006, 59, S219–S227. [Google Scholar] [CrossRef]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.K.; Malcom, G.T.; Smialek, J.; Virmani, R. Healed plaque ruptures and sudden coronary death: Evidence that subclinical rupture has a role in plaque progression. Circulation 2001, 103, 934–940. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, R.; Momiyama, Y.; Taniguchi, H.; Takahashi, R.; Kusuhara, M.; Nakamura, H.; Ohsuzu, F. Plasma osteopontin levels are associated with the presence and extent of coronary artery disease. Atherosclerosis 2003, 170, 333–337. [Google Scholar] [CrossRef] [PubMed]
| Cohort Size | Age [Years] | Study Cohort | Comparator | Comparator Age [Years] | OPG Levels in Study Group | OPG in the Comparator Group | Reference |
|---|---|---|---|---|---|---|---|
| Osteoprotegerin Quantified Using ELISA (BioVendor)—Concentrations Given in pmol/L | |||||||
| 114 | 67.6 ± 6.5 | ICA stenosis > 50% | 50 age-, sex-, and BMI-matched healthy individuals | 65.4 ± 8.9 | 8.00 ± 3.47 * | 5.74 ± 2.39 | [105] |
| 97 | 63.6 ± 9.9 | ICA stenosis > 40% | 52 healthy controls | 60.3 ± 8.8 | 7.54 ± 2.78 *** Symptomatic 8.15 ± 2.46 Asymptomatic 6.97 ± 2.15 | 3.39 ± 1.64 | [102] |
| 140 (70 + 70) | 1. 64.8 ± 7.3 2. 63.2 ± 6.8 | ICA stenosis > 30% (<60% for symptomatic or <70% for asymptomatic) | - | - | 1. 7.00 ± 2.71 before normal lipid-lowering therapy 2. 6.31 ± 2.56 before aggressive lipid-lowering therapy | - | [106] |
| 113 (46 + 67) | 1. 66.8 ± 7.3 2. 64.9 ± 10.4 | Symptomatic with >70% ICA stenosis (1.) or asymptomatic with 30–69% ICA stenosis (2.) | - | - | Symptomatic 8.86 ± 3.47 Asymptomatic 9.05 ± 2.65 | - | [107] |
| 113 | 70 ± 9 | Asymptomatic with 70–99% ICA stenosis (1.) or symptomatic > 50% ICA stenosis (2.) | 38 age-, sex-matched individuals | 66 ± 10 | 8.88 ± 2.74 * Asymptomatic 7.98 ± 2.22 Symptomatic 10.88 ± 3.31 ** | 6.72 ± 2.88 | [108] |
| Osteoprotegerin Quantification ELISA (Method Not Specified)—Concentrations Given in pmol/L | |||||||
| 347 (159 + 188) | 1. 72.2 ± 3.3 2. 72.3 ± 3.9 | Diabetic patients with unstable (1.) or stable (2.) ICA stenosis | 526 diabetic patients without ICA stenosis | 71.8 ± 3.8 | All 6.86 ± 6.55 *** Unstable 7.65 ± 8.12 *** Stable 3.13 ± 2.23 | 3.23 ± 2.25 | [109] |
| Osteoprotegerin Quantified Using ELISA (R&D System)—Concentrations Given in ng/mL | |||||||
| 91 (54 + 37) | 1. 68 ± 6 2. 69 ± 1 | Noncalcified (1.) or calcified (2.) carotid plaque | 54 age-, sex-, BMI- matched individuals | 67 ± 9 | Noncalcified 3.21 (median) Calcified 4.11 (median) * | 3.20 (median) | [110] |
| 73 (24 + 49) | 1. 71 ± 9 2. 69 ± 11 | Symptomatic (1.) and asymptomatic (2.) ICA stenosis | - | - | Symptomatic 2.5 ± 1.6 Asymptomatic 3.2 ± 1.5 | - | [111] |
| 59 | 1. 70.5 (68.4–72.7) 2. 68.7 (66.2–71.3) | Echogenic (1.) or echolucent (2.) carotid plaques | 41 subjects without carotid plaques | 68.1 (66.3–70.0) | Echogenic 1.23 (95%CI: 1.02–1.48) ** Echolucent 1.76 (95%CI: 1.46–2.14) | 1.89 (95%CI: 1.60–2.21) | [112] |
| 114 (51 + 63) | 1. 73.3 ± 7.4 2. 72.1 ± 6.2 | ICA stenosis > 70% with CKD (1.) or without CKD (2.) | - | - | 1. 3.19 ± 0.18 2. 3.12 ± 0.11 | - | [113] |
| 38 (26 + 12) | 1. 72.0 (66.0–77.3) 2. 71.5 (67.3–76.7) | Asymptomatic ICA stenosis > 70% before carotid endarterectomy: treated (1.) or not treated (2.) with statin | - | - | 1. 0.17 (0.06–0.32) # 2. 0.06 (0.06–0.43) # | - | [114] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudek, J.; Pośpiech, M.; Chudek, A.; Holecki, M.; Puzianowska-Kuźnicka, M. Osteoprotegerin as an Emerging Biomarker of Carotid Artery Stenosis? A Scoping Review with Meta-Analysis. Diagnostics 2025, 15, 219. https://doi.org/10.3390/diagnostics15020219
Chudek J, Pośpiech M, Chudek A, Holecki M, Puzianowska-Kuźnicka M. Osteoprotegerin as an Emerging Biomarker of Carotid Artery Stenosis? A Scoping Review with Meta-Analysis. Diagnostics. 2025; 15(2):219. https://doi.org/10.3390/diagnostics15020219
Chicago/Turabian StyleChudek, Jerzy, Marta Pośpiech, Anna Chudek, Michał Holecki, and Monika Puzianowska-Kuźnicka. 2025. "Osteoprotegerin as an Emerging Biomarker of Carotid Artery Stenosis? A Scoping Review with Meta-Analysis" Diagnostics 15, no. 2: 219. https://doi.org/10.3390/diagnostics15020219
APA StyleChudek, J., Pośpiech, M., Chudek, A., Holecki, M., & Puzianowska-Kuźnicka, M. (2025). Osteoprotegerin as an Emerging Biomarker of Carotid Artery Stenosis? A Scoping Review with Meta-Analysis. Diagnostics, 15(2), 219. https://doi.org/10.3390/diagnostics15020219






