Potentials of Presepsin as a Novel Sepsis Biomarker in Critically Ill Adults: Correlation Analysis with the Current Diagnostic Markers
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Infection, Inflammation, and Clinical Parameters as Diagnostic Criteria for Sepsis
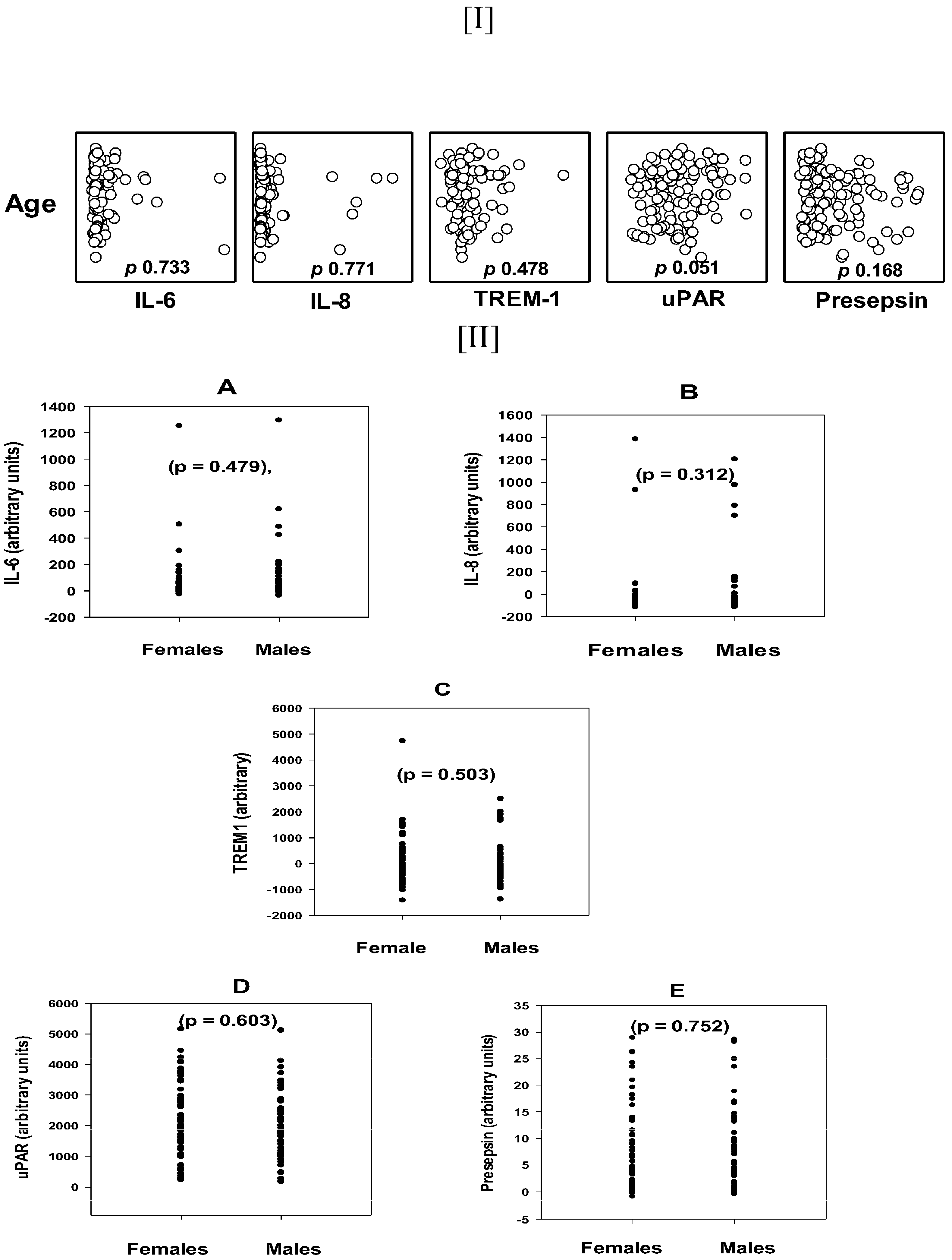
3.2. The Age and Gender Distribution of the Tested Biomarkers
3.3. Correlations of Tested Biomarkers with the Major Diagnostic Parameters of Sepsis
3.4. Correlations of the Tested Biomarkers with the Hemostasis Parameters as Secondary Diagnostic Markers
3.5. Correlations of the Tested Biomarkers with the Organ Dysfunction Markers
3.6. Comparisons of Serum Levels of Test Biomarkers in the Clinical Types of SSI, Sepsis (Sp), Pneumonia (Pn), and Mixed Se/Pn
3.7. Comparisons of Serum Levels of Test Biomarkers Between SSI Patients Based on Severity Markers
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Diagnostic Criteria | D-Index | PLT | Fibrin. | PT | APTT | INR | D-dimer | |
|---|---|---|---|---|---|---|---|---|
| CRP | 100% | p | 0.594 | 0.204 | 0.462 | 0.108 | 0.418 | 0.170 |
| (0–3) mg/L | 73/73 | CC | 0.064 | 0.179 | −0.0886 | 0.195 | −0.0977 | −0.184 |
| HR | 79.2% | p | 0.406 | 0.291 | 0.825 | 0.887 | 0.759 | 0.671 |
| >90 | 99/125 | CC | −0.075 | −0.137 | 0.0200 | −0.0130 | 0.0277 | −0.0537 |
| WBC | 72.1% | p | 0.148 | 0.604 | 0.109 | 0.169 | 0.152 | 0.586 |
| <4000 or >12,000 | 93/129 | CC | 0.127 | −0.0661 | 0.141 | 0.123 | 0.126 | −0.0667 |
| PCT | 70.2% | p | 0.851 | 0.635 | 0.902 | 0.506 | 0.506 | 0.151 |
| (0–0.5) mg/L | 59/84 | CC | 0.0208 | −0.0625 | −0.0136 | −0.0744 | −0.0744 | 0.183 |
| Temp | 36.8%% | p | 0.0438 | 0.0226 | 0.303 | 0.0886 | 0.259 | 0.405 |
| <36 C and >38 C | 46/125 | CC | 0.181 | 0.242 | −0.0929 | −0.155 | −0.102 | −0.116 |
| Lactate | 33.8% | p | 0.650 | 0.00145 | 0.706 | 0.380 | 0.716 | 0.239 |
| (0.5–2.2) mmol/L | 24/71 | CC | −0.0548 | 0.511 | 0.0455 | 0.107 | 0.0440 | 0.177 |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- WHO. Available online: https://www.who.int/news-room/fact-sheets/detail/sepsis (accessed on 19 July 2023).
- Angus, D.C.; Linde-Zwirble, W.T.; Lidicker, J.; Clermont, G.; Carcillo, J.; Pinsky, M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001, 29, 1303–1310. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Jones, T.M.; Hamad, Y.; Pande, A.; Varon, J.; O’Brien, C.; Anderson, D.J.; Warren, D.K.; Dantes, R.B.; Epstein, L.; et al. Centers for Disease Control and Prevention (CDC) prevention epicenters program. Prevalence, Underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw. Open 2019, 2, e187571. [Google Scholar] [CrossRef]
- Larsen, F.F.; Petersen, J.A. Novel biomarkers for sepsis: A narrative review. Eur. J. Intern. Med. 2017, 45, 46–50. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Z.; He, Y.; Shu, C. Pitfalls in the rapid diagnosis of positive blood culture. Rev. Med. Microbiol. 2010, 21, 39–43. [Google Scholar] [CrossRef]
- Mellhammar, L.; Kahn, F.; Whitlow, C.; Kander, T.; Christensson, B.; Linder, A. Bacteremic sepsis leads to higher mortality when adjusting for confounders with propensity score matching. Sci. Rep. 2021, 11, 6972. [Google Scholar] [CrossRef] [PubMed]
- Henriquez-Camacho, C.; Losa, J. Biomarkers for sepsis. BioMed Res. Int. 2014, 2014, 547818. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.J.; Kushner, I. Serum C-reactive protein levels in disease. Ann. N. Y. Acad. Sci. 1982, 389, 406–418. [Google Scholar] [CrossRef]
- Taylor, R.; Jones, A.; Kelly, S.; Simpson, M.; Mabey, J. A Review of the value of procalcitonin as a marker of infection. Cureus 2017, 9, e1148. [Google Scholar] [CrossRef]
- Hou, T.; Huang, D.; Zeng, R.; Ye, Z.; Zhang, Y. Accuracy of serum interleukin (IL)-6 in sepsis diagnosis: A systematic review and meta-analysis. Int. J. Clin. Exp. Med. 2015, 8, 15238–15245. [Google Scholar] [PubMed]
- Matsumoto, H.; Ogura, H.; Shimizu, K.; Ikeda, M.; Hirose, T.; Matsuura, H.; Kang, S.; Takahashi, K.; Tanaka, T.; Shimazu, T. The clinical importance of a cytokine network in the acute phase of sepsis. Sci. Rep. 2018, 8, 13995. [Google Scholar] [CrossRef] [PubMed]
- Jiyong, J.; Tiancha, H.; Wei, C.; Huahao, S. Diagnostic value of the soluble triggering receptor expressed on myeloid cells-1 in bacterial infection: A meta-analysis. Intensive Care Med. 2009, 35, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Eugen-Olsen, J. suPAR as a marker of disease severity and risk of mortality in sepsis. J. Intern. Med. 2011, 270, 29–31. [Google Scholar] [CrossRef]
- Donadello, K.; Covajes, C.; Scolletta, S.; Taccone, F.S.; Santonocito, C.; Brimioulle, S.; Beumier, M.; Vannuffelen, M.; Gottin, L.; Vincent, J.L. Clinical value of suPAR, a new biomarker. Intensive Care Med. 2011, 37, S199. [Google Scholar]
- Chenevier-Gobeaux, C.; Borderie, D.; Weiss, N.; Mallet-Coste, T.; Claessens, Y.E. Presepsin (sCD14-ST), an innate immune response marker in sepsis. Clin. Chim. Acta 2015, 450, 97–103. [Google Scholar] [CrossRef]
- Bone, R.C.; Balk, R.A.; Cerra, F.B.; Dellinger, R.P.; Fein, A.M.; Knaus, W.A.; Schein, R.M.H.; Sibbald, W.J. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992, 101, 1644–1655. [Google Scholar] [CrossRef]
- Sater, M.S.; AlDehaini, D.M.B.; Malalla, Z.H.A.; Ali, M.E.; Giha, H.A. Plasma IL-6, TREM1, uPAR, and IL6/IL8 biomarkers increment further witnessing the chronic inflammation in type 2 diabetes. Horm. Mol. Biol. Clin. Investig. 2023, 44, 259–269. [Google Scholar] [CrossRef]
- Pierrakos, C.; Vincent, J.L. Sepsis biomarkers: A review. Crit. Care 2010, 14, R15. [Google Scholar] [CrossRef]
- Ko, R.-E.; Kang, D.; Cho, J.; Na, S.J.; Chung, C.R.; Lim, S.Y.; Lee, Y.J.; Park, S.; Oh, D.K.; Park, M.H.; et al. Influence of gender on age-associated in-hospital mortality in patients with sepsis and septic shock: A prospective nationwide multicenter cohort study. Crit. Care 2023, 27, 229. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.H.; Kim, E.H.; Kim, H.Y.; Ahn, J.G. Presepsin as a diagnostic marker of sepsis in children and adolescents: A systemic review and meta-analysis. BMC Infect. Dis. 2019, 19, 760. [Google Scholar] [CrossRef] [PubMed]
- Ulla, M.; Pizzolato, E.; Lucchiari, M.; Loiacono, M.; Soardo, F.; Forno, D.; Morello, F.; Lupia, E.; Moiraghi, C.; Mengozzi, G.; et al. Diagnostic and prognostic value of presepsin in the management of sepsis in the emergency department: A multicenter prospective study. Crit. Care 2013, 17, R168. [Google Scholar] [CrossRef] [PubMed]
- Koozi, H.; Lengquist, M.; Frigyesi, A. C-reactive protein as a prognostic factor in intensive care admissions for sepsis: A Swedish multicenter study. J. Crit. Care 2019, 56, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.E.; Park, D.W. Lactate as a Biomarker for Sepsis Prognosis? Infect. Chemother. 2016, 48, 252–253. [Google Scholar] [CrossRef]
- Ibarz, M.; Haas, L.E.M.; Ceccato, A.; Artigas, A. The critically ill older patient with sepsis: A narrative review. Ann. Intensive Care 2024, 14, 6. [Google Scholar] [CrossRef] [PubMed]
- Shozushima, T.; Takahashi, G.; Matsumoto, N.; Kojika, M.; Okamura, Y.; Endo, S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. J. Infect. Chemother. 2011, 17, 764–769. [Google Scholar] [CrossRef] [PubMed]
- Endo, S.; Suzuki, Y.; Takahashi, G.; Shozushima, T.; Ishikura, H.; Murai, A.; Nishida, T.; Irie, Y.; Miura, M.; Iguchi, H.; et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. J. Infect. Chemother. 2012, 18, 891–897. [Google Scholar] [CrossRef]
- Tsantes, A.G.; Parastatidou, S.; Tsantes, E.A.; Bonova, E.; Tsante, K.A.; Mantzios, P.G.; Vaiopoulos, A.G.; Tsalas, S.; Konstantinidi, A.; Houhoula, D.; et al. Sepsis-Induced Coagulopathy: An Update on Pathophysiology, Biomarkers, and Current Guidelines. Life 2023, 13, 350. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, J.; Park, D.W.; Seok, H.; Ahn, S.; Kim, J.; Park, J.; Cho, H.-J.; Moon, S. Diagnostic and prognostic value of presepsin and procalcitonin in non-infectious organ failure, sepsis, and septic shock: A prospective observational study according to the Sepsis-3 definitions. BMC Infect. Dis. 2022, 22, 8. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Massaccesi, L.; de Vecchi, E.; Banfi, G.; Romanelli, M.M.C. Clinical application of presepsin as diagnostic biomarker of infection: Overview and updates. Clin. Chem. Lab. Med. 2019, 58, 11–17. [Google Scholar] [CrossRef]
- Stankovic, S. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. In Heat Illness and Critical Care; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Elebute, E.A.; Stoner, H.B. The grading of sepsis. Br. J. Surg. 1983, 70, 29–31. [Google Scholar] [CrossRef] [PubMed]
- Khwannimit, B.; Bhurayanontachai, R.; Vattanavanit, V. Validation of the Sepsis Severity Score Compared with Updated Severity Scores in Predicting Hospital Mortality in Sepsis Patients. Shock 2017, 47, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Harbarth, S.; Holeckova, K.; Froidevaux, C.; Pittet, D.; Ricou, B.; Grau, G.E.; Vadas, L.; Pugin, J.; The Geneva Sepsis Network. Diagnostic value of procalcitonin, interleukin-6, and interleukin-8 in critically ill patients admitted with suspected sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Rohm, T.V.; Meier, D.T.; Olefsky, J.M.; Donath, M.Y. Inflammation in obesity, diabetes, and related disorders. Immunity 2022, 55, 31–55. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alvarez, M.; Marik, P.; Bellomo, R. Sepsis-associated hyperlactatemia. Crit. Care 2014, 18, 503. [Google Scholar] [CrossRef] [PubMed]
- Luzzani, A.; Polati, E.; Dorizzi, R.; Rungatscher, A.; Pavan, R.; Merlini, A. Comparison of procalcitonin and C-reactive protein as markers of sepsis. Crit. Care Med. 2003, 31, 1737–1741. [Google Scholar] [CrossRef]
- Piccioni, A.; Santoro, M.C.; de Cunzo, T.; Tullo, G.; Cicchinelli, S.; Saviano, A.; Valletta, F.; Pascale, M.M.; Candelli, M.; Covino, M.; et al. Presepsin as Early Marker of Sepsis in Emergency Department: A Narrative Review. Medicina 2021, 57, 770. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, Y.; Sato, N.; Suzuki, Y.; Kojika, M.; Imai, S.; Takahashi, G.; Miyata, M.; Endo, S.; Shirakawa, K.; Furusako, S. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. J. Infect. Chemother. 2005, 11, 234–238. [Google Scholar] [CrossRef]
- Shirakawa, K.; Naitou, K.; Hirose, J.; Takahashi, T.; Furusako, S. Presepsin (sCD14-ST): Development and evaluation of one-step ELISA with a new standard that is similar to the form of presepsin in septic patients. Clin. Chem. Lab. Med. 2011, 49, 937–939. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fravega, M.; Fanos, V. Soluble CD14 subtype presepsin (sCD14-ST) and lipopolysaccharide binding protein (LBP) in neonatal sepsis: New clinical and analytical perspectives for two old biomarkers. J. Matern. Neonatal Med. 2011, 24 (Suppl. S2), 12–14. [Google Scholar] [CrossRef] [PubMed]
- Mussap, M.; Puxeddu, E.; Burrai, P.; Noto, A.; Cibecchini, F.; Testa, M.; Puddu, M.; Ottonello, G.; Dessì, A.; Irmesi, R.; et al. Soluble CD14 subtype (sCD14-ST) presepsin in critically ill preterm newborns: Preliminary reference ranges. J. Matern. Neonatal Med. 2012, 25 (Suppl. S5), 51–53. [Google Scholar] [CrossRef]


| Variables | Sepsis (Se) Patients | Pneumonia (Pn) Patients | Se/Pn Double Infection Patients | p Value |
|---|---|---|---|---|
| Number | 48 | 48 | 33 | |
| Sex (M/F—ratio) | 23/25 | 23/25 | 16/17 | |
| Age (years) | 58.5, 37.5–68.0 | 64.5, 43.25–77.25 | 64.0, 46.5–73.0 | 0.086 |
| Vital functions, Temp and Blood count | ||||
| HR (bpm) | 102.0, 93.25–117.5 | 98.0, 90.5–114.75 | 104.0, 94.0–113.0 | 0.608 |
| RR | 24.5, 21.25–30.0 | 26.0, 22.75–29.0 | 32.0, 28.0–38.5 | 0.049 |
| Temp (°C) | 36.95, 36.05–38.1 | 36.95, 36.5–37.88 | 38.0, 36.5–38.45 | 0.115 |
| WBC (×109/L) | 17.02, 12.49–24.39 | 13.43, 9.04–17.61 | 13.4, 9.03–16.71 | 0.019 |
| Inflammatory markers | ||||
| CRP (mg/L) | 100.0, 42.3–144.0 | 82.4, 24.2–216.5 | 96.1, 63.4–137.5 | 0.992 |
| PCT (mg/L) | 3.53, 0.5–15.6 | 1.21, 0.412–2.58 | 1.83, 0.302–7.4 | 0.150 |
| Lactate (mmol/L) | 1.85, 1.375–3.525 | NK | 1.7, 1.35–2.2 | 0.35 |
| Hemostasis parameters | ||||
| Platelets (×109/L) | 192.0, 93.5–275.75 | 255.5, 169.0–328.0 | 199.0, 127.5–367.5 | 0.052 |
| PT (sec) | 15.25, 13.53–18.28 | 12.9, 12.0–15.08 | 13.9, 12.85–15.45 | <0.001 |
| APTT (sec) | 27.5, 23.4–39.0 | 23.2, 21.0–30.8 | 25.9, 21.05–29.50 | 0.032 |
| INR | 1.325,1.155–1.578 | 1.100, 1.012–1.282 | 1.180, 1.105–1.305 | <0.001 |
| Fibrinogen (g/L) | 403.88 ± 151.49 | 455.68 ± 194.99 | 458.08 ± 200.25 | 0.355 ANOVA |
| D-dimer (mg/L) | 5.67, 1.85–10.08 | 3.42, 1.93–11.44 | 0.631 MW | |
| Organ dysfunction parameters Severity indicators | ||||
| Bilirubin (µmol/L) | 13.5, 7.25–44.0 | 12.0, 9.0–22.0 | 15.0, 8.5–28.0 | 0.508 |
| Creatinine (µmol/L) | 129.0, 54.8–267.8 | 78.0, 38.0–160.0 | NK | 0.076 MW |
| Artificial ventilation | 54.5% (24/44) | 43.5% (20/46) | 60.6% (20/33) | 0.296 Chi-square |
| Test Biomarker | Platelets Count | Fibrinogen | PT | APTT | INR | D-Dimer | Score | |
|---|---|---|---|---|---|---|---|---|
| Presepsin | p | 0.004 | 0.734 | 0.002 | 0.013 | 0.005 | 0.0284 | 5 |
| CC | −0.250 | 0.0357 | 0.265 | 0.221 | 0.248 | 0.291 | ||
| IL-6 | p | 0.017 | 0.576 | 0.000 | 0.237 | 0.000 | 0.000 | 4 |
| CC | −0.225 | 0.0639 | 0.581 | 0.114 | 0.573 | 0.550 | ||
| IL-8 | p | 0.020 | 0.799 | 0.001 | 0.721 | 0.001 | 0.003 | 4 |
| CC | −0.219 | 0.0291 | 0.321 | 0.0346 | 0.320 | 0.418 | ||
| TREM-1 | p | 0.020 | 0.691 | 0.118 | 0.004 | 0.147 | 0.733 | 2 |
| CC | 0.214 | 0.0438 | −0.145 | −0.270 | −0.135 | −0.047 | ||
| uPAR | p | 0.229 | 0.984 | 0.591 | 0.171 | 0.607 | 0.0146 | 2 |
| CC | −0.112 | −0.0022 | 0.0502 | 0.129 | 0.0481 | 0.328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sater, M.S.; Almansour, N.; Malalla, Z.H.A.; Fredericks, S.; Ali, M.E.; Giha, H.A. Potentials of Presepsin as a Novel Sepsis Biomarker in Critically Ill Adults: Correlation Analysis with the Current Diagnostic Markers. Diagnostics 2025, 15, 217. https://doi.org/10.3390/diagnostics15020217
Sater MS, Almansour N, Malalla ZHA, Fredericks S, Ali ME, Giha HA. Potentials of Presepsin as a Novel Sepsis Biomarker in Critically Ill Adults: Correlation Analysis with the Current Diagnostic Markers. Diagnostics. 2025; 15(2):217. https://doi.org/10.3390/diagnostics15020217
Chicago/Turabian StyleSater, Mai S., Nourah Almansour, Zainab Hasan Abdulla Malalla, Salim Fredericks, Muhalab E. Ali, and Hayder A. Giha. 2025. "Potentials of Presepsin as a Novel Sepsis Biomarker in Critically Ill Adults: Correlation Analysis with the Current Diagnostic Markers" Diagnostics 15, no. 2: 217. https://doi.org/10.3390/diagnostics15020217
APA StyleSater, M. S., Almansour, N., Malalla, Z. H. A., Fredericks, S., Ali, M. E., & Giha, H. A. (2025). Potentials of Presepsin as a Novel Sepsis Biomarker in Critically Ill Adults: Correlation Analysis with the Current Diagnostic Markers. Diagnostics, 15(2), 217. https://doi.org/10.3390/diagnostics15020217







