Frequency and Predictors of Pneumonia After Isolated Coronary Artery Bypass Grafting (CABG): A Single-Center Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Preoperative Risk Assessment
2.3. Surgical Technique
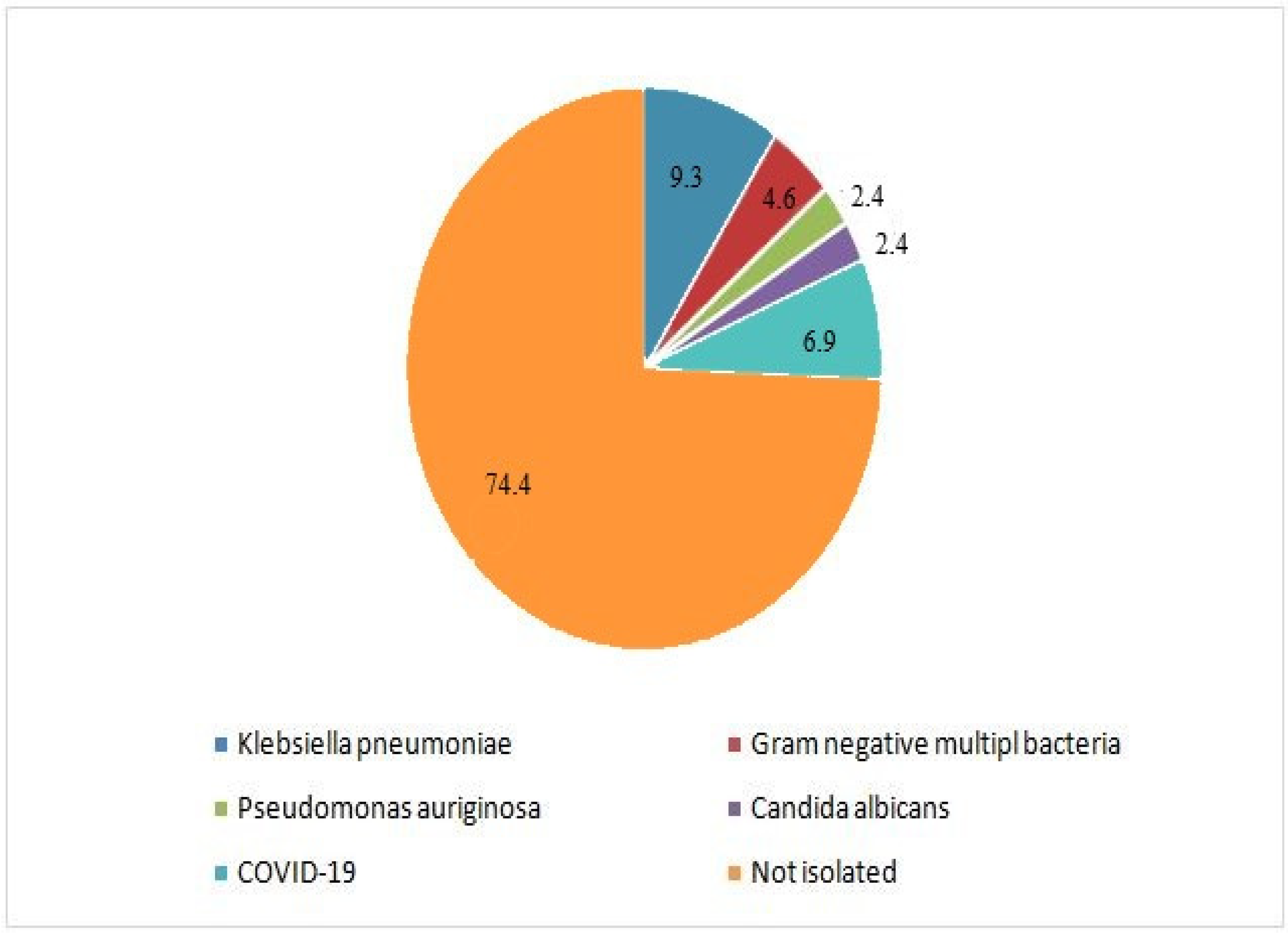
2.4. Definition of Pneumonia
- Evidence of new or progressive infiltrate, consolidation, or cavitation on a chest radiograph obtained at least two days postoperatively.
- The presence of at least one symptom:
- ○
- Fever (>38 °C) without an alternative explanation;
- ○
- Leukopenia (<4000 WBC/mm3) or leukocytosis (≥12,000 WBC/mm3);
- ○
- Altered mental status in patients aged ≥70 years without another identifiable cause.
- Two or more of the following clinical signs:
- ○
- Purulent sputum, changes in sputum quality, or increased respiratory secretions;
- ○
- New or worsening respiratory symptoms (cough, dyspnea, or tachypnea);
- ○
- Wheezing or bronchial breath sounds;
- ○
- Worsening gas exchange (e.g., need of increased oxygen or ventilator demand).
2.5. Statistical Analysis
3. Results
3.1. Postoperative Complications
3.2. Univariate Descriptive Statistics of Postoperative Pneumonia
3.3. Multivariate Binary Logistic Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barnett, N.M.; Liesman, D.R.; Strobel, R.J.; Wu, X.; Paone, G.; DeLucia, A., III; Zhang, M.; Ling, C.; Pagani, F.D.; Likosky, D.S.; et al. The Association of Intraoperative and Early Postoperative Events with Risk of Pneumonia Following Cardiac Surgery. J. Thorac. Cardiovasc. Surg. 2023, 168, 1144. [Google Scholar] [CrossRef] [PubMed]
- Pahwa, S.; Bernabei, A.; Schaff, H.; Stulak, J.; Greason, K.; Pochettino, A.; Daly, R.; Dearani, J.; Bagameri, G.; King, K.; et al. Impact of Postoperative Complications After Cardiac Surgery on Long-Term Survival. J. Card. Surg. 2021, 36, 2045–2052. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, K.S.; Kheirallah, K.A.; Al Manasra, A.R.A.; Megdadi, M.A. Factors Affecting Duration of Stay in the Intensive Care Unit After Coronary Artery Bypass Surgery and Its Impact on In-Hospital Mortality: A Retrospective Study. J. Cardiothorac. Surg. 2024, 19, 45. [Google Scholar] [CrossRef]
- Rotar, E.P.; Beller, J.P.; Smolkin, M.E.; Chancellor, W.Z.; Ailawadi, G.; Yarboro, L.T.; Hulse, M.; Ratcliffe, S.J.; Teman, N.R. Prediction of Prolonged Intensive Care Unit Length of Stay Following Cardiac Surgery. Semin. Thorac. Cardiovasc. Surg. 2022, 34, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Poelaert, J.; Haentjens, P.; Blot, S. Association Among Duration of Mechanical Ventilation, Cuff Material of Endotracheal Tube, and Postoperative Nosocomial Pneumonia in Cardiac Surgical Patients: A Prospective Study. J. Thorac. Cardiovasc. Surg. 2014, 148, 1622–1627. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, P.; Li, H.; Chi, H.; Zheng, N.; Pan, X.; Tang, C. Factors Influencing the Incidence of Pneumonia After Coronary Artery Bypass Grafting. Heart Surg. Forum 2023, 26, E863–E868. [Google Scholar] [CrossRef] [PubMed]
- Ailawadi, G.; Chang, H.L.; O’Gara, P.T.; O’Sullivan, K.; Woo, Y.J.; DeRose, J.J., Jr.; Parides, M.K.; Thourani, V.H.; Robichaud, S.; Gillinov, A.M.; et al. Pneumonia After Cardiac Surgery: Experience of the NIH/CIHR Cardiothoracic Surgical Trials Network. J. Thorac. Cardiovasc. Surg. 2017, 153, 1384. [Google Scholar] [CrossRef]
- Brescia, A.A.; Rankin, J.S.; Cyr, D.D.; Jacobs, J.P.; Prager, R.L.; Zhang, M.; Matsouaka, R.A.; Harrington, S.D.; Dokholyan, R.S.; Bolling, S.F.; et al. Determinants of Variation in Pneumonia Rates After Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2018, 105, 513–520. [Google Scholar] [CrossRef]
- Strobel, R.J.; Liang, Q.; Zhang, M.; Wu, X.; Rogers, M.A.M.; Theurer, P.F.; Fishstrom, A.B.; Harrington, S.D.; DeLucia, A., III; Paone, G.; et al. A Preoperative Risk Model for Postoperative Pneumonia After Coronary Artery Bypass Grafting. Ann. Thorac. Surg. 2016, 102, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Nashef, S.A.; Roques, F.; Michel, P.; Gauducheau, E.; Lemeshow, S.; Salamon, R. European System for Cardiac Operative Risk Evaluation (EuroSCORE). Eur. J. Cardiothorac. Surg. 1999, 16, 9–13. [Google Scholar] [CrossRef]
- Roques, F.; Michel, P.; Goldstone, A.R.; Nashef, S.A.M. The Logistic EuroSCORE. Eur. Heart J. 2003, 24, 881–882. [Google Scholar] [CrossRef]
- Writing Committee Members; Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar]
- Zukowska, A.; Kaczmarczyk, M.; Listewnik, M.; Zukowski, M. Impact of Post-Operative Infection after CABG on Long-Term Survival. J. Clin. Med. 2023, 12, 3125. [Google Scholar] [CrossRef]
- Hadaya, J.; Verma, A.; Marzban, M.; Sanaiha, Y.; Shemin, R.J.; Benharash, P. Impact of Pulmonary Complications on Outcomes and Resource Use After Elective Cardiac Surgery. Ann. Surg. 2023, 278, e661–e666. [Google Scholar] [CrossRef]
- Mathis, M.R.; Duggal, N.M.; Likosky, D.S.; Haft, J.W.; Douville, N.J.; Vaughn, M.T.; Maile, M.D.; Blank, R.S.; Colquhoun, D.A.; Strobel, R.J.; et al. Intraoperative Mechanical Ventilation and Postoperative Pulmonary Complications after Cardiac Surgery. Anesthesiology 2019, 131, 1046–1062. [Google Scholar] [CrossRef]
- Fischer, M.O.; Brotons, F.; Briant, A.R.; Suehiro, K.; Gozdzik, W.; Sponholz, C.; Kirkeby-Garstad, I.; Joosten, A.; Neto, C.N.; Kunstyr, J.; et al. Postoperative Pulmonary Complications After Cardiac Surgery: The VENICE International Cohort Study. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2344–2351. [Google Scholar] [CrossRef] [PubMed]
- As, A.K.; Engin, M. The predictive role of modified stress hyperglycemia rate in predicting early pneumonia after isolated coronary bypass surgery in patients with diabetes mellitus. Biomol. Biomed. 2025, 25, 505–510. [Google Scholar] [CrossRef]
- Thourani, V.H.; Weintraub, W.S.; Stein, B.; Gebhart, S.S.P.; Craver, J.M.; Jones, E.L.; Guyton, R.A. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann. Thorac. Surg. 1999, 67, 1045–1052. [Google Scholar] [CrossRef]
- Ji, Q.; Mei, Y.; Wang, X.; Feng, J.; Cai, J.; Ding, W. Risk factors for pulmonary complications following cardiac surgery with cardiopulmonary bypass. Int. J. Med. Sci. 2013, 10, 1578–1583. [Google Scholar] [CrossRef]
- Dacey, L.J.; Liu, J.Y.; Braxton, J.H.; Weintraub, R.M.; DeSimone, J.; Charlesworth, D.C.; Lahey, S.J.; Ross, C.S.; Hernandez, F., Jr.; Leavitt, B.J.; et al. Long-term survival of dialysis patients after coronary bypass grafting. Ann. Thorac. Surg. 2002, 74, 458–462, discussion 462–463. [Google Scholar] [CrossRef]
- Safaie, N.; Chaichi, P.; Habibzadeh, A.; Nasiri, B. Postoperative outcomes in patients with chronic renal failure undergoing coronary artery bypass grafting in madani heart center: 2000–2010. J. Cardiovasc. Thorac. Res. 2011, 3, 53–56. [Google Scholar] [PubMed]
- Huang, X.; Redfors, B.; Chen, S.; Liu, Y.; Ben-Yehuda, O.; Puskas, J.D.; Kandzari, D.E.; Merkely, B.; Horkay, F.; van Boven, A.J.; et al. Impact of chronic obstructive pulmonary disease on prognosis after percutaneous coronary intervention and bypass surgery for left main coronary artery disease: An analysis from the EXCEL trial. Eur. J. Cardiothorac. Surg. 2019, 55, 1144–1151. [Google Scholar] [CrossRef]
- Magovern, J.A.; Sakert, T.; Magovern, G.J.; Benckart, D.H.; Burkholder, J.A.; Liebler, G.A.; Magovern, G.J., Sr. A model that predicts morbidity and mortality after coronary artery bypass graft surgery. J. Am. Coll. Cardiol. 1996, 28, 1147–1153. [Google Scholar] [CrossRef]
- Gao, J.; Wang, H.; Liu, X.; Song, X.; Zhong, X. Surgical site wound infection, and other postoperative problems after coronary artery bypass grafting in subjects with chronic obstructive pulmonary disease: A meta-analysis. Int. Wound J. 2022, 20, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, H.; Siau, K.; Curtis, J.; Ng, A.; Menon, S.; Luckraz, H.; Brookes, M.J. Preoperative Anemia and Outcomes in Cardiovascular Surgery: Systematic Review and Meta-Analysis. Ann. Thorac. Surg. 2019, 108, 1840–1848. [Google Scholar] [CrossRef]
- Koster, A.; Zittermann, A.; Gummert, J.F.; von Dossow, V.; Deutsch, M.A. Transfusions and early outcomes in anaemic patients undergoing off- or on-pump coronary artery bypass grafting. Interact. Cardiovasc. Thorac. Surg. 2022, 35, ivac276. [Google Scholar] [CrossRef] [PubMed]
- LaPar, D.J.; Hawkins, R.B.; McMurry, T.L.; Isbell, J.M.; Rich, J.B.; Speir, A.M.; Quader, M.A.; Kron, I.L.; Kern, J.A.; Ailawadi, G.; et al. Preoperative anemia versus blood transfusion: Which is the culprit for worse outcomes in cardiac surgery? J. Thorac. Cardiovasc. Surg. 2018, 156, 66–74.e2. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Lu, Y.; Sun, M.; Huang, X.; Du, X.; Jiao, Z.; Sun, F.; Xie, F. Pneumonia After Cardiovascular Surgery: Incidence, Risk Factors and Interventions. Front. Cardiovasc. Med. 2022, 9, 911878. [Google Scholar] [CrossRef] [PubMed]
- Shokr, H.; Marwah, M.K.; Siddiqi, H.; Wandroo, F.; Sanchez-Aranguren, L.; Ahmad, S.; Wang, K.; Marwah, S. Lactate Dehydrogenase/Albumin To-Urea Ratio: A Novel Prognostic Marker for Fatal Clinical Complications in Patients with COVID-19 Infection. J. Clin. Med. 2023, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Huang, Q.; Dai, C.; Ren, W.; Chen, S. Lactic Dehydrogenase to Albumin Ratio Is Associated With the Risk of Stroke-Associated Pneumonia in Patients With Acute Ischemic Stroke. Front. Nutr. 2021, 8, 743216. [Google Scholar] [CrossRef] [PubMed]
- Hendy, R.M.; Elawady, M.A.; El Kareem, H.M.A. Role of lactate dehydrogenase and other biomarkers in predicting prognosis of community-acquired pneumonia. Egypt J. Bronchol. 2019, 13, 539–544. [Google Scholar] [CrossRef]
- Zhao, L.; Bao, J.; Shang, Y.; Zhang, Y.; Yin, L.; Yu, Y.; Xie, Y.; Chen, L.; Zheng, Y.; Xu, Y.; et al. The prognostic value of serum albumin levels and respiratory rate for community-acquired pneumonia: A prospective, multi-center study. PLoS ONE 2021, 16, e0248002. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, Y.; Zhang, K.; Tian, M.; Qin, S.; Li, X. Relationship Between Preoperative Hypoalbuminemia and Postoperative Pneumonia Following Geriatric Hip Fracture Surgery: A Propensity-Score Matched and Conditional Logistic Regression Analysis. Clin. Interv. Aging. 2022, 17, 495. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Chen, L.; Yang, N.; Zhang, J.; Wang, Y.; Chen, X. Relationships between human serum albumin levels and septic shock, in-hospital, and out-of-hospital mortality in elderly patients with pneumonia in different BMI ranges. Pneumonia 2024, 16, 17. [Google Scholar] [CrossRef]
- de la Varga-Martínez, O.; Gómez-Sánchez, E.; Muñoz, M.F.; Lorenzo, M.; Gómez-Pesquera, E.; Poves-Álvarez, R.; Tamayo, E.; Heredia-Rodríguez, M. Impact of nosocomial infections on patient mortality following cardiac surgery. J. Clin. Anesth. 2021, 69, 110104. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Chen, B.; Li, W.; Yan, J.; Chen, L.; Wang, X.; Xiao, Y. Ventilator-associated pneumonia after cardiac surgery: A meta-analysis and systematic review. J. Thorac. Cardiovasc. Surg. 2014, 148, 3148–3155.e5. [Google Scholar] [CrossRef] [PubMed]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Patients undergoing isolated CABG | Prior cardiac surgery Emergency CABG procedures |
| Age ≥ 18 years | Incomplete medical records |
| Complete preoperative and postoperative data | Active infection at the time of surgery Malignancy |
| Demographic characteristics | ||
| Age, years | mean ± SD | 65.4 ± 8.7 |
| Gender, n (%) | Women | 111 (25.8%) |
| Men | 319 (74.2%) | |
| Smoking history, n (%) | Non-smoker | 194 (45.1%) |
| Current smoker | 109 (25.4%) | |
| Former smoker | 127 (29.5%) | |
| Presence of any comorbidity, n (%) | 370 (86%) | |
| Hypertension | 296 (68.8%) | |
| Diabetes Mellitus | 193 (44.9%) | |
| Chronic Renal Disease | 36 (8.4%) | |
| COPD | 30 (7%) | |
| Euro SCORE, n (%) | Low risk | 393 (91.4%) |
| Moderate risk | 30 (7%) | |
| High risk | 7 (1.6%) | |
| Preoperative EF (%) | mean ± SD | 56 ± 12.4 |
| Surgical procedures | ||
| Surgical method, n (%) | OPCAB | 112 (26%) |
| CPB | 318 (74%) | |
| Number of grafts, n (%) | 1 | 12 (2.8%) |
| 2 | 48 (11.2%) | |
| 3 | 160 (37.2%) | |
| 4 | 152 (35.3%) | |
| 5 | 49 (11.4%) | |
| 6 | 8 (1.9%) | |
| 7 | 1 (0.2%) | |
| CPB time, min | mean ± SD | 118.4 ± 35.8 |
| Cross clamp time, min | mean ± SD | 69.2 ± 21.1 |
| Extubation time, h | median (25th–75th percentile) | 9 (7–13) |
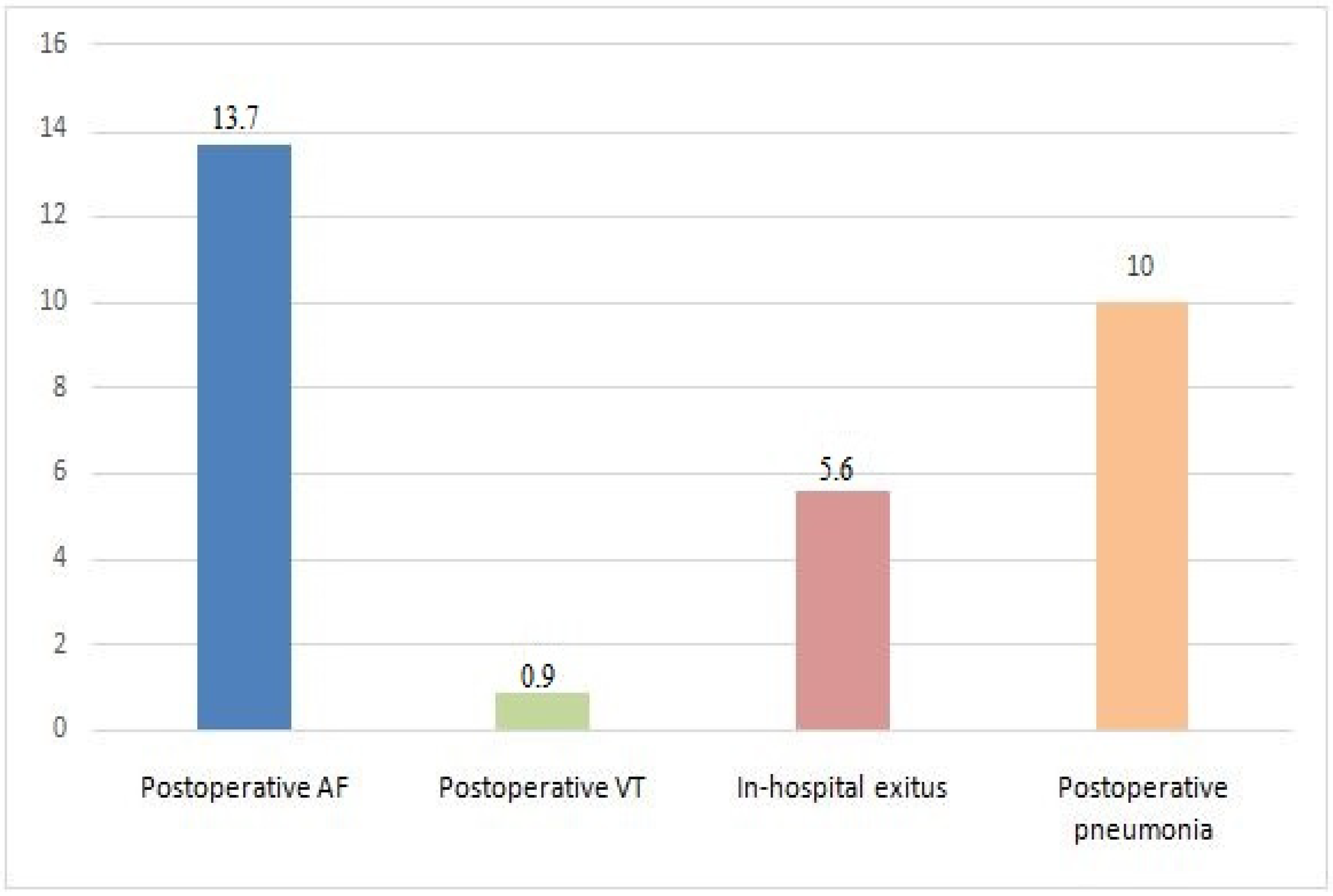
| Postoperative complications | ||
| Postoperative AF | 59 (13.3%) | |
| Postoperative VT | 4 (0.9%) | |
| Postoperative pneumonia | 43 (10%) | |
| In-hospital exitus | 24 (5.6%) | |
| Postoperative Pneumonia | ||||
|---|---|---|---|---|
| (+) | (−) | p | ||
| Demographic characteristics | ||||
| Gender | Female | 10 (23.3%) | 101 (26.1%) | 0.69 |
| Male | 33 (76.7%) | 286 (73.9%) | ||
| Age, year | mean ± SD | 65.7 ± 8.9 | 65.4 ± 8.7 | 0.82 |
| Smoking history, n (%) | Non-smoker | 18 (41.9%) | 176 (45.5%) | 0.27 |
| Current smoker | 8 (18.6%) | 101 (26.1%) | ||
| Former smoker | 17 (39.5%) | 110 (28.4%) | ||
| Comorbidity | (+) | 40 (93%) | 330 (85.3%) | 0.16 |
| (−) | 3 (7%) | 57 (14.7%) | ||
| Hypertension | 34 (79.1%) | 262 (67.7%) | 0.13 | |
| Diabetes mellitus | 26 (60.5%) | 167 (43.2%) | 0.03 | |
| Chronic renal disease | 7 (16.3 %) | 29 (7.5%) | 0.048 | |
| COPD | 6 (14%) | 24 (6.2%) | 0.058 | |
| EuroSCORE, n (%) | Low risk | 38 (88.4%) | 355 (91.7%) | 0.76 |
| Moderate risk | 4 (9.3%) | 26 (6.7%) | ||
| High risk | 1(2.3%) | 6 (1.6%) | ||
| Preoperative EF (%) | mean ± SD | 56.4 ± 13.04 | 55.99 ± 12.34 | 0.83 |
| Surgical procedure | ||||
| Surgical method, n (%) | OPCAB | 11 (25.6%) | 101 (26.1%) | 0.94 |
| CPB | 32 (74.4%) | 286 (73.9%) | ||
| Number of grafts | 1 | 1 (2.3%) | 11 (2.8%) | 0.98 |
| 2 | 5 (11.6%) | 43 (11.1%) | ||
| 3 | 16 (37.2%) | 144 (37.2%) | ||
| 4 | 16 (37.2%) | 136 (35.1%) | ||
| 5 | 5 (11.6%) | 44 (11.4%) | ||
| 6 | 0 | 8 (2.1%) | ||
| 7 | 0 | 1 (0.3%) | ||
| CPB time, min | mean ± SD | 138.84 ± 60.99 | 116.14 ± 31.1 | 0.01 |
| Cross clamp time, min | mean ± SD | 79.56 ± 25.7 | 68.02 ± 20.3 | 0.003 |
| Extubation time, h | Median/(25th–75th percentiles) | 9.25 (6.13–12.8) | 9 (7–13) | 0.87 |
| LOS in ICU, day | Median/(25th–75th percentiles) | 4 (2–5) | 3.5 (3–5) | 0.41 |
| LOS in hospital, day | Median/(25th–75th percentiles) | 9 (7–13) | 8 (7–10) | 0.053 |
| Post operative complications | ||||
| Postoperative AF | 7 (16.3%) | 52 (13.4%) | 0.61 | |
| Postoperative VT | 0 | 4 (1%) | 0.5 | |
| In-hospital exitus | 4 (9.3%) | 20 (5.2%) | 0.26 | |
| Laboratory tests at the time of diagnosis | ||||
| WBC, ×103 | mean ± SD | 8.10 ± 1.98 | 8.31 ± 2.45 | 0.59 |
| Leukocyte count, ×103 | Median/(25th–75th percentiles) | 5 (4–6) | 4.9 (3.9–6.2) | 0.54 |
| Leukocyte, % | Median/(25th–75th percentiles) | 64.6 (58.3–70.8) | 62 (56.9–68) | 0.27 |
| Lymphocyte count, ×103 | Median/(25th–75th percentiles) | 1.8 (1.3–2.2) | 1.9 (1.4–2.5) | 0.085 |
| Lymphocyte, % | mean ± SD | 22.8 ± 7.08 | 25.27 ± 8.9 | 0.04 |
| Hemoglobin, (g/dL) | mean ± SD | 12.25 ± 2.21 | 13.07 ± 1.99 | 0.012 |
| Platelet, ×103 | mean ± SD | 248,095 ± 71,575 | 241,037 ± 67,514 | 0.52 |
| MPV, (fL) | mean ± SD | 9.96 ± 1.26 | 9.85 ± 1.16 | 0.57 |
| Albumin, (g/L) | mean ± SD | 37.35 ± 5.2 | 39.4 ± 5.2 | 0.015 |
| LDH, (U/L) | Median/(25th × 75th percentiles) | 231(201–316) | 206 (168–265) | 0.017 |
| CRP, (mg/L) | Median/(25th × 75th percentiles) | 7 (3–24) | 5.6 (1.8–17.5) | 0.11 |
| Procalcitonin, (ng/mL) | Median/(25th × 75th percentiles) | 0.12 (0.57–0.76) | 0.12 (0.05–0.53) | 0.32 |
| Variable | OR [95% CI] | p |
|---|---|---|
| Diabetes mellitus | 1.243 [0.483–3.194] | 0.652 |
| Chronic renal disease | 1.904 [0.488–7.435] | 0.354 |
| COPD | 4.383 [1.106–17.363] | 0.035 |
| Length of stay in hospital | 1.052 [1.004–1.103] | 0.035 |
| Lymphocyte,% | 1.003 [0.95–1.059] | 0.913 |
| Haemoglobin | 0.801 [0.594–1.079] | 0.145 |
| LDH | 1.0 [0.995–1.004] | 0.898 |
| Albumin | 0.956 [0.871–1.049] | 0.342 |
| CPB time | 1.013 [1.001–1.025] | 0.030 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baris, O.; Onyilmaz, T.A.; Kaya, H. Frequency and Predictors of Pneumonia After Isolated Coronary Artery Bypass Grafting (CABG): A Single-Center Study. Diagnostics 2025, 15, 195. https://doi.org/10.3390/diagnostics15020195
Baris O, Onyilmaz TA, Kaya H. Frequency and Predictors of Pneumonia After Isolated Coronary Artery Bypass Grafting (CABG): A Single-Center Study. Diagnostics. 2025; 15(2):195. https://doi.org/10.3390/diagnostics15020195
Chicago/Turabian StyleBaris, Ozgur, Tugba Asli Onyilmaz, and Huseyin Kaya. 2025. "Frequency and Predictors of Pneumonia After Isolated Coronary Artery Bypass Grafting (CABG): A Single-Center Study" Diagnostics 15, no. 2: 195. https://doi.org/10.3390/diagnostics15020195
APA StyleBaris, O., Onyilmaz, T. A., & Kaya, H. (2025). Frequency and Predictors of Pneumonia After Isolated Coronary Artery Bypass Grafting (CABG): A Single-Center Study. Diagnostics, 15(2), 195. https://doi.org/10.3390/diagnostics15020195






