Assessment of the Severity of Intermediate Coronary Artery Stenosis Using the Systemic Inflammatory Response Index
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vedanthan, R.; Seligman, B.; Fuster, V. Global perspective on acute coronary syndrome: A burden on the young and poor. Circ. Res. 2014, 114, 1959–1975. [Google Scholar] [CrossRef] [PubMed]
- Task Force Members; Montalescot, G.; Sechtem, U.; Achenbach, S.; Andreotti, F.; Arden, C.; Budaj, A.; Bugiardini, R.; Crea, F.; Cuisset, T.; et al. 2013 ESC guidelines on the management of stable coronary artery disease: The task force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 2013, 34, 2949–3003. [Google Scholar] [PubMed]
- Tobis, J.; Azarbal, B.; Slavin, L. Assessment of intermediate severity coronary lesions in the catheterization laboratory. J. Am. Coll. Cardiol. 2007, 49, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Hansson, G.K. Inflammation, atherosclerosis, and coronary artery disease. N. Engl. J. Med. 2005, 352, 1685–1695. [Google Scholar] [CrossRef]
- Hilgendorf, I.; Swirski, F.K.; Robbins, C.S. Monocyte fate in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Kundnani, N.R.; Sharma, A.; Lighezan, D.F.; Georgescu, D.; Morariu, S.I.; Nisulescu, D.D.; Bita, R.G.; Rosca, C.I. Use of Neutrophil-to-Lymphocyte Ratio to Predict In-Hospital Mortality in Patients Admitted with Acute Decompensation of Atrial Fibrillation. J. Clin. Med. 2024, 13, 4719. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, T.; Chen, L.; Jin, T.; Sheng, Y.; Wu, G.; Zong, G. The systemic immune-inflammation index predicts coronary stenosis severity in coronary heart disease patients. Coron. Artery Dis. 2021, 32, 715–720. [Google Scholar] [CrossRef] [PubMed]
- Canpolat, U.; Çetin, E.H.; Cetin, S.; Aydin, S.; Akboga, M.K.; Yayla, C.; Turak, O.; Aras, D.; Aydogdu, S. The association of monocyte-to-HDL cholesterol ratio with slow coronary flow is linked to systemic inflammation. Clin. Appl. Thromb. Hemost. 2016, 22, 476–482. [Google Scholar] [CrossRef]
- Möckel, M.; Danne, O.; Müller, R.; Vollert, J.O.; Müller, C.; Lueders, C.; Störk, T.; Frei, U.; Koenig, W.; Dietz, R.; et al. Development of an optimized multimarker strategy for early risk assessment of patients with acute coronary syndromes. Clin. Chim. Acta 2008, 393, 103–109. [Google Scholar] [CrossRef]
- Hatmi, Z.N.; Saeid, A.K.; Broumand, M.A. Multiple inflammatory prognostic factors in acute coronary syndromes: A prospective inception cohort study. Acta Med. Iran. 2010, 48, 51–57. [Google Scholar]
- Blaschke, F.; Bruemmer, D.; Yin, F.; Takata, Y.; Wang, W.; Fishbein, M.C.; Okura, T.; Higaki, J.; Graf, K.; Fleck, E.; et al. C-reactive protein induces apoptosis in human coronary vascular smooth muscle cells. Circulation 2004, 110, 579–587. [Google Scholar] [CrossRef]
- de Lemos, J.A.; Morrow, D.A.; Blazing, M.A.; Jarolim, P.; Wiviott, S.D.; Sabatine, M.S.; Califf, R.M.; Braunwald, E. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: A to Z trial results. J. Am. Coll. Cardiol. 2007, 50, 2117–2124. [Google Scholar] [CrossRef]
- Kaptoge, S.; Di Angelantonio, E.; Pennells, L.; Pennells, L.; Wood, A.M.; White, I.R.; Gao, P.; Walker, M.; Thompson, A.; Sarwar, N.; et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N. Engl. J. Med. 2012, 367, 1310–1320. [Google Scholar]
- Shah, A.D.; Denaxas, S.; Nicholas, O.; Hingorani, A.D.; Hemingway, H. Low eosinophil and low lymphocyte counts and the incidence of 12 cardiovascular diseases: A CALIBER cohort study. Open Heart 2016, 3, e000477. [Google Scholar] [CrossRef] [PubMed]
- Gratchev, A.; Sobenin, I.; Orekhov, A.; Kzhyshkowska, J. Monocytes as a diagnostic marker of cardiovascular diseases. Immunobiology 2012, 217, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-Y.; Li, C.-J.; Hou, M.-F.; Chu, P.-Y. New Insights into the Role of Inflammation in the Pathogenesis of Atherosclerosis. Int. J. Mol. Sci. 2017, 18, 2034. [Google Scholar] [CrossRef] [PubMed]
- Berg, K.E.; Ljungcrantz, I.; Andersson, L.; Bryngelsson, C.; Hedblad, B.; Fredrikson, G.N.; Nilsson, J.; Björkbacka, H. Elevated CD14++CD16− monocytes predict cardiovascular events. Circ. Cardiovasc. Genet. 2012, 5, 122–131. [Google Scholar] [CrossRef]
- Rogacev, K.S.; Seiler, S.; Zawada, A.M.; Reichart, B.; Herath, E.; Roth, D.; Ulrich, C.; Fliser, D.; Heine, G.H. CD14++CD16+ monocytes and cardiovascular outcome in patients with chronic kidney disease. Eur. Heart J. 2011, 32, 84–92. [Google Scholar] [CrossRef]
- Shah, A.D.; Denaxas, S.; Nicholas, O.; Hingorani, A.D.; Hemingway, H. Neutrophil counts and initial presentation of 12 cardiovascular diseases: A CALIBER cohort study. J. Am. Coll. Cardiol. 2017, 69, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ruiz, I. Neutrophil-driven SMC death destabilizes atherosclerotic plaques. Nat. Rev. Cardiol. 2019, 16, 455. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, F.M.; Cole, P.G.; Ramage, D. Leukocyte adhesion to the coronary microvasculature during ischemia and reperfusion in an in vivo canine model. Circulation 1996, 93, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Miñana, G.; Bodí, V.; Núñez, E.; Sanchis, J.; Husser, O.; Llàcer, A. Low lymphocyte count and cardiovascular diseases. Curr. Med. Chem. 2011, 18, 3226–3233. [Google Scholar] [CrossRef]
- Ommen, S.R.; Gibbons, R.J.; Hodge, D.O.; Thomson, S.P. The usefulness of the lymphocyte concentration as a prognostic marker in coronary artery disease. Am. J. Cardiol. 1997, 79, 812–814. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Núñez, J.; Núñez, E.; Bodí, V.; Sanchis, J.; Mainar, L.; Miñana, G.; Fácila, L.; Bertomeu, V.; Merlos, P.; Darmofal, H.; et al. Low lymphocyte count in the acute ST-segment elevation myocardial infarction phase predicts long-term recurrent myocardial infarction. Coron. Artery Dis. 2010, 21, 1–7. [Google Scholar] [CrossRef]
- Massberg, S.; Brand, K.; Grüner, S.; Page, S.; Müller, E.; Müller, I.; Bergmeier, W.; Richter, T.; Lorenz, M.; Konrad, I.; et al. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J. Exp. Med. 2002, 196, 887–896. [Google Scholar] [CrossRef]
- Gawaz, M.; Langer, H.; May, A.E. Platelets in inflammation and atherogenesis. J. Clin. Investig. 2005, 115, 3378–3384. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.; Park, K.S.; Jang, H.C.; Choi, S.H. Total, and differential WBC counts are related to coronary artery atherosclerosis and increase Koreans’ cardiovascular risk. PLoS ONE 2017, 12, e0180332. [Google Scholar] [CrossRef]
- Wu, T.-H.; Chien, K.-L.; Lin, H.-J.; Hsu, H.-C.; Su, T.-C.; Chen, M.-F.; Lee, Y.-T. Total white blood cell count or neutrophil count predict ischemic stroke events among adult Taiwanese: Report from a community-based cohort study. BMC Neurol. 2013, 13, 7. [Google Scholar] [CrossRef]
- Fan, Z.; Ji, H.; Li, Y.; Jian, X.; Li, L.; Liu, T. Relationship between monocyte-to-lymphocyte ratio and coronary plaque vulnerability in patients with stable angina. Biomark. Med. 2017, 11, 979–990. [Google Scholar] [CrossRef] [PubMed]
- Ateş, A.H.; Aytemir, K.; Koçyiğit, D.; Yalcin, M.U.; Gürses, K.M.; Yorgun, H.; Canpolat, U.; Hazırolan, T.; Özer, N. Association of Neutrophil-to-Lymphocyte Ratio with the Severity and Morphology of Coronary Atherosclerotic Plaques Detected by Multidetector Computerized Tomography. Acta Cardiol. Sin. 2016, 32, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Balta, S.; Ozturk, C. The platelet-lymphocyte ratio: A simple, inexpensive and rapid prognostic marker for cardiovascular events. Platelets 2015, 26, 680–681. [Google Scholar] [CrossRef]
- Hua, Y.; Sun, J.Y.; Lou, Y.X.; Sun, W.; Kong, X.Q. Monocyte-to-lymphocyte ratio predicts mortality and cardiovascular mortality in the general population. Int. J. Cardiol. 2023, 379, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, Y.; Ji, H.; Jian, X. Prognostic utility of the combination of monocyte-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio in patients with NSTEMI after primary percutaneous coronary intervention: A retrospective cohort study. BMJ Open 2018, 8, e023459. [Google Scholar] [CrossRef] [PubMed]
- Nunez, J.; Nunez, E.; Bodi, V.; Sanchis, J.; Minana, G.; Mainar, L.; Santas, E.; Merlos, P.; Rumiz, E.; Darmofal, H.; et al. The usefulness of the neutrophil to lymphocyte ratio in predicting long-term mortality in ST-segment elevation myocardial infarction. Am. J. Cardiol. 2008, 101, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Cong, B.L.; Wang, M.; Abdullah, M.; Wang, X.L.; Zhang, Y.H.; Xu, S.J.; Cui, L. Neutrophil to lymphocyte ratio as a predictor of myocardial damage and cardiac dysfunction in acute coronary syndrome patients. Integr. Med. Res. 2018, 7, 192–199. [Google Scholar] [CrossRef] [PubMed]
- William, H.A.; Harianto, J.C.; Cipta, H. Platelet-to-Lymphocyte Ratio at Admission as a Predictor of In-Hospital and Long-Term Outcomes in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Cardiol. Res. 2021, 12, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Luke, K.; Purwanto, B.; Herawati, L.; Al-Farabi, M.J.; Oktaviono, Y.H. Predictive Value of Hematologic Indices in Diagnosing Acute Coronary Syndrome. Open Access Maced. J. Med. Sci. 2019, 7, 2428–2433. [Google Scholar] [CrossRef] [PubMed]
- Ye, G.-L.; Chen, Q.; Chen, X.; Yin, T.-T.; Meng, Q.-H.; Liu, Y.-C.; Wei, H.-Q.; Zhou, Q.-H. The prognostic role of platelet-to-lymphocyte ratio in patients with acute heart failure: A cohort study. Sci. Rep. 2019, 9, 10639. [Google Scholar] [CrossRef]
- Turcato, G.; Sanchis-Gomar, F.; Cervellin, G.; Zorzi, E.; Sivero, V.; Salvagno, G.L.; Tenci, A.; Lippi, G. Evaluation of Neutrophil-lymphocyte and platelet-lymphocyte ratios as predictors of 30-day mortality in patients hospitalized for an episode of acute decompensated heart failure. J. Med. Biochem. 2019, 38, 452–460. [Google Scholar] [CrossRef] [PubMed]
- Candemir, M.; Kiziltunç, E.; Nurkoç, S.; Şahinarslan, A. Relationship Between Systemic Immune-Inflammation Index (SII) and the Severity of Stable Coronary Artery Disease. Angiology 2021, 72, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Erdoğan, M.; A Erdöl, M.; Öztürk, S.; Durmaz, T. The systemic immune-inflammation index is a novel marker to predict functionally significant coronary artery stenosis. Biomark. Med. 2020, 14, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-L.; Wu, C.-H.; Hsu, P.-F.; Chen, S.-C.; Huang, S.-S.; Chan, W.L.; Lin, S.-J.; Chou, C.-Y.; Chen, J.-W.; Pan, J.-P.; et al. Systemic immune-inflammation index (SII) predicted clinical outcomes in coronary artery disease patients. Eur. J. Clin. Investig. 2020, 50, e13230. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Ma, X.; Shao, Q.; Yang, Z.; Wang, Y.; Gao, F.; Zhou, Y.; Yang, L.; Wang, Z. Prognostic Impact of Multiple Lymphocyte-Based Inflammatory Indices in Acute Coronary Syndrome Patients. Front. Cardiovasc. Med. 2022, 9, 811790. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wu, Q.; Chen, S.; Gao, J.; Li, X.; Zhang, X.; Zhou, Y.; He, D.; Cheng, Z.; Zhu, Y.; et al. The Associations of Two Novel Inflammation Indexes, SII, and SIRI with the Risks for Cardiovascular Diseases and All-Cause Mortality: A Ten-Year Follow-Up Study in 85,154 Individuals. J. Inflamm. Res. 2021, 14, 131–140. [Google Scholar] [CrossRef]
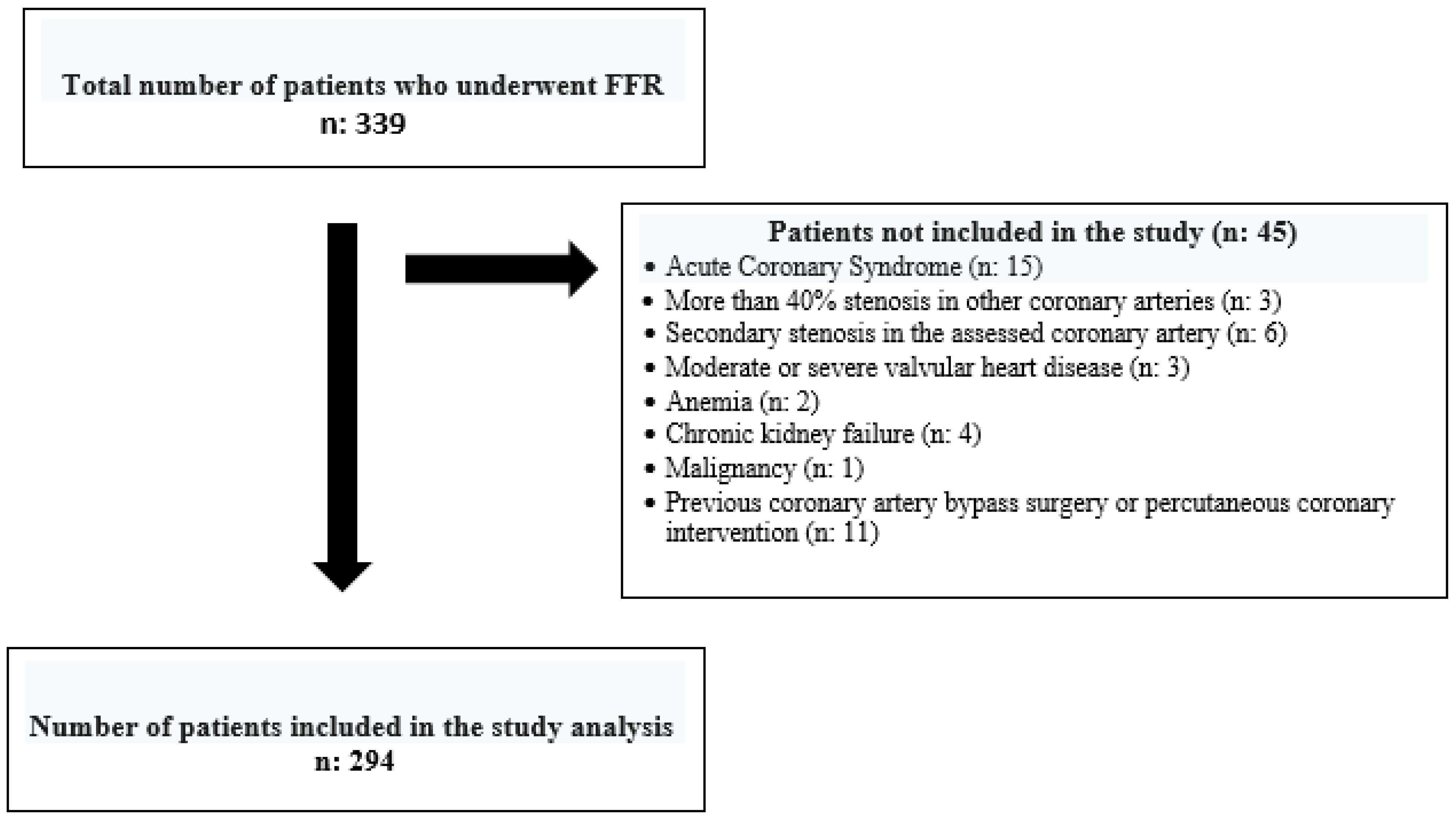
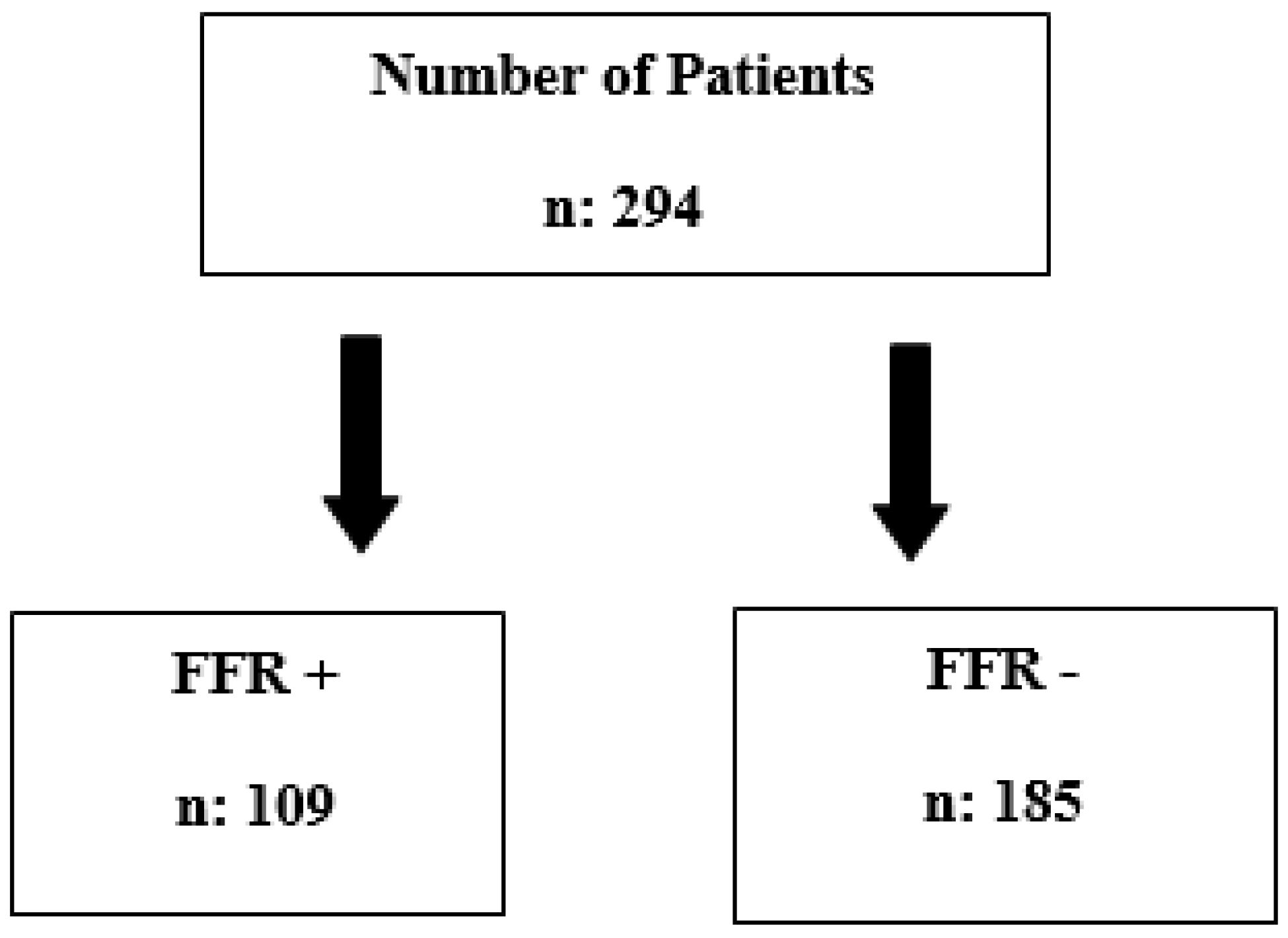
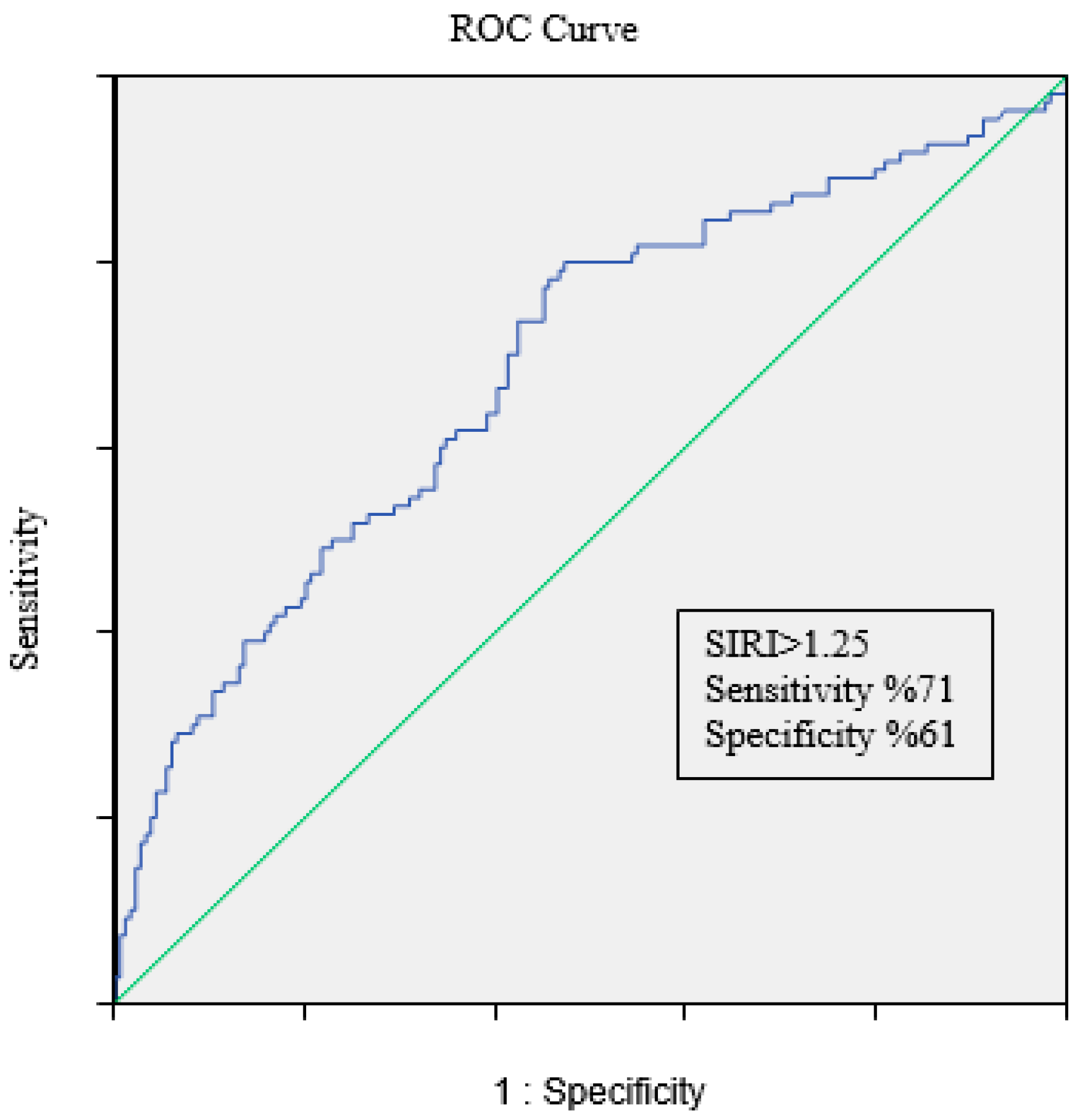
|
| FFR Non-Significant | FFR Significant | p Value | |
|---|---|---|---|
| Age | 61 (54–70) | 59 (51–66) | 0.054 |
| Male gender | 128 (70%) | 87 (79%) | 0.079 |
| DM2 | 57 (31%) | 26 (24%) | 0.227 |
| HT | 72 (39%) | 40 (37%) | 0.709 |
| Smoking | 94 (51%) | 48 (44%) | 0.277 |
| FFR Non-Significant | FFR Significant | p Value | |
|---|---|---|---|
| Glucose | 108 (94–151) | 107 (91–135) | 0.719 |
| Urea | 34 (27–40) | 33 (26–39) | 0.635 |
| Creatinine | 0.89 (0.77–1.03) | 0.93 (0.78–1.04) | 0.249 |
| eGFR | 82 (63–101) | 79 (64–95) | 0.234 |
| Hemoglobin | 14.1 (13–15.1) | 14.5 (13.4–15.4) | 0.057 |
| RDW | 13.7 (13–14.6) | 13.5 (13.1–14.1) | 0.153 |
| MPV | 8.6 (8.1–9.5) | 8.7 (8–9.3) | 0.780 |
| PCT | 0.21 (0.17–0.24) | 0.21 (0.16–0.23) | 0.108 |
| PDW | 16.9 (16.3–18) | 16.8 (16.2–17.3) | 0.140 |
| PLT | 238 (207–275) | 242 (197–293) | 0.549 |
| WBC | 7.81 (6.73–9.30) | 8.20 (6.79–9.7) | 0.182 |
| Neutrophils | 4.62 (3.69–5.68) | 5.32 (3.80–6.50) | 0.003 * |
| Lymphocytes | 2.26 (1.75–2.73) | 2.13 (1.75–2.63) | 0.218 |
| Monocytes | 0.56 (0.5–0.7) | 0.65 (0.56–0.78) | <0.001 * |
| SII | 477 (328–753) | 575 (390–882) | 0.009 |
| SIRI | 1.10 (0.87–1.54) | 1.56 (1.18–2.13) | <0.001 * |
| Total cholesterol | 185 (151–214) | 204 (162–234) | 0.008 |
| LDL | 111 (86–143) | 122 (96–143) | 0.068 |
| HDL | 41 (35–49) | 41 (34–46) | 0.142 |
| Triglycerides | 127 (93–178) | 146 (104–244) | 0.018 |
| TG/HDLc | 4.15 (0.81–7.49) | 4.80 (1.24–8.36) | 0.198 |
| Initial FFR | 0.94 (0.92–0.95) | 0.88 (0.83–0.92) | <0.001 * |
| Final FFR | 0.88 (0.84–0.91) | 0.74 (0.71–0.78) | <0.001 * |
| Amount of adenosine | 250 (150–300) | 200 (104–250) | 0.003 * |
| Odds Ratio | 95% Confidence Interval | p Value | |
|---|---|---|---|
| SIRI | 2.805 | 1.877–4.192 | <0.001 * |
| Male gender | 0.689 | 0.353–1.343 | 0.274 |
| Age | 0.984 | 0.958–1.010 | 0.232 |
| Triglyceride | 1.002 | 0.999–1.005 | 0.223 |
| Total cholesterol | 1.006 | 0.999–1.012 | 0.075 |
| Hemoglobin | 0.979 | 0.874–1.096 | 0.708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akyel, S.; Korkmaz, A.; Yıldız, A. Assessment of the Severity of Intermediate Coronary Artery Stenosis Using the Systemic Inflammatory Response Index. Diagnostics 2025, 15, 162. https://doi.org/10.3390/diagnostics15020162
Akyel S, Korkmaz A, Yıldız A. Assessment of the Severity of Intermediate Coronary Artery Stenosis Using the Systemic Inflammatory Response Index. Diagnostics. 2025; 15(2):162. https://doi.org/10.3390/diagnostics15020162
Chicago/Turabian StyleAkyel, Serdar, Ahmet Korkmaz, and Abdülkadir Yıldız. 2025. "Assessment of the Severity of Intermediate Coronary Artery Stenosis Using the Systemic Inflammatory Response Index" Diagnostics 15, no. 2: 162. https://doi.org/10.3390/diagnostics15020162
APA StyleAkyel, S., Korkmaz, A., & Yıldız, A. (2025). Assessment of the Severity of Intermediate Coronary Artery Stenosis Using the Systemic Inflammatory Response Index. Diagnostics, 15(2), 162. https://doi.org/10.3390/diagnostics15020162






