Prevalence and Molecular Characterization of Carbapenemase-Producing Multidrug-Resistant Bacteria in Diabetic Foot Ulcer Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Clinical Assessment and Ulcer Grading
2.4. Microbiological Processing
2.5. Molecular Characterization of Resistance Mechanisms
2.6. Modified Hodge Test (MHT)
2.7. Carba NP Test
2.8. Molecular Detection of Carbapenemase Genes
2.9. Statistical Analysis
3. Results
- (A)
- Study of association between demographical and clinical characteristics with MDR and Non-MDR bacterial isolates in DFU patients
- (B)
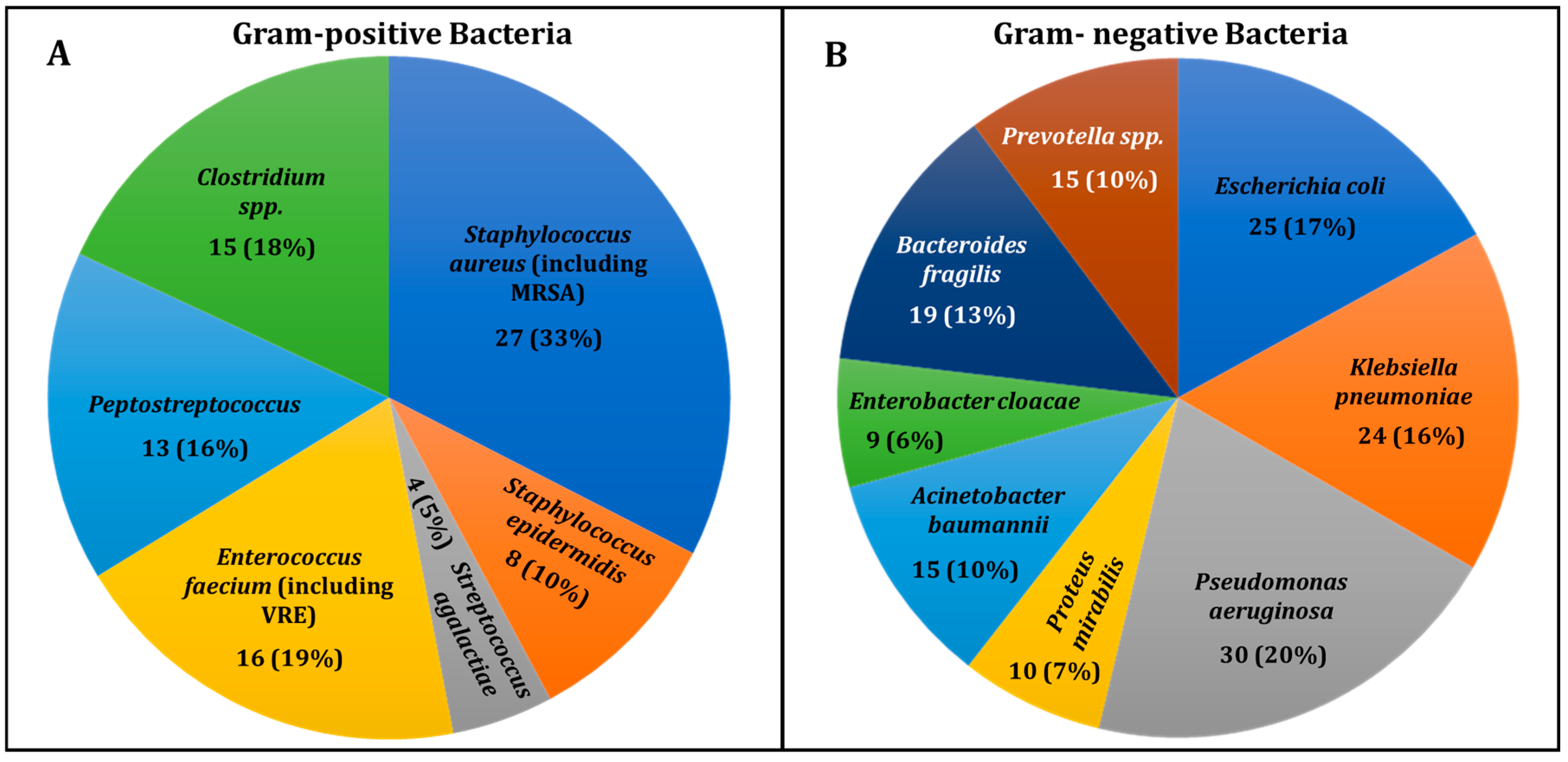
- Bacterial Diversity in DFU Infections
- (C)
- Renal Status in Patients with Diabetic Foot Ulcer Infections
- (D)
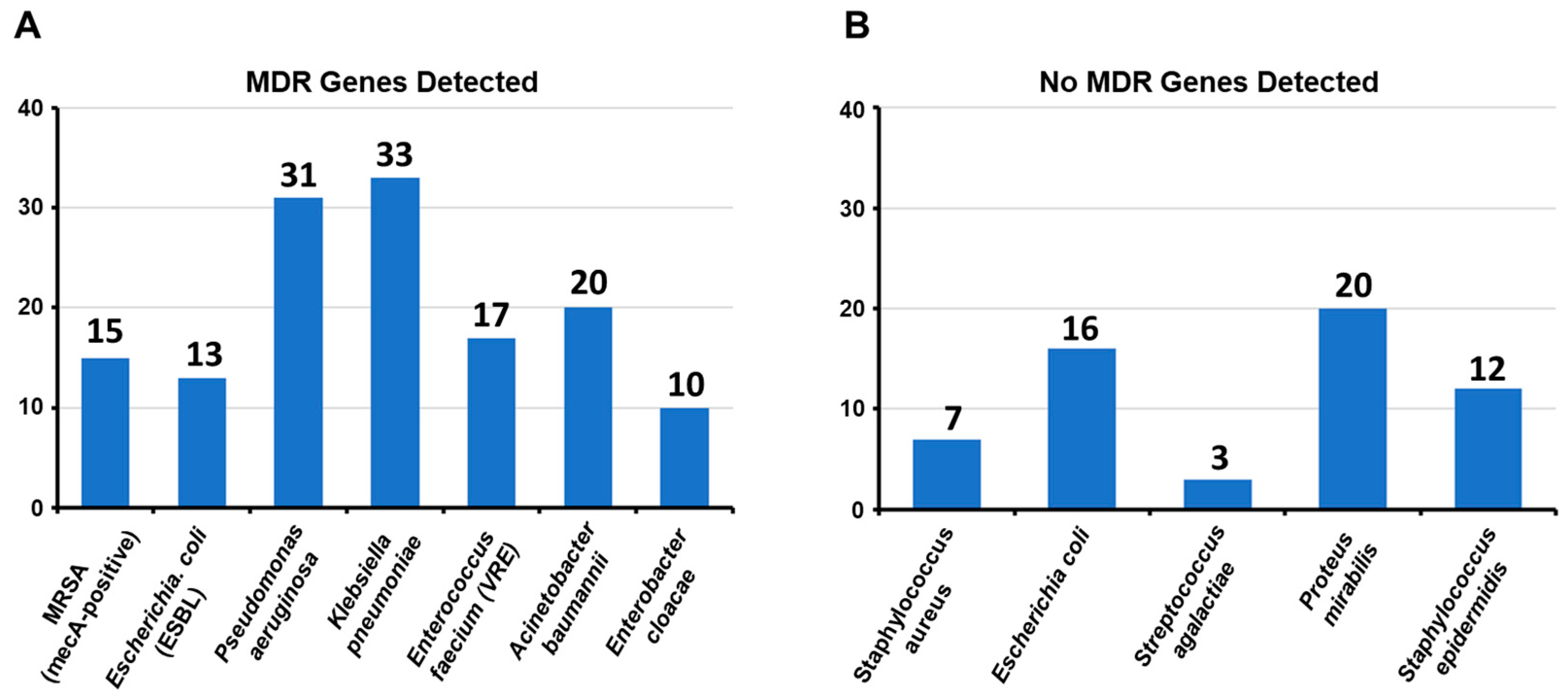
- Distribution of multidrug resistance genes in clinically significant bacterial pathogens
- (E)
- MHT, Carba NP Test, and Molecular Detection of Carbapenemase Genes from E. coli Isolates
- (i)
- Carbapenemase Production in Escherichia coli Isolates
- (ii)
- Phenotypic and Molecular Detection of Carbapenemase Production and Prevalence of Carbapenemase Genes
- (F)
- MHT, Carba NP Test, and Molecular Detection of Carbapenemase Genes of Pseudomonas aeruginosa Isolates
- (i)
- Phenotypic detection of carbapenemase production
- (ii)
- Carbapenemase Genes Detected by PCR and Their Correlation with Antibiotic Susceptibility to Imipenem and Meropenem
- (G)
- MHT, Carba NP Test, and Molecular Detection of Carbapenemase Genes of Klebsiella pneumoniae Isolates
- (i)
- Phenotypic and Molecular Detection of Carbapenemase Production and Genes in Klebsiella pneumoniae isolates
- (ii)
- Antibiotic Susceptibility to Carbapenems in Non-Carbapenemase-Producing Isolates
- (H)
- MHT, Carba NP test, and molecular detection of carbapenemase genes of Acinetobacter baumannii isolates
- (i)
- Phenotypic and Molecular Detection of Carbapenemase Production and Genes in Acinetobacter baumannii isolates
- (ii)
- Antibiotic Susceptibility and Resistance Mechanisms in Non-Carbapenemase Producers
- (I)
- MHT, Carba NP Test, and Molecular Detection of Carbapenemase Genes of Enterobacter cloacae isolates
- (i)
- Phenotypic and Molecular Detection of Carbapenemase Production and Antibiotic Susceptibility to Carbapenems
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yazdanpanah, L.; Shahbazian, H.; Nazari, I.; Arti, H.R.; Ahmadi, F.; Mohammadianinejad, S.E.; Cheraghian, B.; Hesam, S. Incidence and Risk Factors of Diabetic Foot Ulcer: A Population-Based Diabetic Foot Cohort (ADFC Study)—Two-Year Follow-Up Study. Int. J. Endocrinol. 2018, 2018, 7631659. [Google Scholar] [CrossRef]
- Edmonds, M.; Manu, C.; Vas, P. The current burden of diabetic foot disease. J. Clin. Orthop. Trauma 2021, 17, 88–93. [Google Scholar] [CrossRef]
- Jodheea-Jutton, A.; Hindocha, S.; Bhaw-Luximon, A. Health economics of diabetic foot ulcer and recent trends to accelerate treatment. Foot 2022, 52, 101909. [Google Scholar] [CrossRef] [PubMed]
- Akkus, G.; Sert, M. Diabetic foot ulcers: A devastating complication of diabetes mellitus continues non-stop in spite of new medical treatment modalities. World J. Diabetes 2022, 13, 1106–1121. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial Antibiotic Resistance: The Most Critical Pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Song, Q.; Mei, S.; Xue, Z.; Li, J.; Ning, T. Distribution of multidrug-resistant bacterial infections in diabetic foot ulcers and risk factors for drug resistance: A retrospective analysis. PeerJ 2023, 11, e16162. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 8 December 2024).
- Reina-Bueno, M.; Palomo-Toucedo, I.; Castro-Méndez, A.; Domínguez-Maldonado, G.; Vázquez-Bautista, M. Methicillin-Resistant Staphylococcus aureus Diabetic Foot Crossed Infection: A Case Report. Pathogens 2020, 9, 549. [Google Scholar] [CrossRef] [PubMed]
- Abebe, A.; Birhanu, A. Methicillin Resistant Staphylococcus aureus: Molecular Mechanisms Underlying Drug Resistance Development and Novel Strategies to Combat. Infect. Drug Resist. 2023, 16, 7641–7662. [Google Scholar] [CrossRef] [PubMed]
- Rawat, D.; Nair, D. Extended-spectrum ß-lactamases in gram negative bacteria. J. Glob. Infect. Dis. 2010, 2, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Mączyńska, B.; Jama-Kmiecik, A.; Sarowska, J.; Woronowicz, K.; Choroszy-Król, I.; Piątek, D.; Frej-Mądrzak, M. Changes in Antibiotic Resistance of Acinetobacter baumannii and Pseudomonas aeruginosa Clinical Isolates in a Multi-Profile Hospital in Years 2017–2022 in Wroclaw, Poland. J. Clin. Med. 2023, 12, 5020. [Google Scholar] [CrossRef]
- Yuan, P.-B.; Dai, L.-T.; Zhang, Q.-K.; Zhong, Y.-X.; Liu, W.-T.; Yang, L.; Chen, D.-Q.; Ponraj, V.P. Global emergence of double and multi-carbapenemase producing organisms: Epidemiology, clinical significance, and evolutionary benefits on antimicrobial resistance and virulence. Microbiol. Spectr. 2024, 12, e00008-24. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The Versatile β-Lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, Y.; Tang, G.; Qiao, F.; McNally, A.; Zong, Z. NDM Metallo-β-Lactamases and Their Bacterial Producers in Health Care Settings. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef] [PubMed]
- Karampatakis, T.; Tsergouli, K.; Behzadi, P. Carbapenem-Resistant Klebsiella pneumoniae: Virulence Factors, Molecular Epidemiology and Latest Updates in Treatment Options. Antibiotics 2023, 12, 234. [Google Scholar] [CrossRef] [PubMed]
- Patil, H.V.; Patil, V.C.; Patange, A.P.; Asim Khan, M. Antibiotic Resistance Profiles of Extended-Spectrum β-Lactamase (ESBL)- and Metallo-β-Lactamase (MBL)-Producing Klebsiella pneumoniae Isolates from Diabetic Foot Ulcers: Implications for Treatment Strategies. Cureus 2024, 16, e66089. [Google Scholar] [CrossRef] [PubMed]
- Codjoe, F.; Donkor, E. Carbapenem Resistance: A Review. Med. Sci. 2017, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Inturi, R.; Anne, D.; Jadhav, D.; Viswambharan, V.; Khadilkar, R.; Dnyanmote, A.; Shahi, S. Wagner’s Classification as a Tool for Treating Diabetic Foot Ulcers: Our Observations at a Suburban Teaching Hospital. Cureus 2022, 14, e21501. [Google Scholar] [CrossRef] [PubMed]
- Mills, J.P.; Patel, P.; Broekhuizen, E.; Burdick, S.; DeGeorge, C.; Gallagher, K.A.; Haase, S.C.; Holmes, C.M.; Jacobson, J.A.; Nagel, J.L. Diabetic Foot Infections; Michigan Medicine University of Michigan: Ann Arbor, MI, USA, 2019. [Google Scholar]
- Ramsay, S.; Cowan, L.; Davidson, J.M.; Nanney, L.; Schultz, G. Wound samples: Moving towards a standardised method of collection and analysis. Int. Wound J. 2015, 13, 880–891. [Google Scholar] [CrossRef]
- Chagla, A.H.; Borczyk, A.A.; Facklam, R.R.; Lovgren, M. Breast abscess associated with Helcococcus kunzii. J. Clin. Microbiol. 1998, 36, 2377–2379. [Google Scholar] [CrossRef]
- Bauer, A.; Kirby, W.; Sherris, J.C.; Turck, M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Moses, V.K.; Kandi, V.; Rao, S.K.D. Minimum Inhibitory Concentrations of Vancomycin and Daptomycin Against Methicillin-resistant Staphylococcus aureus Isolated from Various Clinical Specimens: A Study from South India. Cureus 2020, 12, e6749. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Cosgrove, S.E.; Maragakis, L.L. Combination Therapy for Treatment of Infections with Gram-Negative Bacteria. Clin. Microbiol. Rev. 2012, 25, 450–470. [Google Scholar] [CrossRef]
- Rafif Khairullah, A.; Rehman, S.; Agus Sudjarwo, S.; Helmi Effendi, M.; Chasyer Ramandinianto, S.; Aega Gololodo, M.; Widodo, A.; Hendriana Priscilia Riwu, K.; Ayu Kurniawati, D. Detection of mecA gene and methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and risk factors from farms in Probolinggo, Indonesia. F1000Research 2022, 11, 722. [Google Scholar] [CrossRef]
- Mathers, A.J.; Carroll, J.; Sifri, C.D.; Hazen, K.C. Modified Hodge Test versus Indirect Carbapenemase Test: Prospective Evaluation of a Phenotypic Assay for Detection of Klebsiella pneumoniae Carbapenemase (KPC) in Enterobacteriaceae. J. Clin. Microbiol. 2013, 51, 1291–1293. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bonomo, R.A.; Burd, E.M.; Conly, J.; Limbago, B.M.; Poirel, L.; Segre, J.A.; Westblade, L.F. Carbapenemase-Producing Organisms: A Global Scourge. Clin. Infect. Dis. 2018, 66, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Bouslah, Z. Carba NP test for the detection of carbapenemase-producing Pseudomonas aeruginosa. Médecine Mal. Infect. 2020, 50, 466–479. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Saeed, N.K.; Ahmad, N.; Shadab, M.; Joji, R.M.; Al-Mahmeed, A.; Bindayna, K.M.; Tabbara, K.S.; Ismaeel, A.Y.; Dar, F.K. Molecular Screening of Carbapenem-Resistant K. pneumoniae (CRKP) Clinical Isolates for Concomitant Occurrence of Beta-Lactam Genes (CTX-M, TEM, and SHV) in the Kingdom of Bahrain. J. Clin. Med. 2023, 12, 7522. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes/Metab. Res. Rev. 2020, 36, e3280. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Jahan, N.; Khatoon, R.; Ansari, F.M.; Ahmad, S. Diabetic Foot Ulcer: Aerobic Bacterial Isolates and Their Antimicrobial Resistance Profile. Biochem. Cell. Arch. 2024, 24, 1681. [Google Scholar] [CrossRef]
- Alkhatieb, M.; Alrayiqi, R.; Alsulami, O.A.; Albassam, Z.M.; Wali, S.M.; Alnahdi, H. Common Pathogens Isolated from Infected Diabetic Foot Ulcers at King Abdulaziz University Hospital, Saudi Arabia: A Retrospective Study. J. Med. Res. Surg. 2022, 3, 71–78. [Google Scholar] [CrossRef]
- Malik, A.; Mohammad, Z.; Ahmad, J. The diabetic foot infections: Biofilms and antimicrobial resistance. Diabetes Metab. Syndr. Clin. Res. Rev. 2013, 7, 101–107. [Google Scholar] [CrossRef]
- Xie, X.; Bao, Y.; Ni, L.; Liu, D.; Niu, S.; Lin, H.; Li, H.; Duan, C.; Yan, L.; Huang, S.; et al. Bacterial Profile and Antibiotic Resistance in Patients with Diabetic Foot Ulcer in Guangzhou, Southern China: Focus on the Differences among Different Wagner’s Grades, IDSA/IWGDF Grades, and Ulcer Types. Int. J. Endocrinol. 2017, 2017, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.E. Prevalence of Acinetobacter baumannii in Saudi Arabia: Risk factors, antimicrobial resistance patterns and mechanisms of carbapenem resistance. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 1. [Google Scholar] [CrossRef]
- Hassan, H.; Yassin, N.; Saadi, A. Molecular Detection of the Genes bla OXA, bla KPC and bla NDM Among Carbapenem-Resistant Klebsiella Pneumoniae Isolated from Different Hospitals in Duhok City, Iraq. Internet J. Microbiol. 2020, 17, 1. [Google Scholar]
- Mokhtari, M.; Mojtahedi, A.; Mahdieh, N.; Jafari, A.; Arya, M.J. High Prevalence of blaOXA-48 and blaNDM-Producing Carbapenem-Resistant Klebsiella pneumoniae Isolated from Clinical Samples in Shahid Rajaei Hospital in Tehran, Iran. Jundishapur J. Microbiol. 2022, 15, e130804. [Google Scholar] [CrossRef]
- Haji, S.H.; Aka, S.T.H.; Ali, F.A. Prevalence and characterisation of carbapenemase encoding genes in multidrug-resistant Gram-negative bacilli. PLoS ONE 2021, 16, e0259005. [Google Scholar] [CrossRef]
- Rotondo, C.; Venditti, C.; Butera, O.; Dimartino, V.; Messina, F.; Properzi, M.; Caparrelli, C.; Antonelli, V.; D’Arezzo, S.; Selleri, M.; et al. Molecular Characterization of Multidrug-Resistant and Hypervirulent New Delhi Metallo-Beta-Lactamase Klebsiella pneumoniae in Lazio, Italy: A Five-Year Retrospective Study. Antibiotics 2024, 13, 1045. [Google Scholar] [CrossRef] [PubMed]
- Chegini, Z.; Khoshbayan, A.; Taati Moghadam, M.; Farahani, I.; Jazireian, P.; Shariati, A. Bacteriophage therapy against Pseudomonas aeruginosa biofilms: A review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 45. [Google Scholar] [CrossRef] [PubMed]
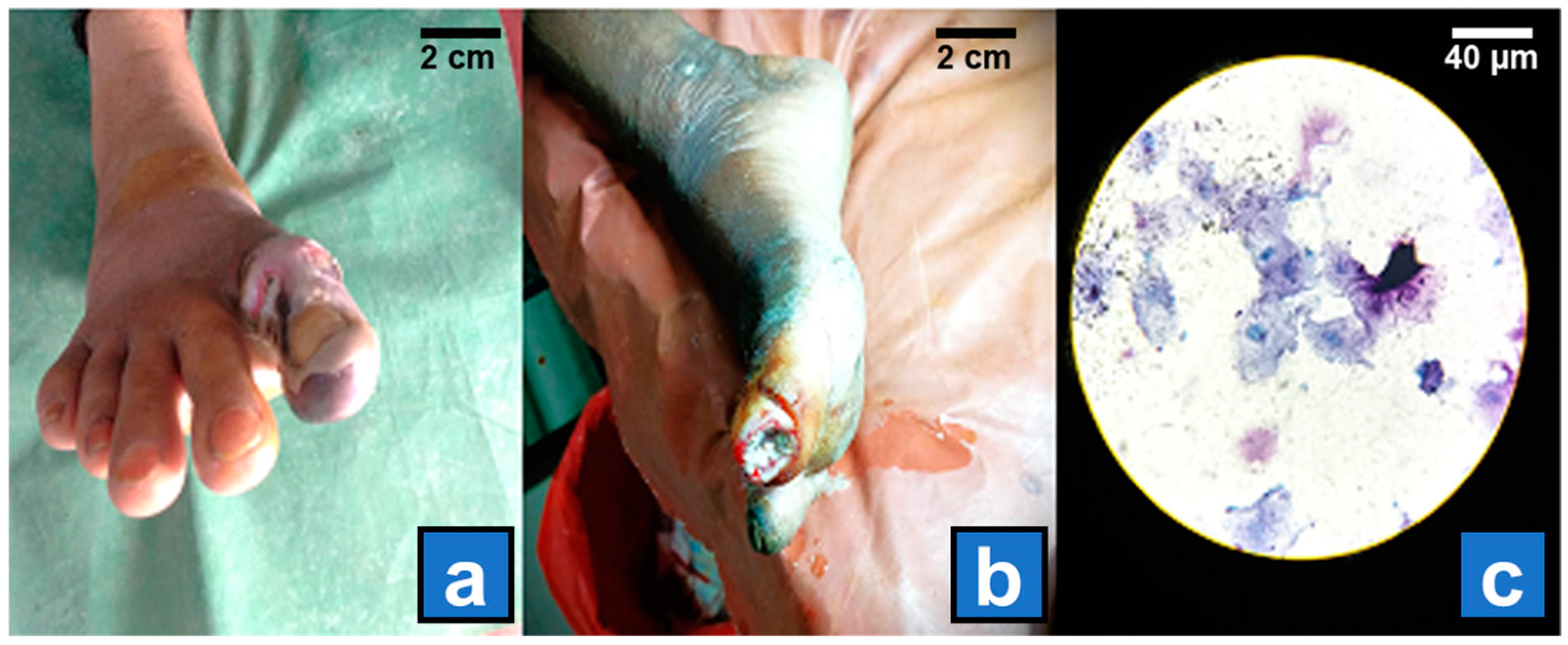
| Characteristics | Number of Isolates (%) | |
|---|---|---|
| Sex | Male | 120 (60%) |
| Female | 80 (40%) | |
| Age (years) | 63.5 ± 10.5 | |
| Body mass index (BMI) Kg/m2 | 24.9 ± 5.7 | |
| Duration of diabetes (years) | 8.6 ± 1.5 | |
| HbA1c % | 8.6 ± 1.5 | |
| Amputation | Minor | 47 (64.4%) |
| Major | 26 (35.6%) | |
| Mortality | 9 (4.5%) | |
| Wagner ulcer grade | Grade II | 80 (40%) |
| Grade III | 60 (30%) | |
| Grade IV | 40 (20%) | |
| Grade V | 20 (10%) | |
| Ulcer size (cm2) | <5 cm2 | 47 (23.5%) |
| >5 cm2 | 153 (76.5%) | |
| Duration of ulcers | <1months | 65 (32.5%) |
| >1 months | 135 (67.5%) | |
| Clinical complications | Neuropathy | 166 (83%) |
| Retinopathy | 155 (77.5%) | |
| Nephropathy | 57 (28.5%) | |
| Hypertension | 108 (54%) | |
| CVD | 43 (21.5%) | |
| Type of infection | Monomicrobial infection | 83 (41.5%) |
| Polymicrobial infection | 117 (58.5%) | |
| Biofilm production | Strong | 50 (25%) |
| Moderate | 80 (40%) | |
| Weak | 50 (25%) | |
| None | 20 (10%) | |
| Antibiotics used in last 1 month | Yes | 81 (40.5%) |
| No | 119 (59.5%) | |
| Nature of ulcer | Necrotic | 74 (37%) |
| Non-necrotic | 126 (63%) | |
| Sample type | Wound swab | 128 (64%) |
| Tissue biopsy | 72 (36%) | |
| Pyrexia | Yes | 151 (75.5%) |
| No | 49 (24.5%) | |
| Hospital stays (in days) | 27.9 ± 6.2 |
| Characteristics | MDR n (%) | Non-MDR n (%) | p-Value | |
|---|---|---|---|---|
| Sex | Male | 35 (70%) | 85 (56.7%) | 0.095 |
| Female | 15 (30%) | 65 (43.3%) | ||
| Age (years) | <40 | 10 (20%) | 26 (17.3%) | 0.670 |
| >40 | 40 (80%) | 124 (82.7%) | ||
| Duration of diabetes (years) | <2 | 9 (18%) | 31 (20.7%) | 0.683 |
| >2 | 41 (82%) | 119 (79.3%) | ||
| Body mass index (kg/m2) | <16.7 | 6 (12%) | 13 (8.7%) | 0.488 |
| >16.7 | 44 (88%) | 137 (91.3%) | ||
| HbA1c (%) | <7 | 4 (8%) | 17 (11.3%) | 0.505 |
| >7 | 46 (92%) | 133 (88.7%) | ||
| Wound size (cm2) | <5 | 11 (22%) | 36 (24%) | 0.772 |
| >5 | 39 (78%) | 114 (76%) | ||
| Pyrexia | Yes | 44 (88%) | 107 (71.3%) | 0.017 ** |
| No | 6 (12%) | 43 (28.7%) | ||
| Minor amputation | Yes | 38 (76%) | 9 (6%) | <0.001 ** |
| No | 12 (24%) | 141 (94%) | ||
| Major amputation | Yes | 19 (38%) | 7 (4.7%) | <0.001 ** |
| No | 31 (62%) | 143 (95.3%) | ||
| Wagner ulcer grade | <II | 11 (22%) | 69 (46%) | 0.002 ** |
| >II | 39 (78%) | 81 (54%) | ||
| Duration of ulcers (months) | <1 | 13 (26%) | 52 (48.9%) | 0.257 |
| >1 | 37 (74%) | 98 (65.3%) | ||
| Sample type | Wound swab | 34 (68%) | 94 (62.7%) | 0.496 |
| Tissue biopsy | 16 (32%) | 56 (37.3%) | ||
| Type of infection | Polymicrobial | 41 (82%) | 76 (50.7%) | <0.001 ** |
| Monomicrobial | 9 (18%) | 74 (49.3%) | ||
| Nature of ulcer | Necrotic | 14 (28%) | 60 (40%) | 0.127 |
| Non-necrotic | 36 (72%) | 90 (60%) | ||
| Biofilm | Biofilm producer | 42 (84%) | 138 (92%) | 0.102 |
| Biofilm non-producer | 8 (16%) | 12 (8%) | ||
| Antibiotic used in last 1 month | Yes | 29 (58%) | 52 (34.7%) | 0.003 ** |
| No | 21 (42%) | 98 (65.3%) |
| Isolate No. | IMP (mm) | MRP (mm) | ETP (mm) | MHT | Carba NP Test | KPC | NDM | VIM | IMP | OXA-48 | Carbapenemase Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 20 | 18 | + | + | - | + | - | - | - | NDM |
| 2 | 21 | 18 | 14 | + | + | + | - | - | - | - | KPC |
| 3 | 23 | 22 | 16 | - | - | - | - | - | - | - | None |
| 4 | 24 | 19 | 18 | + | + | - | + | - | - | - | NDM |
| 5 | 19 | 17 | 15 | + | + | + | - | - | - | - | KPC |
| 6 | 22 | 21 | 20 | - | - | - | - | - | - | - | None |
| 7 | 16 | 14 | 11 | + | + | - | + | - | - | - | NDM |
| 8 | 20 | 18 | 17 | + | + | + | - | - | - | - | KPC |
| 9 | 18 | 16 | 15 | + | + | - | + | - | - | - | NDM |
| 10 | 23 | 21 | 19 | - | - | - | - | - | - | - | None |
| 11 | 17 | 14 | 13 | + | + | + | - | - | - | - | KPC |
| 12 | 19 | 17 | 15 | + | + | - | + | - | - | - | NDM |
| 13 | 24 | 22 | 18 | - | - | - | - | - | - | - | None |
| 14 | 22 | 19 | 17 | + | + | + | - | - | - | - | KPC |
| 15 | 19 | 17 | 15 | + | + | - | + | - | - | - | NDM |
| 16 | 23 | 20 | 19 | - | - | - | - | - | - | - | None |
| 17 | 21 | 18 | 16 | + | + | + | - | - | - | - | KPC |
| 18 | 16 | 15 | 14 | + | + | - | + | - | - | - | NDM |
| 19 | 19 | 18 | 17 | + | + | + | - | - | - | - | KPC |
| 20 | 24 | 22 | 19 | - | - | - | - | - | - | - | None |
| 21 | 18 | 17 | 16 | + | + | - | + | - | - | - | NDM |
| 22 | 22 | 21 | 20 | + | + | + | - | - | - | - | KPC |
| 23 | 20 | 18 | 17 | + | + | - | + | - | - | - | NDM |
| 24 | 23 | 21 | 20 | - | - | - | - | - | - | - | None |
| 25 | 21 | 19 | 18 | + | + | + | - | - | - | - | KPC |
| 26 | 17 | 16 | 15 | + | + | - | + | - | - | - | NDM |
| 27 | 18 | 17 | 16 | + | + | + | - | - | - | - | KPC |
| 28 | 16 | 15 | 14 | + | + | - | + | - | - | - | NDM |
| 29 | 24 | 22 | 19 | - | - | - | - | - | - | - | None |
| Isolate No. | IMP (mm) | MRP (mm) | ETP (mm) | MHT | Carba NP Test | KPC | NDM | VIM | IMP | OXA-48 | Carbapenemase Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 20 | 18 | N/A | + | + | - | + | - | - | - | NDM |
| 2 | 16 | 14 | N/A | + | + | - | - | + | - | - | VIM |
| 3 | 22 | 19 | N/A | - | - | - | - | - | - | - | None |
| 4 | 18 | 17 | N/A | + | + | - | - | - | - | - | IMP |
| 5 | 15 | 12 | N/A | + | + | + | - | - | - | - | KPC |
| 6 | 24 | 20 | N/A | - | - | - | - | - | - | - | None |
| 7 | 17 | 15 | N/A | + | + | - | + | - | - | - | NDM |
| 8 | 19 | 16 | N/A | + | + | - | - | + | - | - | VIM |
| 9 | 14 | 12 | N/A | + | + | + | - | - | - | - | KPC |
| 10 | 12 | 20 | N/A | - | - | - | - | - | - | - | None |
| 11 | 18 | 15 | N/A | + | + | - | + | - | - | - | NDM |
| 12 | 16 | 14 | N/A | + | + | - | - | + | - | - | VIM |
| 13 | 21 | 18 | N/A | - | - | - | - | - | - | - | None |
| 14 | 14 | 22 | N/A | + | + | + | - | - | - | - | KPC |
| 15 | 20 | 16 | N/A | + | + | - | - | + | - | - | VIM |
| 16 | 15 | 12 | N/A | + | + | - | + | - | - | - | NDM |
| 17 | 24 | 21 | N/A | - | - | - | - | - | - | - | None |
| 18 | 19 | 17 | N/A | + | + | - | - | + | - | - | VIM |
| 19 | 14 | 13 | N/A | + | + | + | - | - | - | - | KPC |
| 20 | 17 | 14 | N/A | + | + | - | + | - | - | - | NDM |
| 21 | 15 | 12 | N/A | + | + | - | - | + | - | - | VIM |
| 22 | 18 | 16 | N/A | - | - | - | - | - | - | - | None |
| 23 | 16 | 14 | N/A | + | + | - | + | - | - | - | NDM |
| 24 | 20 | 17 | N/A | + | + | + | - | - | - | - | KPC |
| 25 | 14 | 12 | N/A | + | + | - | - | + | - | - | VIM |
| 26 | 22 | 19 | N/A | - | - | - | - | - | - | - | None |
| 27 | 17 | 15 | N/A | + | + | + | - | - | - | - | KPC |
| 28 | 18 | 16 | N/A | + | + | - | + | - | - | - | NDM |
| 29 | 21 | 18 | N/A | - | - | - | - | - | - | - | None |
| 30 | 19 | 17 | N/A | + | + | + | - | - | - | - | KPC |
| 31 | 14 | 13 | N/A | + | + | - | - | + | - | - | VIM |
| Isolate No. | IMP (mm) | MRP (mm) | ETP (mm) | MHT | Carba NP Test | KPC | NDM | VIM | IMP | OXA-48 | Carbapenemase Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 18 | 15 | 14 | + | + | + | - | - | - | - | KPC |
| 2 | 16 | 14 | 13 | + | + | - | + | - | - | - | NDM |
| 3 | 14 | 12 | 10 | + | + | + | - | - | - | - | None |
| 4 | 14 | 12 | 10 | + | + | + | - | - | - | - | KPC |
| 5 | 19 | 16 | 15 | + | + | - | + | - | - | - | NDM |
| 6 | 22 | 20 | 19 | - | - | - | - | - | - | - | None |
| 7 | 15 | 12 | 11 | + | + | + | - | - | - | - | KPC |
| 8 | 20 | 18 | 17 | + | + | - | - | + | - | - | VIM |
| 9 | 13 | 11 | 10 | + | + | + | - | - | - | - | KPC |
| 10 | 22 | 19 | 18 | - | - | - | - | - | - | - | None |
| 11 | 17 | 14 | 12 | + | + | + | - | - | - | - | KPC |
| 12 | 16 | 13 | 11 | + | + | - | + | - | - | - | NDM |
| 13 | 21 | 19 | 17 | - | - | - | - | - | - | - | None |
| 14 | 15 | 12 | 10 | + | + | + | - | - | - | - | KPC |
| 15 | 18 | 16 | 14 | + | + | - | + | - | - | - | NDM |
| 16 | 22 | 20 | 19 | - | - | - | - | - | - | - | None |
| 17 | 14 | 12 | 10 | + | + | + | - | - | - | - | KPC |
| 18 | 19 | 17 | 15 | + | + | - | - | + | - | - | VIM |
| 19 | 13 | 11 | 9 | + | + | + | - | - | - | - | KPC |
| 20 | 21 | 19 | 17 | - | - | - | - | - | - | - | None |
| 21 | 17 | 14 | 13 | + | + | + | - | - | - | - | KPC |
| 22 | 18 | 16 | 14 | + | + | - | + | - | - | - | NDM |
| 23 | 21 | 18 | 17 | - | - | - | - | - | - | - | None |
| 24 | 13 | 11 | 9 | + | + | + | - | - | - | - | KPC |
| 25 | 15 | 13 | 11 | + | + | - | + | - | - | - | NDM |
| 26 | 19 | 17 | 15 | + | + | + | - | - | - | - | KPC |
| 27 | 22 | 20 | 18 | - | - | - | - | - | - | - | None |
| 28 | 16 | 14 | 12 | + | + | + | - | - | - | - | KPC |
| 29 | 18 | 16 | 14 | + | + | - | + | - | - | - | NDM |
| 30 | 22 | 19 | 17 | - | - | - | - | - | - | - | None |
| 31 | 15 | 12 | 10 | + | + | + | - | - | - | - | KPC |
| 32 | 17 | 14 | 13 | + | + | - | + | - | - | - | NDM |
| 33 | 21 | 19 | 17 | - | - | - | - | - | - | - | None |
| Isolate No. | IMP (mm) | MRP (mm) | ETP (mm) | MHT | Carba NP Test | KPC | NDM | VIM | IMP | OXA-48 | Carbapenemase Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 15 | 12 | 10 | + | + | - | - | - | + | - | IMP |
| 2 | 14 | 13 | 11 | + | + | - | + | - | - | - | NDM |
| 3 | 17 | 15 | 12 | + | + | + | - | - | - | - | KPC |
| 4 | 13 | 11 | 9 | + | + | - | - | - | + | - | IMP |
| 5 | 20 | 18 | 17 | - | - | - | - | - | - | - | None |
| 6 | 14 | 12 | 10 | + | + | - | - | - | + | - | IMP |
| 7 | 16 | 14 | 11 | + | + | - | + | - | - | - | NDM |
| 8 | 18 | 16 | 15 | + | + | + | - | - | - | - | KPC |
| 9 | 12 | 10 | 8 | + | + | - | - | - | + | - | IMP |
| 10 | 22 | 19 | 18 | - | - | - | - | - | - | - | None |
| 11 | 13 | 11 | 9 | + | + | - | + | - | - | - | NDM |
| 12 | 12 | 13 | 10 | + | + | - | - | - | + | - | IMP |
| 13 | 18 | 16 | 14 | + | + | + | - | - | - | - | KPC |
| 14 | 12 | 10 | 8 | + | + | - | - | - | + | - | IMP |
| 15 | 20 | 18 | 17 | - | - | - | - | - | - | - | None |
| 16 | 16 | 12 | 10 | + | + | - | + | - | - | - | NDM |
| 17 | 19 | 17 | 16 | - | - | - | - | - | - | - | None |
| 18 | 16 | 13 | 12 | + | + | + | - | - | - | - | KPC |
| 19 | 11 | 9 | 7 | + | + | - | - | - | + | - | IMP |
| 20 | 22 | 19 | 18 | - | - | - | - | - | - | - | None |
| Isolate No. | IMP (mm) | MRP (mm) | ETP (mm) | MHT | Carba NP Test | KPC | NDM | VIM | IMP | OXA-48 | Carbapenemase Type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 16 | 14 | 13 | + | + | + | - | - | - | - | KPC |
| 2 | 15 | 13 | 11 | + | + | - | + | - | - | - | NDM |
| 3 | 19 | 17 | 16 | + | + | - | - | + | - | - | VIM |
| 4 | 12 | 10 | 8 | + | + | - | - | - | + | - | IMP |
| 5 | 21 | 19 | 17 | - | - | - | - | - | - | - | None |
| 6 | 15 | 13 | 11 | + | + | + | - | - | - | - | KPC |
| 7 | 18 | 16 | 14 | + | + | - | + | - | - | - | NDM |
| 8 | 20 | 18 | 17 | - | - | - | - | - | - | - | None |
| 9 | 13 | 11 | 10 | + | + | - | - | - | + | - | IMP |
| 10 | 14 | 12 | 11 | + | + | - | + | - | - | - | NDM |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, M.; Moursi, S.A.; Altamimi, T.N.A.; Alharbi, M.S.; Alaskar, A.M.; Hammam, S.A.H.; Rakha, E.; Syed Muhammad, O.I.; Almalaq, H.A.; Alshammari, M.N.; et al. Prevalence and Molecular Characterization of Carbapenemase-Producing Multidrug-Resistant Bacteria in Diabetic Foot Ulcer Infections. Diagnostics 2025, 15, 141. https://doi.org/10.3390/diagnostics15020141
Saleem M, Moursi SA, Altamimi TNA, Alharbi MS, Alaskar AM, Hammam SAH, Rakha E, Syed Muhammad OI, Almalaq HA, Alshammari MN, et al. Prevalence and Molecular Characterization of Carbapenemase-Producing Multidrug-Resistant Bacteria in Diabetic Foot Ulcer Infections. Diagnostics. 2025; 15(2):141. https://doi.org/10.3390/diagnostics15020141
Chicago/Turabian StyleSaleem, Mohd, Soha Abdallah Moursi, Tahani Nasser Almofeed Altamimi, Mohammed Salem Alharbi, Alwaleed Mohammad Alaskar, Sahar Adly Hassan Hammam, Ehab Rakha, Ozair Ilyas Syed Muhammad, Hamoud Abdulmohsin Almalaq, Metab Nasser Alshammari, and et al. 2025. "Prevalence and Molecular Characterization of Carbapenemase-Producing Multidrug-Resistant Bacteria in Diabetic Foot Ulcer Infections" Diagnostics 15, no. 2: 141. https://doi.org/10.3390/diagnostics15020141
APA StyleSaleem, M., Moursi, S. A., Altamimi, T. N. A., Alharbi, M. S., Alaskar, A. M., Hammam, S. A. H., Rakha, E., Syed Muhammad, O. I., Almalaq, H. A., Alshammari, M. N., & Syed Khaja, A. S. (2025). Prevalence and Molecular Characterization of Carbapenemase-Producing Multidrug-Resistant Bacteria in Diabetic Foot Ulcer Infections. Diagnostics, 15(2), 141. https://doi.org/10.3390/diagnostics15020141






