Impact of Nasal Anatomical Variation Subtype on Surgical Outcomes for Rhinogenic Contact Point Headache
Abstract
1. Introduction
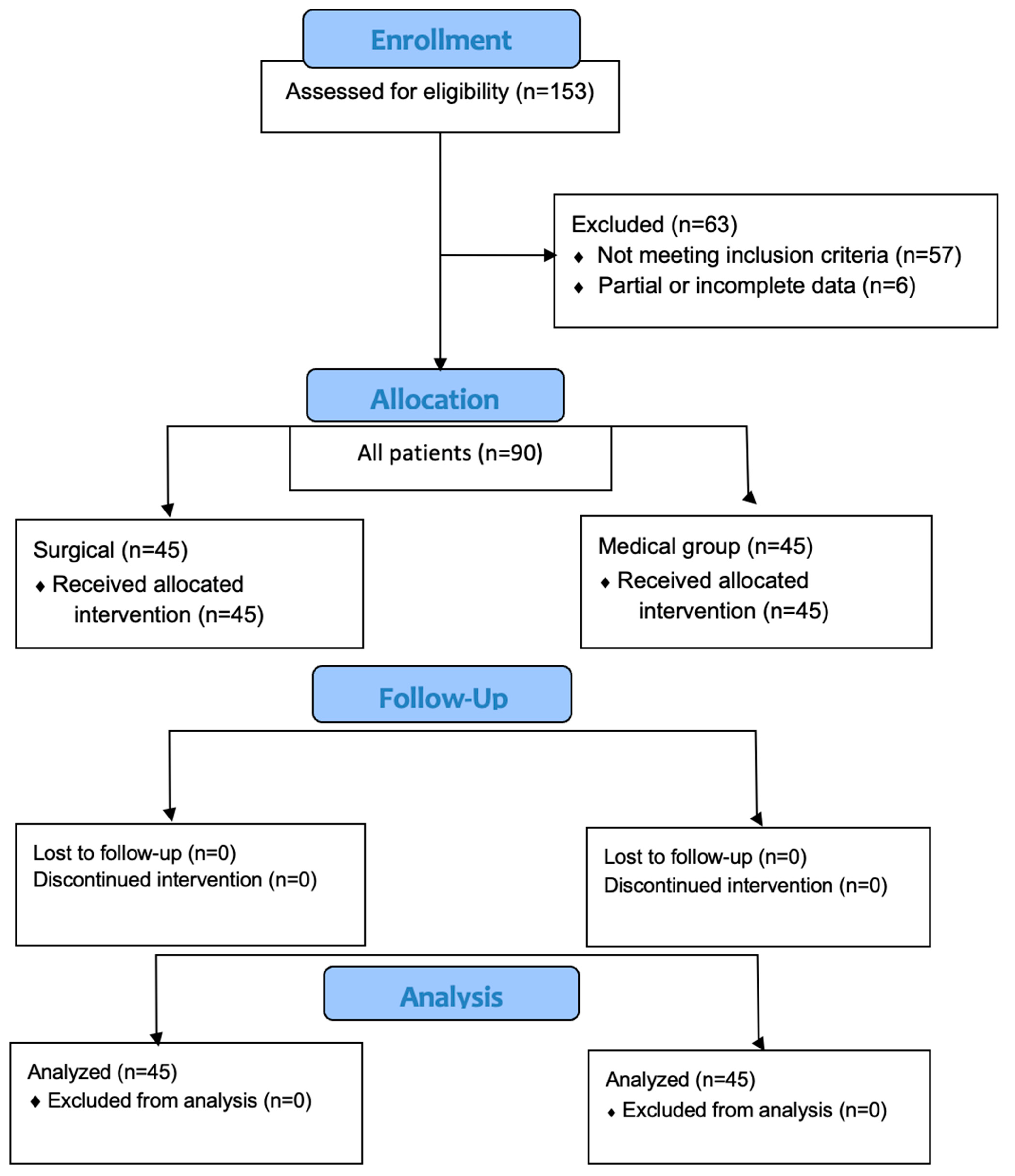
2. Materials and Methods
2.1. Study Design and Ethics
- Initial evaluation included CT scanning, nasal endoscopy, and lidocaine testing.
- Patients meeting inclusion criteria were counseled about both surgical and medical treatment options.
- Treatment allocation was based on the following:
- -
- Severity of anatomical variation as determined by CT imaging;
- -
- Patient preference after detailed discussion of risks and benefits;
- -
- Presence of significant functional nasal obstruction;
- -
- Prior response to medical therapy.
- To ensure balanced representation, subjects were stratified by anatomical variation type (concha bullosa, septal deviation, septal spur) with equal distribution (15 patients per variation) in both surgical and medical groups.
- Regular monitoring was established for both groups with identical follow-up schedules [2].
- Reported chronic headache or facial pain unresponsive to analgesics.
- Had a confirmed diagnosis of septal deviation, septal spur, or concha bullosa.
- Were aged 20 years or above.
- Exhibited a positive response to a lidocaine test, where pain relief was achieved upon lidocaine application to the nasal cavity.
- Were undergoing medical or surgical treatment for RCPH.
- Less than one year of consecutive clinical and diagnostic follow-up.
- Presence of comorbid conditions including allergies, other sinonasal disorders, migraines, cluster headaches, ophthalmologic or vascular disorders, hypertension, pregnancy, or temporomandibular joint disorders.
- A history of previous sinonasal surgeries.
2.2. Diagnostic Workup
2.3. Treatment and Interventions
- -
- Group A (surgical intervention): patients underwent endoscopic surgical procedures by the same surgeons (A.M., M.L.), focusing on the removal of mucosal contact points, lateral resection of concha bullosa, and septoplasty or submucous resection for septal deviations and spurs.
- -
- Group B (medical comparison): Patients received non-surgical treatment consisting of a standardized treatment consisting of intranasal fluticasone propionate, 125 mg per dose, with two sprays in each nostril every morning for 15-day cycles each month, without addressing contact points surgically. The patients were given detailed written instructions on how to take the medication properly, thus guaranteeing uniformity of delivery.
2.4. Surgical Protocol and Postoperative Care
2.5. Statistical Analysis
3. Results
3.1. Demographic Features
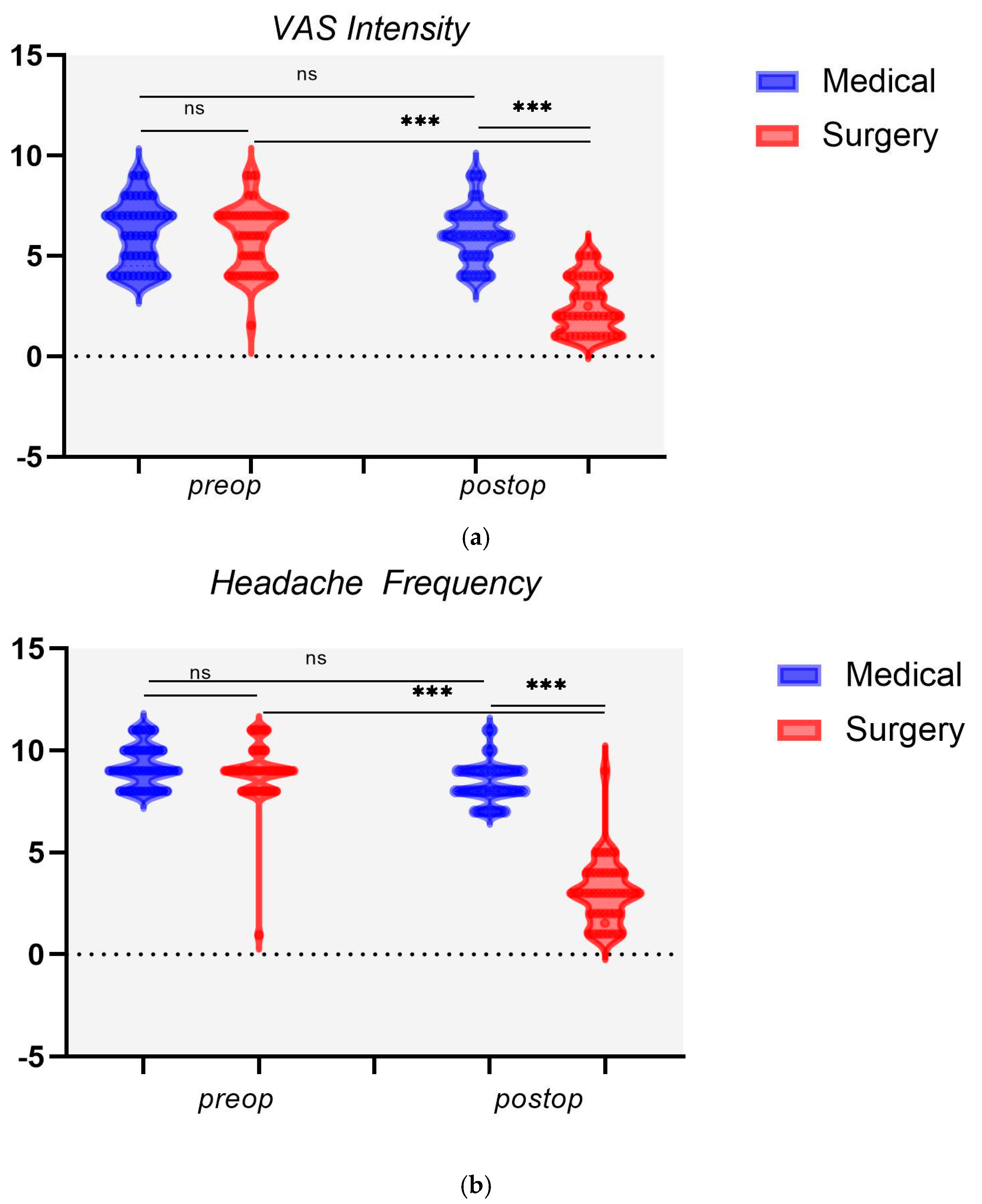
3.2. Surgical vs. Medical Outcomes
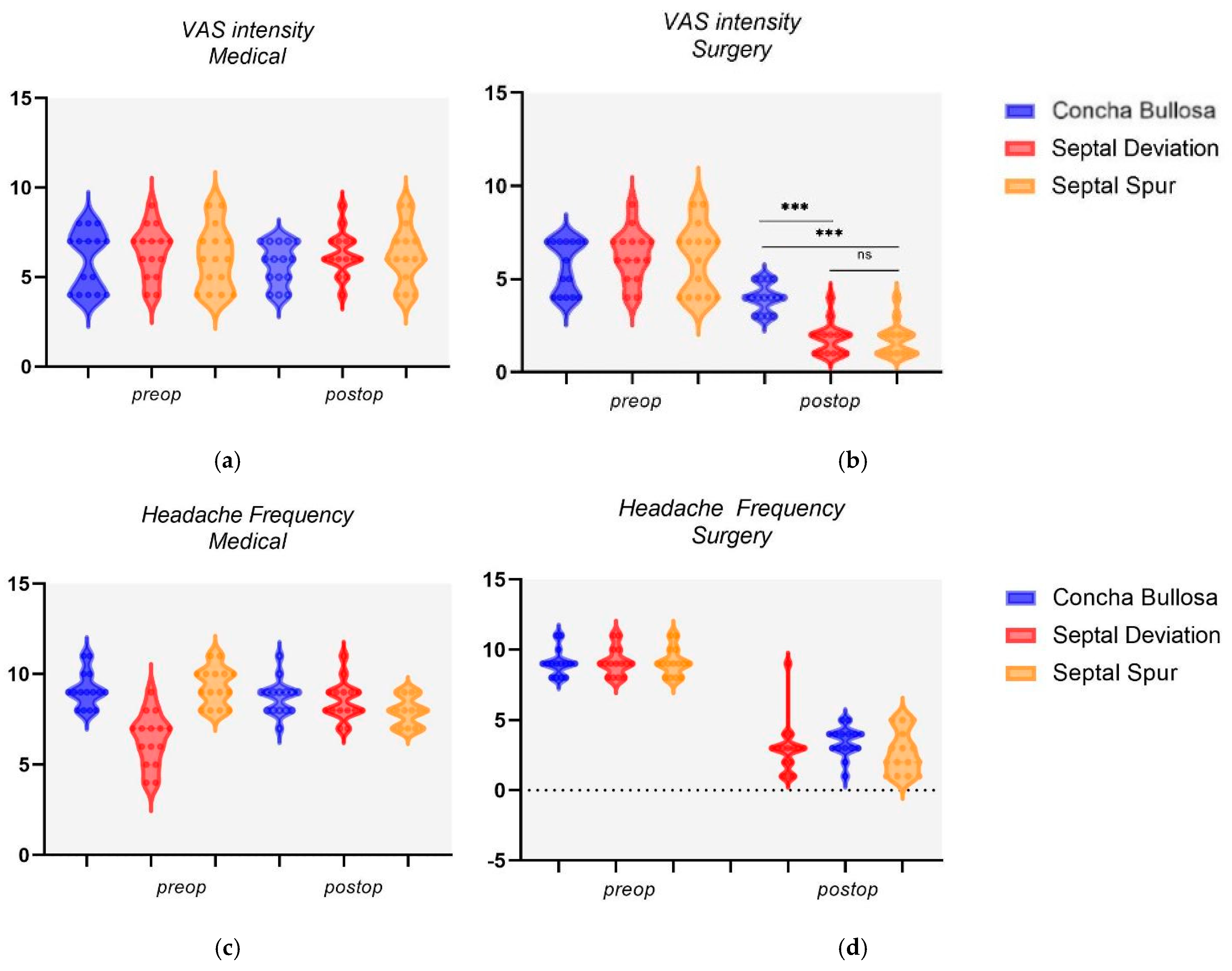
3.3. Nasal Variation Outcomes
3.4. Predictors of Treatment Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicholas, M.; Vlaeyen, J.W.S.; Rief, W.; Barke, A.; Aziz, Q.; Benoliel, R.; Cohen, M.; Evers, S.; Giamberardino, M.A.; Goebel, A.; et al. The IASP classification of chronic pain for ICD-11: Chronic primary pain. Pain 2019, 160, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Benoliel, R.; Svensson, P.; Evers, S.; Wang, S.J.; Barke, A.; Korwisi, B.; Rief, W.; Treede, R.D.; The IASP Taskforce for the Classification of Chronic Pain. The IASP classification of chronic pain for ICD-11: Chronic secondary headache or orofacial pain. Pain 2019, 160, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Subcommittee of the International Headache Society. The international classification of headache disorders: 2nd edition. Cephalalgia 2004, 24 (Suppl. S1), 9–160. [Google Scholar] [CrossRef]
- Herzallah, I.R.; Hamed, M.A.; Salem, S.M.; Suurna, M.V. Mucosal contact points and paranasal sinus pneumatization: Does radiology predict headache causality? Laryngoscope 2015, 125, 2021–2026. [Google Scholar] [CrossRef]
- Sollini, G.; Mazzola, F.; Iandelli, A.; Carobbio, A.; Barbieri, A.; Mora, R.; Peretti, G. Sino-nasal anatomical variations in rhinogenic headache pathogenesis. J. Craniofac. Surg. 2019, 30, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Pasha, R.; Soleja, R.Q.; Ijaz, M.N. Imaging for headache: What the otolaryngologist looks for. Otolaryngol. Clin. N. Am. 2014, 47, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mariotti, L.J.; Setliff, R.C., III; Ghaderi, M.; Voth, S. Patient history and CT findings in predicting surgical outcomes for patients with rhinogenic headache. Ear Nose Throat J. 2009, 88, 926–929. [Google Scholar] [CrossRef] [PubMed]
- Barinsky, G.L.; Hanba, C.; Svider, P.F. Rhinogenic Headache in Children and Adolescents. Curr. Pain Headache Rep. 2020, 24, 7. [Google Scholar] [CrossRef] [PubMed]
- Bektas, D.; Alioglu, Z.; Akyol, N.; Ural, A.; Abu-Samra, M.; Gawad, O.A.; Agha, M. The outcomes for nasal contact point surgeries in patients with unsatisfactory response to chronic daily headache medications. Eur. Arch. Otorhinolaryngol. 2011, 268, 1299–1304. [Google Scholar] [CrossRef]
- Maniaci, A.; Lechien, J.R.; Calvo-Henriquez, C.; Iannella, G.; Leigh, S.; Ingrassia, A.; Merlino, F.; Bannò, V.; Cocuzza, S.; La Mantia, I. Long-term stability of outcomes of endoscopic surgery for rhinogenic contact point headache (Sluder’s neuralgia). Am. J. Otolaryngol. 2022, 43, 103368. [Google Scholar] [CrossRef]
- Maniaci, A.; Merlino, F.; Cocuzza, S.; Iannella, G.; Vicini, C.; Cammaroto, G.; Lechien, J.R.; Calvo-Henriquez, C.; La Mantia, I. Endoscopic surgical treatment for rhinogenic contact point headache: Systematic review and meta-analysis. Eur. Arch. Otorhinolaryngol. 2021, 278, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Cantone, E.; Castagna, G.; Ferranti, I.; Cimmino, M.; Sicignano, S.; Rega, F.; Di Rubbo, V.; Iengo, M. Concha bullosa related headache disability. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 2327–2330. [Google Scholar]
- Guyuron, B.; Kriegler, J.S.; Davis, J.; Amini, S.B. Five-year outcome of surgical treatment of migraine headaches. Plast. Reconstr. Surg. 2011, 127, 603–608. [Google Scholar] [CrossRef] [PubMed]
- West, B.; Jones, N.S. Endoscopy-negative, computed tomography-negative facial pain in a nasal clinic. Laryngoscope 2001, 111, 581–586. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Mohebbi, A.; Memari, F.; Mohebbi, S. Endonasal endoscopic management of contact point headache and diagnostic criteria. Headache 2010, 50, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Wee, J.H.; Lee, J.E.; Hong, S.L.; Shin, J.M.; Kim, D.Y. Prospective study on the characteristics and postoperative improvement of rhinogenic headache. J. Rhinol. 2015, 22, 6–10. [Google Scholar] [CrossRef][Green Version]
- Bahadir, O.; Caylan, R. Surgical outcomes for rhinogenic contact point headaches. Med. Princ. Pract. 2011, 20, 29–33. [Google Scholar] [CrossRef]
- Behin, F.; Behin, B.; Bigal, M.E.; Lipton, R.B. Surgical treatment of patients with refractory migraine headaches and intranasal contact points. Cephalalgia 2005, 25, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Peric, A.; Rasic, D.; Grgurevic, U. Surgical Treatment of Rhinogenic Contact Point Headache: An Experience from a Tertiary Care Hospital. Int. Arch. Otorhinolaryngol. 2016, 20, 166–171. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cocuzza, S.; Maniaci, A.; Di Luca, M.; La Mantia, I.; Grillo, C.; Spinato, G.; Motta, G.; Testa, D.; Ferlito, S. Long-term results of nasal surgery: Comparison of mini-invasive turbinoplasty. J. Biol. Regul. Homeost. Agents 2020, 34, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Altin, F.; Haci, C.; Alimoglu, Y.; Yilmaz, S. Is septoplasty effective rhinogenic headache in patients with isolated contact point between inferior turbinate and septal spur? Am. J. Otolaryngol. 2019, 40, 364–367. [Google Scholar] [CrossRef]
- Welge-Luessen, A.; Hauser, R.; Schmid, N.; Kappos, L.; Probst, R. Endonasal surgery for contact point headaches: A 10-year longitudinal study. Laryngoscope 2003, 113, 2151–2156. [Google Scholar] [CrossRef] [PubMed]
| Preoperative | Postoperative | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| VAS Intensity | Head Frequency | MIDAS Grade% | VAS Intensity | Head Frequency | MIDAS Grade % | ||||
| Surgery (n = 45) | mean | 6.02 a | 9.11 a | I–II | 24.44 | 2.51 a,b | 3.04 a,b | I–II | 53.33 |
| sd | 1.54 | 0.96 | III–IV | 75.55 | 1.34 | 1.54 | III–IV | 46.67 | |
| Medical (n = 45) | mean | 5.96 | 9.22 | I–II | 20 | 6.22 | 8.36 | I–II | 15.55 |
| sd | 1.52 | 0.99 | III–IV | 80 | 1.33 | 0.96 | III–IV | 84.44 | |
| VAS Intensity | ||||||
|---|---|---|---|---|---|---|
| Concha Bullosa | Septal Deviation | p-Value | Concha Bullosa | Septal Spur | p-Value | |
| Surgery | 4 ± 0.73 | 1.8 ± 0.83 | p < 0.001 | 4 ± 0.73 | 1.73 ± 0.85 | p < 0.001 |
| Medical | 5.73 ± 1.12 | 6.33 ± 1.19 | 0.166 | 5.73 ± 1.12 | 6.26 ± 1.56 | 0.294 |
| Headache Frequency | ||||||
| Concha Bullosa | Septal Deviation | p-Value | Concha Bullosa | Septal Spur | p-Value | |
| Surgery | 3 ± 1.86 | 3.46 ± 1.02 | 0.408 | 3 ± 1.86 | 2.66 ± 1.44 | 0.580 |
| Medical | 8.73 ± 0.92 | 8.53 ± 1.02 | 0.577 | 8.73 ± 0.92 | 7.93 ± 0.77 | 0.015 |
| VAS Intensity | Headache Frequency | |||
|---|---|---|---|---|
| Variable | F | Sig. | F | Sig. |
| Nasal anomaly subtype | 30.909 | <0.001 | 1.330 | 0.255 |
| Age <>35 y | 0.036 | 0.850 | 0.043 | 0.914 |
| Sex M/F | 1.271 | 0.266 | 2.333 | 0.134 |
| VAS intensity preop <>4 | 1.378 | 0.247 | 0.940 | 0.338 |
| Headache frequency preop <>9 | 4.756 | 0.035 | 0.155 | 0.696 |
| MIDAS low–high grade | 1.378 | 0.247 | 1.487 | 0.229 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavalle, S.; Pace, A.; Magliulo, G.; Lentini, M.; Lechien, J.R.; Calvo-Henriquez, C.; Parisi, F.M.; Iannella, G.; Maniaci, A.; Messineo, D. Impact of Nasal Anatomical Variation Subtype on Surgical Outcomes for Rhinogenic Contact Point Headache. Diagnostics 2025, 15, 121. https://doi.org/10.3390/diagnostics15020121
Lavalle S, Pace A, Magliulo G, Lentini M, Lechien JR, Calvo-Henriquez C, Parisi FM, Iannella G, Maniaci A, Messineo D. Impact of Nasal Anatomical Variation Subtype on Surgical Outcomes for Rhinogenic Contact Point Headache. Diagnostics. 2025; 15(2):121. https://doi.org/10.3390/diagnostics15020121
Chicago/Turabian StyleLavalle, Salvatore, Annalisa Pace, Giuseppe Magliulo, Mario Lentini, Jerome Rene Lechien, Christian Calvo-Henriquez, Federica Maria Parisi, Giannicola Iannella, Antonino Maniaci, and Daniela Messineo. 2025. "Impact of Nasal Anatomical Variation Subtype on Surgical Outcomes for Rhinogenic Contact Point Headache" Diagnostics 15, no. 2: 121. https://doi.org/10.3390/diagnostics15020121
APA StyleLavalle, S., Pace, A., Magliulo, G., Lentini, M., Lechien, J. R., Calvo-Henriquez, C., Parisi, F. M., Iannella, G., Maniaci, A., & Messineo, D. (2025). Impact of Nasal Anatomical Variation Subtype on Surgical Outcomes for Rhinogenic Contact Point Headache. Diagnostics, 15(2), 121. https://doi.org/10.3390/diagnostics15020121










