Investigation of CD47 Expression in Renal Cell Tumors and Evaluation of Its Relationship with Prognostic Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Immunohistochemical Staining
2.3. Data Analysis
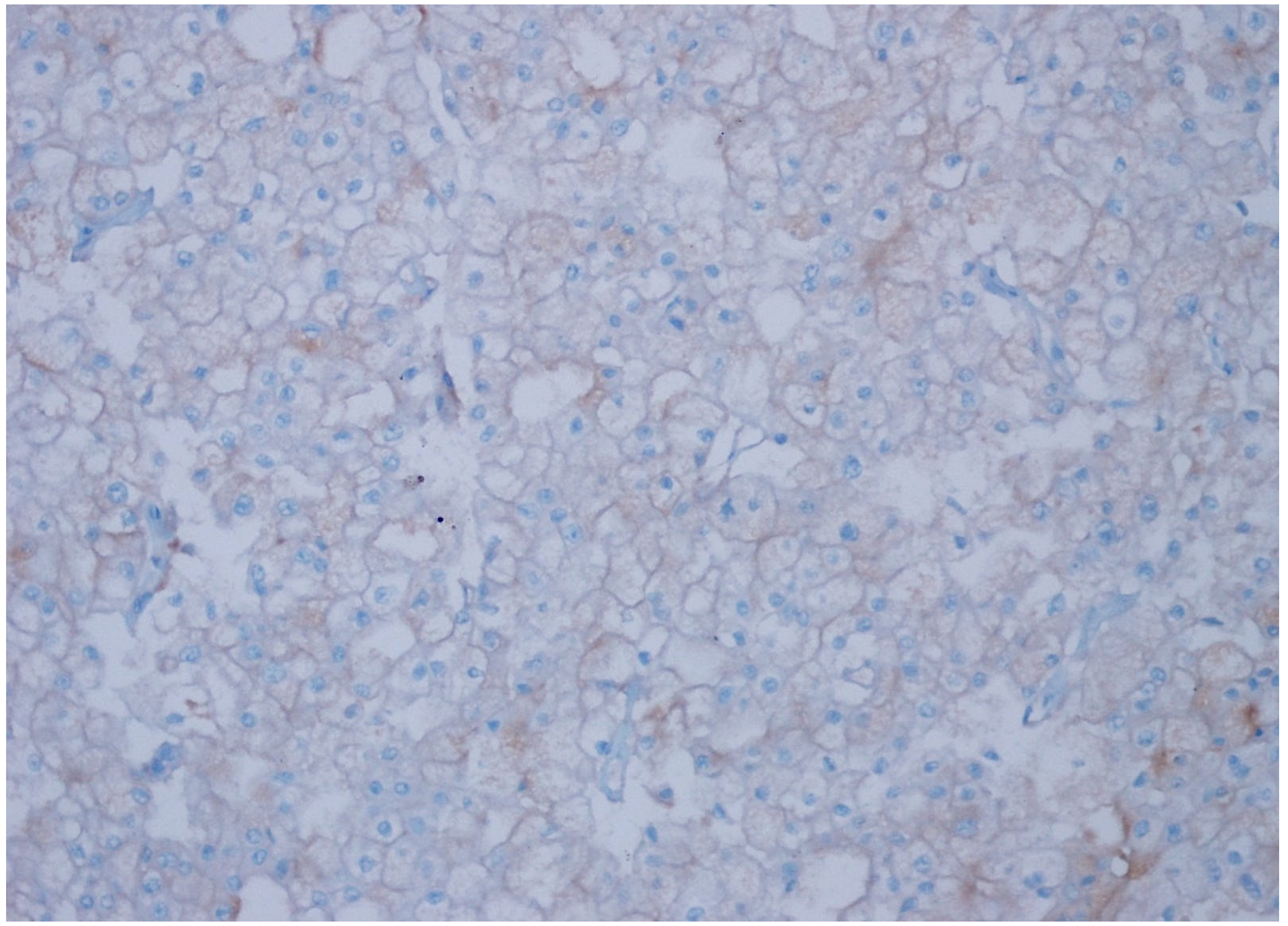
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsieh, J.J.; Purdue, M.P.; Signoretti, S.; Swanton, C.; Albiges, L.; Schmidinger, M.; Heng, D.Y.; Larkin, J.; Ficarra, V. Renal cell carcinoma. Nat. Rev. Dis. Primers 2017, 3, 17009. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cohen, H.T.; McGovern, F.J. Renal-Cell Carcinoma. N. Engl. J. Med. 2005, 353, 2477–2490. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Ward, E.; Murray, T.; Xu, J.; Thun, M.J. Cancer Statistics, 2007. CA Cancer J. Clin. 2007, 57, 43–66. [Google Scholar] [CrossRef] [PubMed]
- Overall Evaluations of Carcinogenicity: An Updating of IARC Monographs Volumes 1 to 42. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Supplement; World Health Organization: Geneva, Switzerland, 1987; Volume 7, pp. 1–440.
- Blettner, M. Hormonal Contraception and Post-Menopausal Hormonal Therapy. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; Supplement; World Health Organization: Geneva, Switzerland, 1999; Volume 72, ISBN 978-92-832-1272-0. [Google Scholar]
- Chow, W.-H.; Gridley, G.; Fraumeni, J.F.; Järvholm, B. Obesity, Hypertension, and the Risk of Kidney Cancer in Men. N. Engl. J. Med. 2000, 343, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, D.F.R.; Verghese, A.; Golash, A.; Kynaston, H.G.; Matthews, P.N.; Hart, A.J.L.; Court, J.B. Contribution of Grade, Vascular Invasion and Age to Outcome in Clinically Localized Renal Cell Carcinoma. BJU Int. 2002, 90, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Janzen, N.K.; Kim, H.L.; Figlin, R.A.; Belldegrun, A.S. Surveillance After Radical or Partial Nephrectomy for Localized Renal Cell Carcinoma and Management of Recurrent Disease. Urol. Clin. N. Am. 2003, 30, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.S.; Leppert, J.T.; Figlin, R.A.; Belldegrun, A.S. Surveillance Following Radical or Partial Nephrectomy for Renal Cell Carcinoma. Curr. Urol. Rep. 2005, 6, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Lane, B.R.; Kattan, M.W. Predicting Outcomes in Renal Cell Carcinoma. Curr. Opin. Urol. 2005, 15, 289–297. [Google Scholar] [CrossRef]
- Leach, R.E.; Miller, J.K. Lateral and Medial Epicondylitis of the Elbow. Clin. Sports Med. 1987, 6, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Isenberg, J.S.; Frazier, W.A.; Roberts, D.D. Thrombospondins: From Structure to Therapeutics. Cell. Mol. Life Sci. 2008, 65, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.-G.; Lindberg, F.P.; Finn, M.B.; Blystone, S.D.; Brown, E.J.; Frazier, W.A. Integrin-Associated Protein Is a Receptor for the C-Terminal Domain of Thrombospondin. J. Biol. Chem. 1996, 271, 21–24. [Google Scholar] [CrossRef]
- Lindberg, F.P.; Bullard, D.C.; Caver, T.E.; Gresham, H.D.; Beaudet, A.L.; Brown, E.J. Decreased Resistance to Bacterial Infection and Granulocyte Defects in IAP-Deficient Mice. Science 1996, 274, 795–798. [Google Scholar] [CrossRef]
- Brown, E. Integrin-Associated Protein (CD47) and Its Ligands. Trends Cell Biol. 2001, 11, 130–135. [Google Scholar] [CrossRef]
- Liu, Y.; Merlin, D.; Burst, S.L.; Pochet, M.; Madara, J.L.; Parkos, C.A. The Role of CD47 in Neutrophil Transmigration. J. Biol. Chem. 2001, 276, 40156–40166. [Google Scholar] [CrossRef]
- Miyashita, M.; Ohnishi, H.; Okazawa, H.; Tomonaga, H.; Hayashi, A.; Fujimoto, T.-T.; Furuya, N.; Matozaki, T. Promotion of Neurite and Filopodium Formation by CD47: Roles of Integrins, Rac, and Cdc42. Mol. Biol. Cell 2004, 15, 3950–3963. [Google Scholar] [CrossRef] [PubMed]
- Oldenborg, P.-A.; Zheleznyak, A.; Fang, Y.-F.; Lagenaur, C.F.; Gresham, H.D.; Lindberg, F.P. Role of CD47 as a Marker of Self on Red Blood Cells. Science 2000, 288, 2051–2054. [Google Scholar] [CrossRef]
- Blazar, B.R.; Lindberg, F.P.; Ingulli, E.; Panoskaltsis-Mortari, A.; Oldenborg, P.-A.; Iizuka, K.; Yokoyama, W.M.; Taylor, P.A. Cd47 (Integrin-Associated Protein) Engagement of Dendritic Cell and Macrophage Counterreceptors Is Required to Prevent the Clearance of Donor Lymphohematopoietic Cells. J. Exp. Med. 2001, 194, 541–550. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.-A.; Michalak, M.; Henson, P.M. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through Trans-Activation of LRP on the Phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Chao, M.P.; Weissman, I.L.; Majeti, R. The CD47–SIRPα Pathway in Cancer Immune Evasion and Potential Therapeutic Implications. Curr. Opin. Immunol. 2012, 24, 225–232. [Google Scholar] [CrossRef]
- Edris, B.; Weiskopf, K.; Volkmer, A.K.; Volkmer, J.-P.; Willingham, S.B.; Contreras-Trujillo, H.; Liu, J.; Majeti, R.; West, R.B.; Fletcher, J.A.; et al. Antibody Therapy Targeting the CD47 Protein Is Effective in a Model of Aggressive Metastatic Leiomyosarcoma. Proc. Natl. Acad. Sci. USA 2012, 109, 6656–6661. [Google Scholar] [CrossRef]
- Willingham, S.B.; Volkmer, J.-P.; Gentles, A.J.; Sahoo, D.; Dalerba, P.; Mitra, S.S.; Wang, J.; Contreras-Trujillo, H.; Martin, R.; Cohen, J.D.; et al. The CD47-Signal Regulatory Protein Alpha (SIRPa) Interaction Is a Therapeutic Target for Human Solid Tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 6662–6667. [Google Scholar] [CrossRef]
- Barclay, A.N.; van den Berg, T.K. The Interaction Between Signal Regulatory Protein Alpha (SIRPα) and CD47: Structure, Function, and Therapeutic Target. Annu. Rev. Immunol. 2014, 32, 25–50. [Google Scholar] [CrossRef]
- Rendtlew Danielsen, J.M.; Knudsen, L.M.; Dahl, I.M.; Lodahl, M.; Rasmussen, T. Dysregulation of CD47 and the Ligands Thrombospondin 1 and 2 in Multiple Myeloma. Br. J. Haematol. 2007, 138, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Jamieson, C.H.M.; Pang, W.W.; Park, C.Y.; Chao, M.P.; Majeti, R.; Traver, D.; van Rooijen, N.; Weissman, I.L. CD47 Is Upregulated on Circulating Hematopoietic Stem Cells and Leukemia Cells to Avoid Phagocytosis. Cell 2009, 138, 271–285. [Google Scholar] [CrossRef]
- Majeti, R.; Chao, M.P.; Alizadeh, A.A.; Pang, W.W.; Jaiswal, S.; Gibbs, K.D.; van Rooijen, N.; Weissman, I.L. CD47 Is an Adverse Prognostic Factor and Therapeutic Antibody Target on Human Acute Myeloid Leukemia Stem Cells. Cell 2009, 138, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Myklebust, J.H.; Varghese, B.; Gill, S.; Jan, M.; Cha, A.C.; Chan, C.K.; Tan, B.T.; et al. Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-Hodgkin Lymphoma. Cell 2010, 142, 699–713. [Google Scholar] [CrossRef]
- Ye, X.; Wang, X.; Lu, R.; Zhang, J.; Chen, X.; Zhou, G. CD47 as a Potential Prognostic Marker for Oral Leukoplakia and Oral Squamous Cell Carcinoma. Oncol. Lett. 2018, 15, 9075–9080. [Google Scholar] [CrossRef] [PubMed]
- Akel, R.; Kurban, M.; Abbas, O. CD47 Expression for in Situ and Invasive Cutaneous Epithelial Lesions. J. Am. Acad. Dermatol. 2016, 75, 434–436. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, H.; Jee, S.; Bang, S.; Son, H.; Cha, H.; Myung, J.; Sim, J.; Kim, Y.; Paik, S.; Kim, H. CD47 Expression Predicts Unfavorable Prognosis in Clear Cell Renal Cell Carcinoma After Curative Resection. Diagnostics 2022, 12, 2291. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, T.; Hardarson, S.; Petursdottir, V.; Thoroddsen, A.; Magnusson, J.; Einarsson, G.V. Renal Oncocytoma: A Clinicopathological Analysis of 45 Consecutive Cases. BJU Int. 2005, 96, 1275–1279. [Google Scholar] [CrossRef]
- Delahunt, B.; Cheville, J.C.; Martignoni, G.; Humphrey, P.A.; Magi-Galluzzi, C.; McKenney, J.; Egevad, L.; Algaba, F.; Moch, H.; Grignon, D.J.; et al. The International Society of Urological Pathology (ISUP) Grading System for Renal Cell Carcinoma and Other Prognostic Parameters. Am. J. Surg. Pathol. 2013, 37, 1490–1504. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Paner, G.P.; Alvarado-Cabrero, I.; Young, A.N.; Stricker, H.J.; Lyles, R.H.; Moch, H. Chromophobe Renal Cell Carcinoma: Histomorphologic Characteristics and Evaluation of Conventional Pathologic Prognostic Parameters in 145 Cases. Am. J. Surg. Pathol. 2008, 32, 1822–1834. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Rajesh, A.; Mayer, N.J.; Mulcahy, K.A.; Haroon, A. Renal Oncocytoma: CT Features Cannot Reliably Distinguish Oncocytoma from Other Renal Neoplasms. Clin. Radiol. 2009, 64, 517–522. [Google Scholar] [CrossRef]
- Fu, W.; Li, J.; Zhang, W.; Li, P. High Expression of CD47 Predicts Adverse Prognosis in Chinese Patients and Suppresses Immune Response in Melanoma. Biomed. Pharmacother. 2017, 93, 1190–1196. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Kong, X.; Li, E.; Liu, Y.; Du, X.; Kang, Z.; Tang, Y.; Kuang, Y.; Yang, Z.; et al. CD47 Promotes Tumor Invasion and Metastasis in Non-Small Cell Lung Cancer. Sci. Rep. 2016, 6, 29719. [Google Scholar] [CrossRef]
- Sudo, T.; Takahashi, Y.; Sawada, G.; Uchi, R.; Mimori, K.; Akagi, Y. Significance of CD47 Expression in Gastric Cancer. Oncol. Lett. 2017, 14, 801–809. [Google Scholar] [CrossRef]
- Yoshida, K.; Tsujimoto, H.; Matsumura, K.; Kinoshita, M.; Takahata, R.; Matsumoto, Y.; Hiraki, S.; Ono, S.; Seki, S.; Yamamoto, J.; et al. CD47 is an Adverse Prognostic Factor and a Therapeutic Target in Gastric Cancer. Cancer Med. 2015, 4, 1322–1333. [Google Scholar] [CrossRef]
- Nagahara, M.; Mimori, K.; Kataoka, A.; Ishii, H.; Tanaka, F.; Nakagawa, T.; Sato, T.; Ono, S.; Sugihara, K.; Mori, M. Correlated Expression of CD47 and SIRPA in Bone Marrow and in Peripheral Blood Predicts Recurrence in Breast Cancer Patients. Clin. Cancer Res. 2010, 16, 4625–4635. [Google Scholar] [CrossRef]
- Li, Y.; Lu, S.; Xu, Y.; Qiu, C.; Jin, C.; Wang, Y.; Liu, Z.; Kong, B. Overexpression of CD47 Predicts Poor Prognosis and Promotes Cancer Cell Invasion in High-Grade Serous Ovarian Carcinoma. Am. J. Transl. Res. 2017, 9, 2901–2910. [Google Scholar]
- Kingsley, L.A.; Fournier, P.G.J.; Chirgwin, J.M.; Guise, T.A. Molecular Biology of Bone Metastasis. Mol. Cancer Ther. 2007, 6, 2609–2617. [Google Scholar] [CrossRef]
- Mundy, G.R. Metastasis to Bone: Causes, Consequences and Therapeutic Opportunities. Nat. Rev. Cancer 2002, 2, 584–593. [Google Scholar] [CrossRef]
- Kozlow, W.; Guise, T.A. Breast Cancer Metastasis to Bone: Mechanisms of Osteolysis and Implications for Therapy. J. Mammary Gland. Biol. Neoplasia 2005, 10, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Roodman, G.D. Mechanisms of Bone Metastasis. N. Engl. J. Med. 2004, 350, 1655–1664. [Google Scholar] [CrossRef]
- Vignery, A. Macrophage Fusion. J. Exp. Med. 2005, 202, 337–340. [Google Scholar] [CrossRef]
- Lundberg, P.; Koskinen, C.; Baldock, P.A.; Löthgren, H.; Stenberg, Å.; Lerner, U.H.; Oldenborg, P.-A. Osteoclast Formation Is Strongly Reduced Both In Vivo and In Vitro in the Absence of CD47/SIRPα-Interaction. Biochem. Biophys. Res. Commun. 2007, 352, 444–448. [Google Scholar] [CrossRef]
- Uluçkan, O.; Becker, S.N.; Deng, H.; Zou, W.; Prior, J.L.; Piwnica-Worms, D.; Frazier, W.A.; Weilbaecher, K.N. CD47 Regulates Bone Mass and Tumor Metastasis to Bone. Cancer Res. 2009, 69, 3196–3204. [Google Scholar] [CrossRef] [PubMed]
- Baccelli, I.; Stenzinger, A.; Vogel, V.; Pfitzner, B.M.; Klein, C.; Wallwiener, M.; Scharpff, M.; Saini, M.; Holland-Letz, T.; Sinn, H.-P.; et al. Co-Expression of MET and CD47 Is a Novel Prognosticator for Survival of Luminal-Type Breast Cancer Patients. Oncotarget 2014, 5, 8147–8160. [Google Scholar] [CrossRef]
- Chao, M.P.; Alizadeh, A.A.; Tang, C.; Jan, M.; Weissman-Tsukamoto, R.; Zhao, F.; Park, C.Y.; Weissman, I.L.; Majeti, R. Therapeutic Antibody Targeting of CD47 Eliminates Human Acute Lymphoblastic Leukemia. Cancer Res. 2011, 71, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wang, J.; Willingham, S.B.; Martin, R.; Wernig, G.; Weissman, I.L. Anti-CD47 Antibodies Promote Phagocytosis and Inhibit the Growth of Human Myeloma Cells. Leukemia 2012, 26, 2538–2545. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.S.; Espinosa, I.; Chao, M.; Wong, D.; Ailles, L.; Diehn, M.; Gill, H.; Presti, J.; Chang, H.Y.; van de Rijn, M.; et al. Identification, Molecular Characterization, Clinical Prognosis, and Therapeutic Targeting of Human Bladder Tumor-Initiating Cells. Proc. Natl. Acad. Sci. USA 2009, 106, 14016–14021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hutter, G.; Kahn, S.A.; Azad, T.D.; Gholamin, S.; Xu, C.Y.; Liu, J.; Achrol, A.S.; Richard, C.; Sommerkamp, P.; et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS ONE 2016, 11, e0153550. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Hori, M. Five-year relative survival rate of kidney and renal pelvis cancer in the USA, Europe and Japan. Jpn. J. Clin. Oncol. 2015, 45, 136. [Google Scholar] [CrossRef]
- Motzer, R.J.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Shah, A.Y.; Suárez, C.; Hamzaj, A.; Porta, C.; Hocking, C.M.; et al. Nivolumab plus cabozantinib versus sunitinib in first-line treatment for advanced renal cell carcinoma (CheckMate 9ER): Long-term follow-up results from an open-label, randomised, phase 3 trial. Lancet Oncol. 2022, 23, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Mandarà, M.; Giuliani, J.; Durante, E.; Buttigliero, C.; Turco, F.; Palesandro, E.; Campisi, I.; Singh, N.; Muraro, M.; et al. Treatment options in first-line metastatic renal carcinoma: A meta-analysis of 2556 patients treated with immune checkpoint inhibitors-based combinations in randomised controlled trials. Cancer Treat. Rev. 2024, 127, 102745. [Google Scholar] [CrossRef]
- Highlights of Prescribing Information:Keytruda (Pembrolizumab) Injection, for Intravenous Use; Merck Sharp&Dohme: Rahway, NJ, USA, 2024; Available online: https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf (accessed on 17 November 2021).
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Pu, Y.; Cron, K.; Deng, L.; Kline, J.; Frazier, W.A.; Xu, H.; Peng, H.; Fu, Y.-X.; Xu, M.M. CD47 Blockade Triggers T Cell–Mediated Destruction of Immunogenic Tumors. Nat. Med. 2015, 21, 1209–1215. [Google Scholar] [CrossRef]
- Murata, Y.; Saito, Y.; Kotani, T.; Matozaki, T. CD47-signal Regulatory Protein α Signaling System and Its Application to Cancer Immunotherapy. Cancer Sci. 2018, 109, 2349–2357. [Google Scholar] [CrossRef]
- Dotsikas, G.; Konowalchuk, T.; Major, P.; Kovac, P.; Ward, G.; Stewart, S.; Price, G.; Elhilali, M.; Mackillop, W. Cellular Heterogeneity in Normal and Neoplastic Human Urothelium: A Study Using Murine Monoclonal Antibodies. Br. J. Cancer 1987, 56, 439–444. [Google Scholar] [CrossRef] [PubMed]
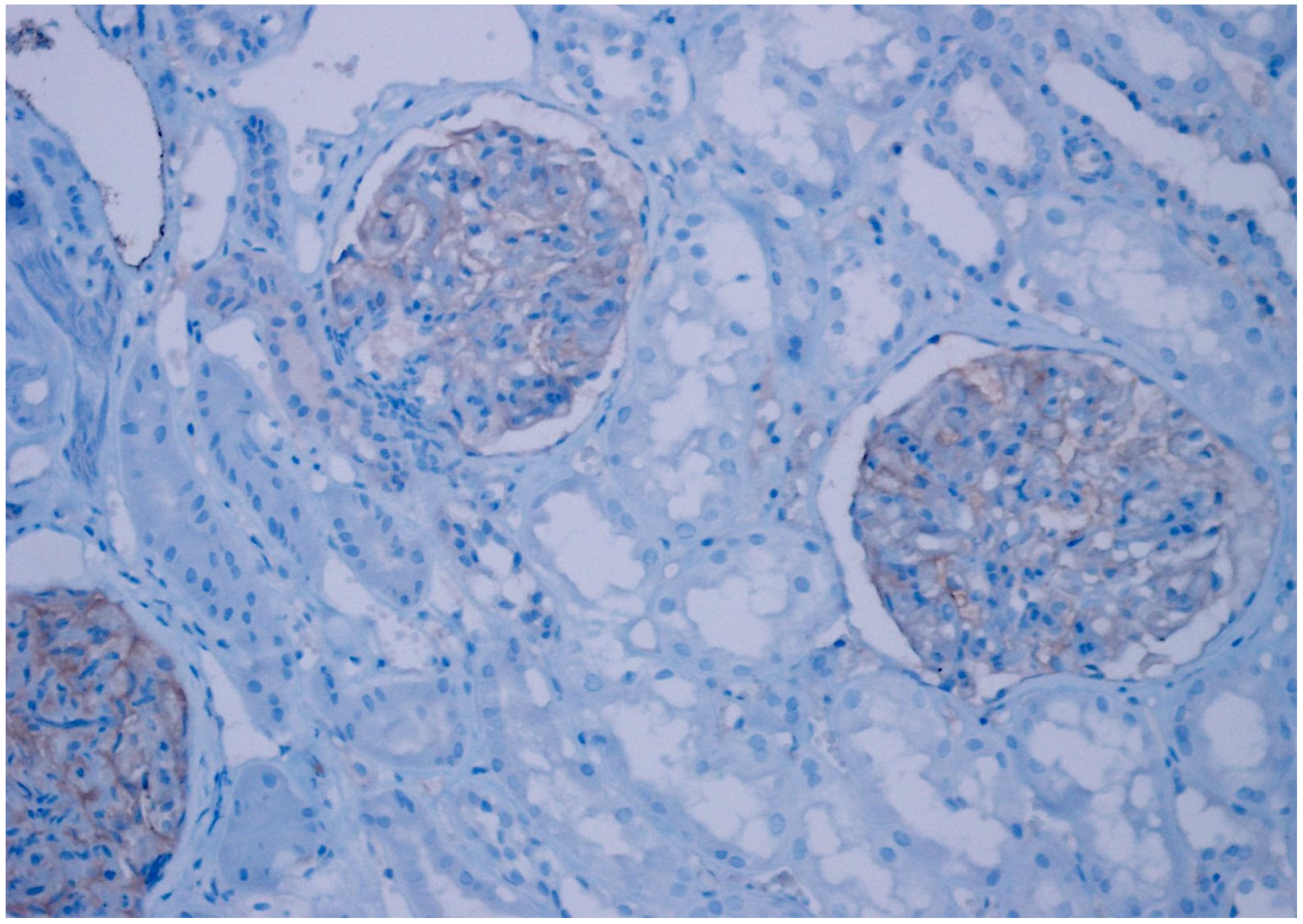
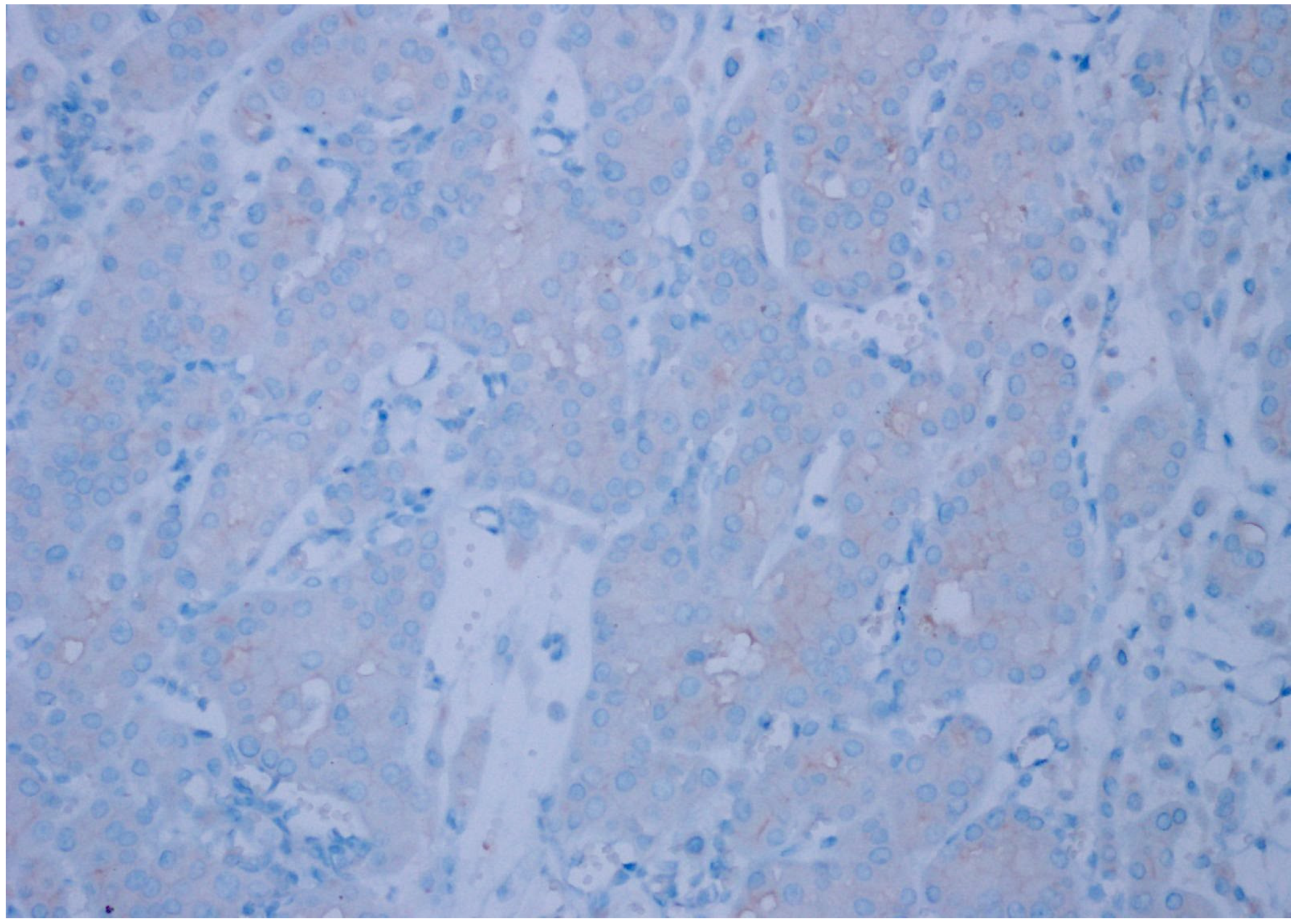
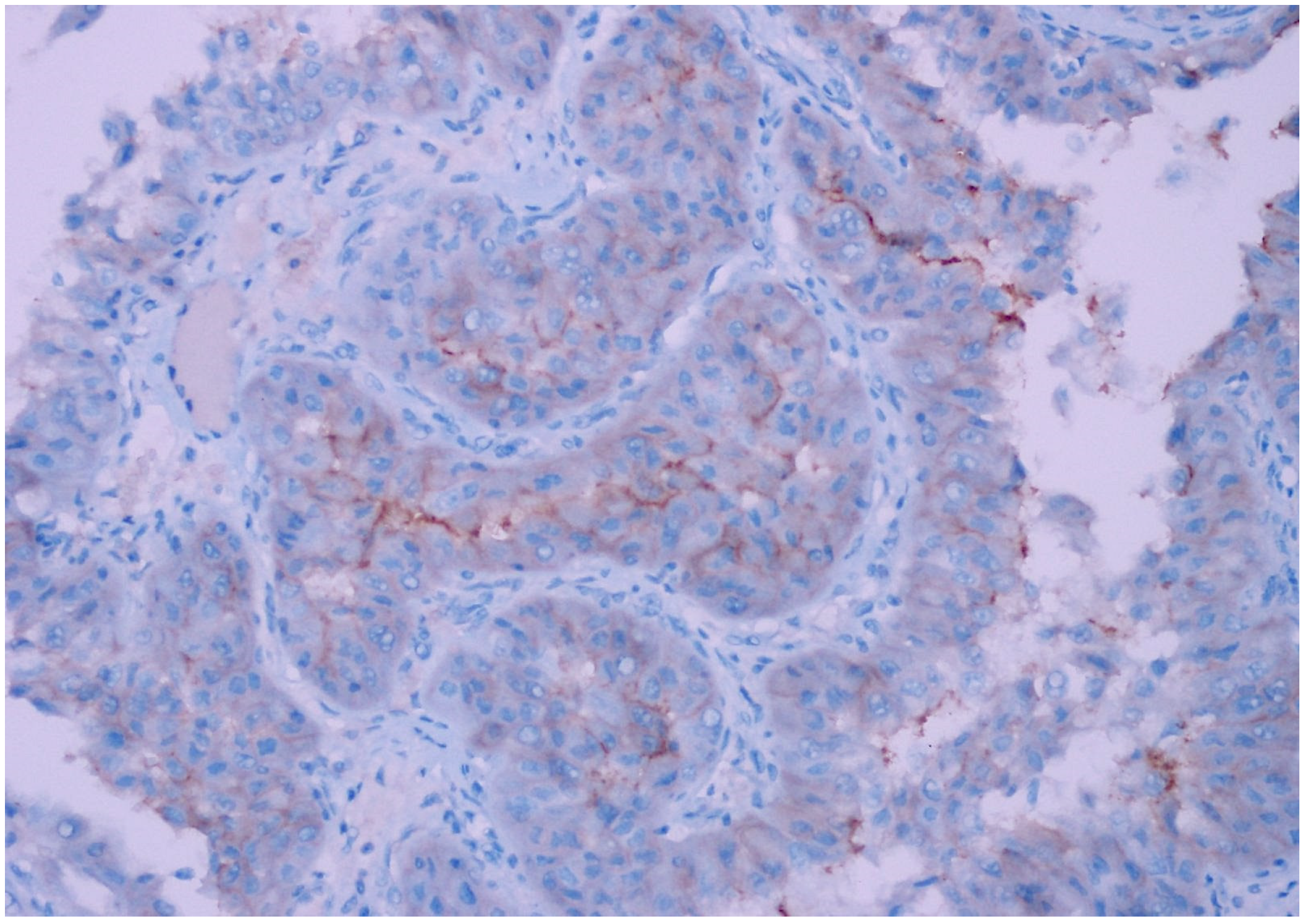
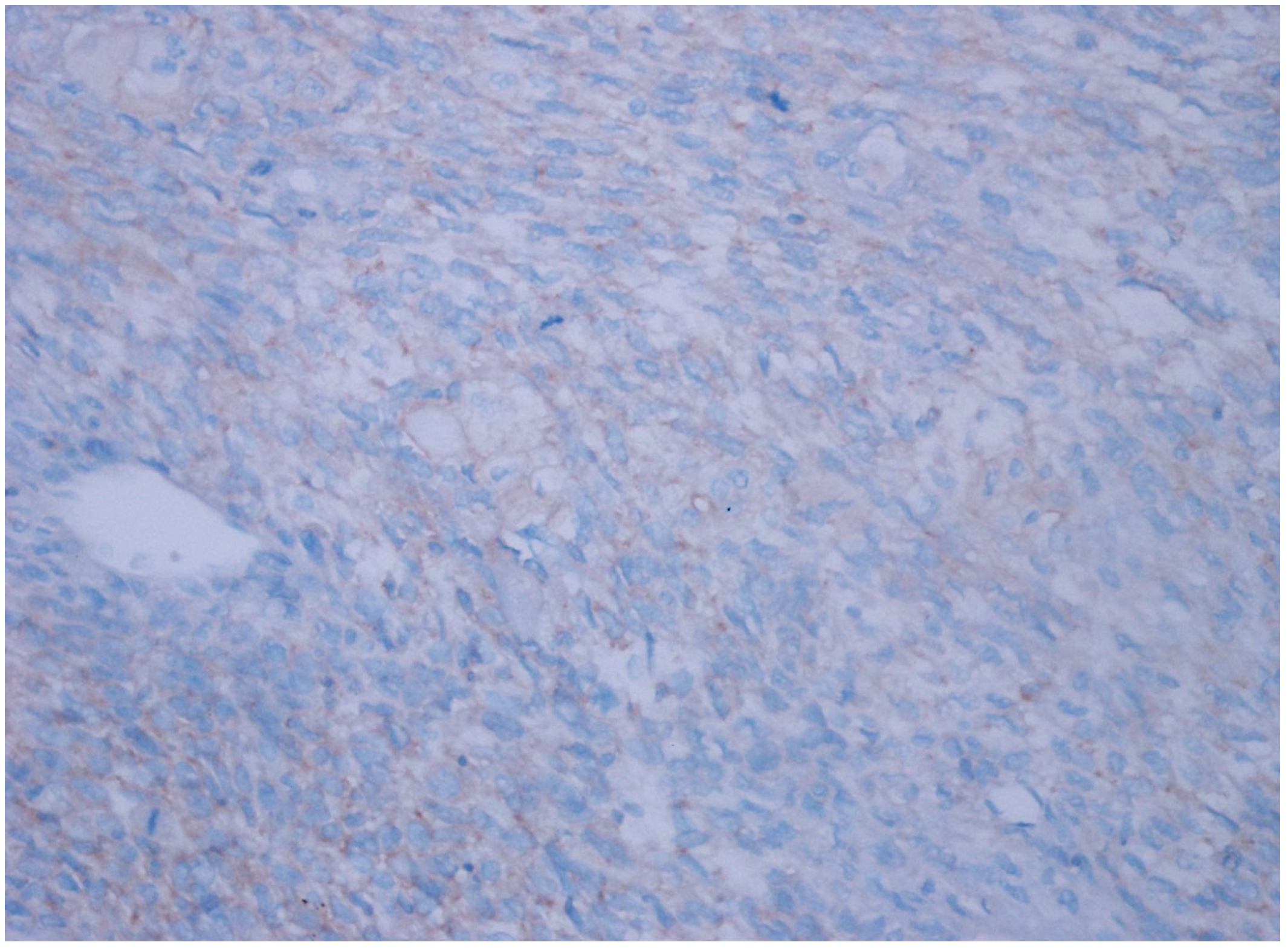

| Patient Group | ||||||
|---|---|---|---|---|---|---|
| Variable | Clear Cell RCC | Chromophobe RCC | Papillary Type 1 RCC | Papillary Type 2 RCC | Oncocytoma | Unclassified RCC |
| Age average | ||||||
| Female | 60.1 | 53.2 | 75 | 59.5 | 62.4 | 46.6 |
| Male | 53.1 | 58.2 | 60.7 | 60.2 | 59.1 | 61.8 |
| Gender | ||||||
| Female | 14 (38%) | 13 | 1 | 4 | 9 | 14 |
| −43% | −3% | −35% | −41% | |||
| −14% | ||||||
| Male | 23 (62%) | 17 | 29 | 25 | 17 | 20 |
| −57% | −97% | −65% | −59% | |||
| −86% | ||||||
| T Phase | ||||||
| T1 | 12 | 11 | 20 | 13 | 24 | 11 |
| T2 | 13 | 16 | 7 | 13 | 2 | 15 |
| T3 | 11 | 3 | 2 | 1 | 0 | 5 |
| T4 | 1 | 0 | 1 | 2 | 0 | 3 |
| Capsule invasion | ||||||
| Present | 8 | 6 | 10 | 8 | 1 | 10 |
| Absent | 29 | 24 | 20 | 21 | 25 | 24 |
| Lymphovascular invasion | ||||||
| Present | 4 | 3 | 0 | 4 | 0 | 0 |
| Absent | 33 | 27 | 30 | 25 | 26 | 34 |
| Distant organ metastasis | ||||||
| Present | 12 | 6 | 0 | 3 | 0 | 16 |
| Absent | 25 | 24 | 30 | 26 | 26 | 18 |
| 5-year survey | ||||||
| Ex | 19 | 6 | 0 | 12 | 0 | 17 |
| Alive | 18 | 24 | 30 | 17 | 26 | 17 |
| Patient Group | |||||||
|---|---|---|---|---|---|---|---|
| CD47 Expression | Clear Cell RCC | Chromophobe RCC | Papillary Type 1 RCC | Papillary Type 2 RCC | Oncocytoma | Unclassified RCC | p Value |
| Negative | 34 (92%) | 6 (20%) | 30 (100%) | 22 (76%) | 21 (81%) | 16 (47%) | |
| Weak | 3 (8%) | 23 (77%) | 0 | 6 (21%) | 5 (19%) | 17 (50%) | 0.001 |
| Strong | 0 | 1 (3%) | 0 | 1 (3%) | 0 | 1 (3%) | |
| Staining Severity | Staining Prevalence | Number of Positivity | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Negative (0) | Low (1+) | Moderate (2+) | Severe (3+) | 0 | 1+ (<10%) | 2+ (10–25%) | 3+ (26–50%) | 4+ (>50%) | ||
| Clear Cell | 34 | 3 | 0 | 0 | 34 | 3 | 0 | 0 | 0 | 3 |
| Chromophobe | 6 | 23 | 1 | 0 | 6 | 4 | 5 | 10 | 5 | 24 |
| Papillary Type 1 | 30 | 0 | 0 | 0 | 30 | 0 | 0 | 0 | 0 | 0 |
| Papillary Type 2 | 22 | 6 | 1 | 0 | 22 | 1 | 3 | 3 | 0 | 7 |
| Oncocytoma | 21 | 5 | 0 | 0 | 21 | 1 | 2 | 1 | 1 | 5 |
| Unclassified | 16 | 17 | 1 | 0 | 16 | 3 | 6 | 4 | 5 | 18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dizibüyük, Ö.F.; Bozdağ, Z.; Karakök, M. Investigation of CD47 Expression in Renal Cell Tumors and Evaluation of Its Relationship with Prognostic Parameters. Diagnostics 2025, 15, 53. https://doi.org/10.3390/diagnostics15010053
Dizibüyük ÖF, Bozdağ Z, Karakök M. Investigation of CD47 Expression in Renal Cell Tumors and Evaluation of Its Relationship with Prognostic Parameters. Diagnostics. 2025; 15(1):53. https://doi.org/10.3390/diagnostics15010053
Chicago/Turabian StyleDizibüyük, Ömer Faruk, Zehra Bozdağ, and Metin Karakök. 2025. "Investigation of CD47 Expression in Renal Cell Tumors and Evaluation of Its Relationship with Prognostic Parameters" Diagnostics 15, no. 1: 53. https://doi.org/10.3390/diagnostics15010053
APA StyleDizibüyük, Ö. F., Bozdağ, Z., & Karakök, M. (2025). Investigation of CD47 Expression in Renal Cell Tumors and Evaluation of Its Relationship with Prognostic Parameters. Diagnostics, 15(1), 53. https://doi.org/10.3390/diagnostics15010053




