Diagnostic Difficulties of Erosive Lichen Planus in a Pediatric Patient
Abstract
:1. Introduction
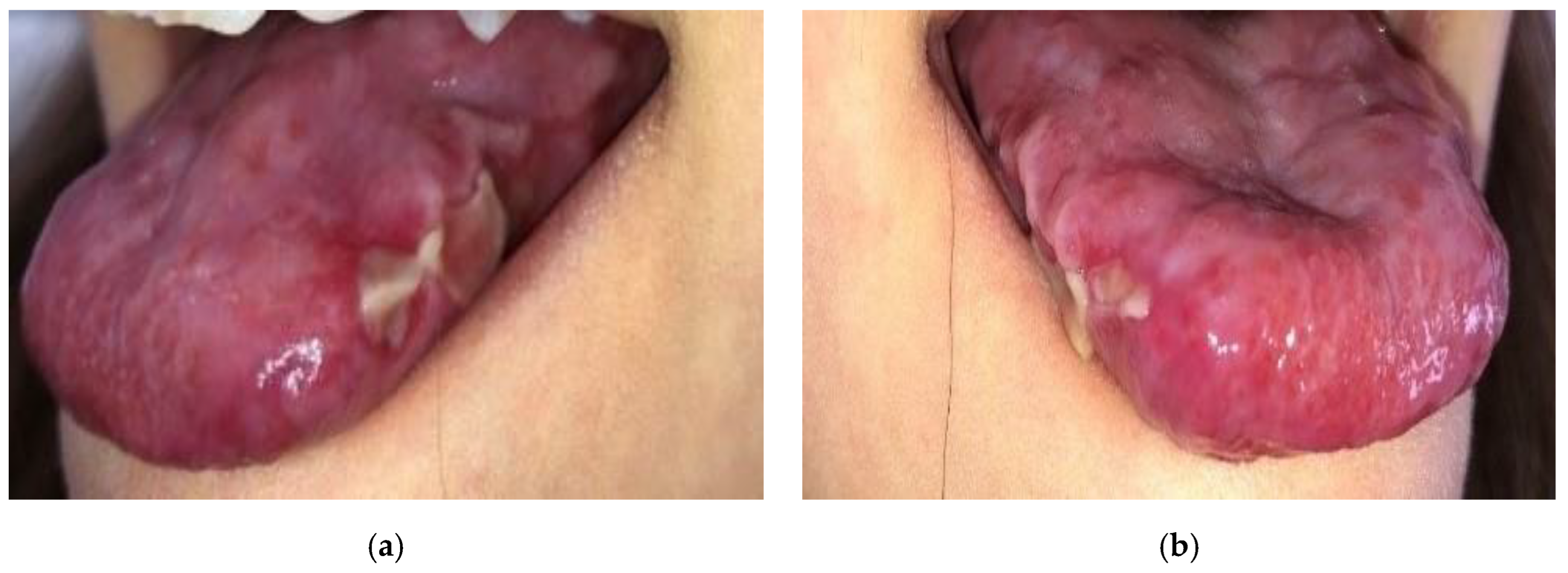
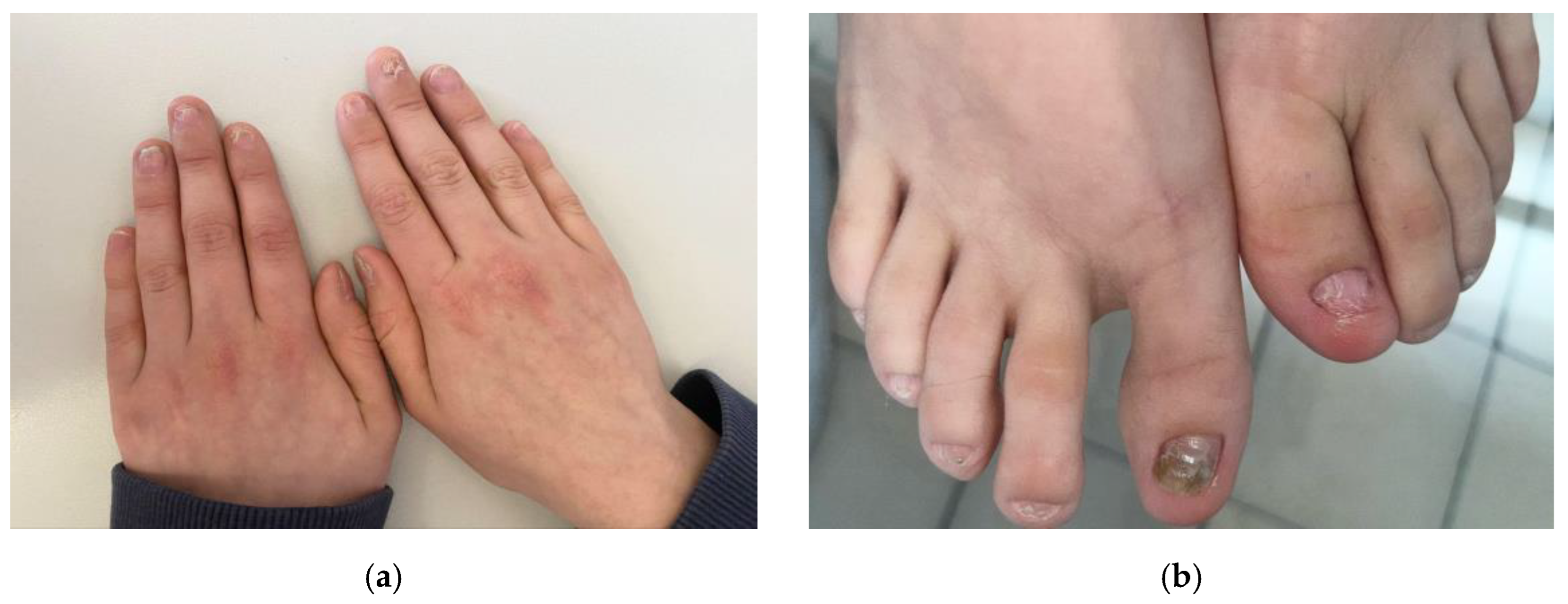
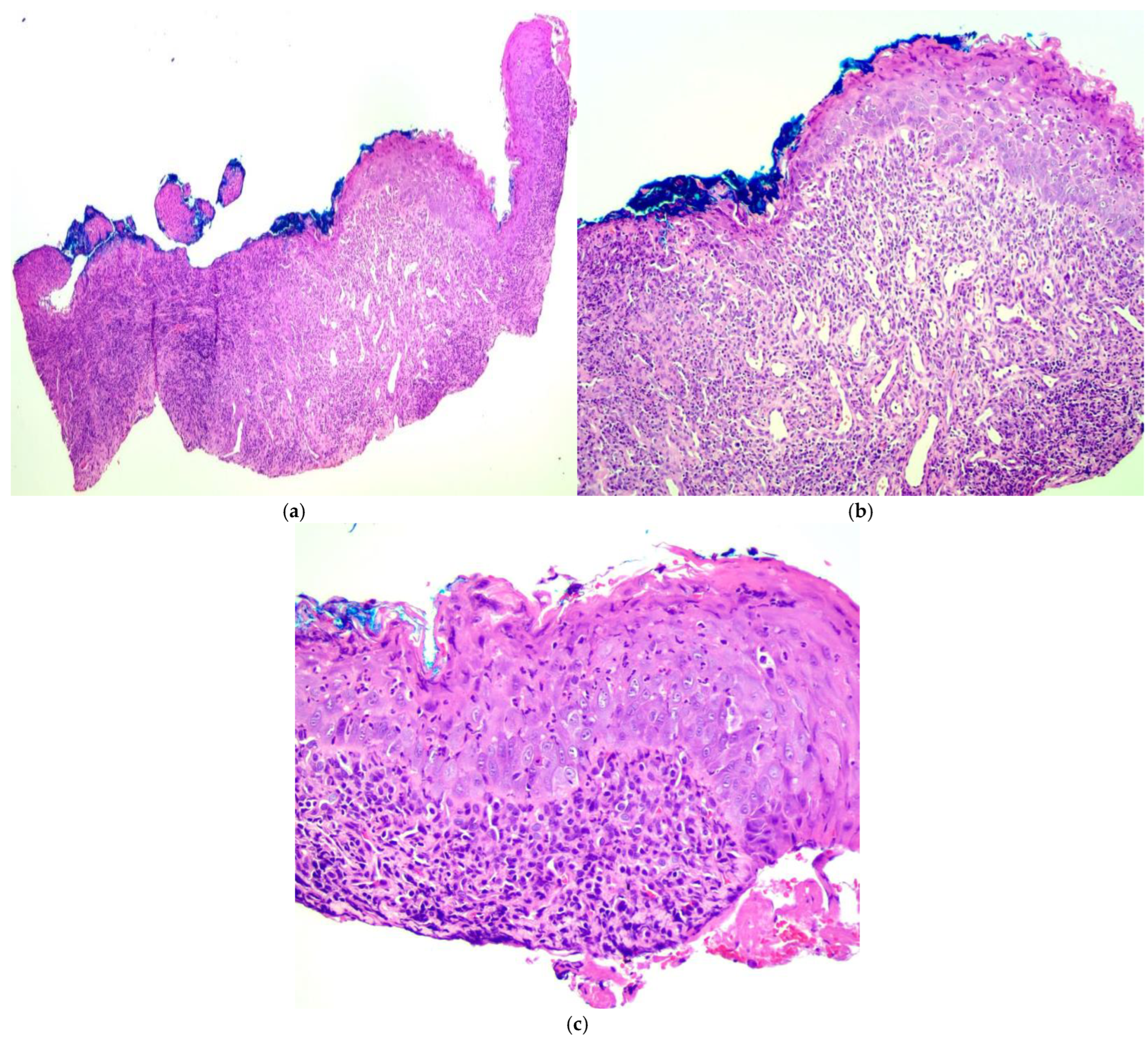
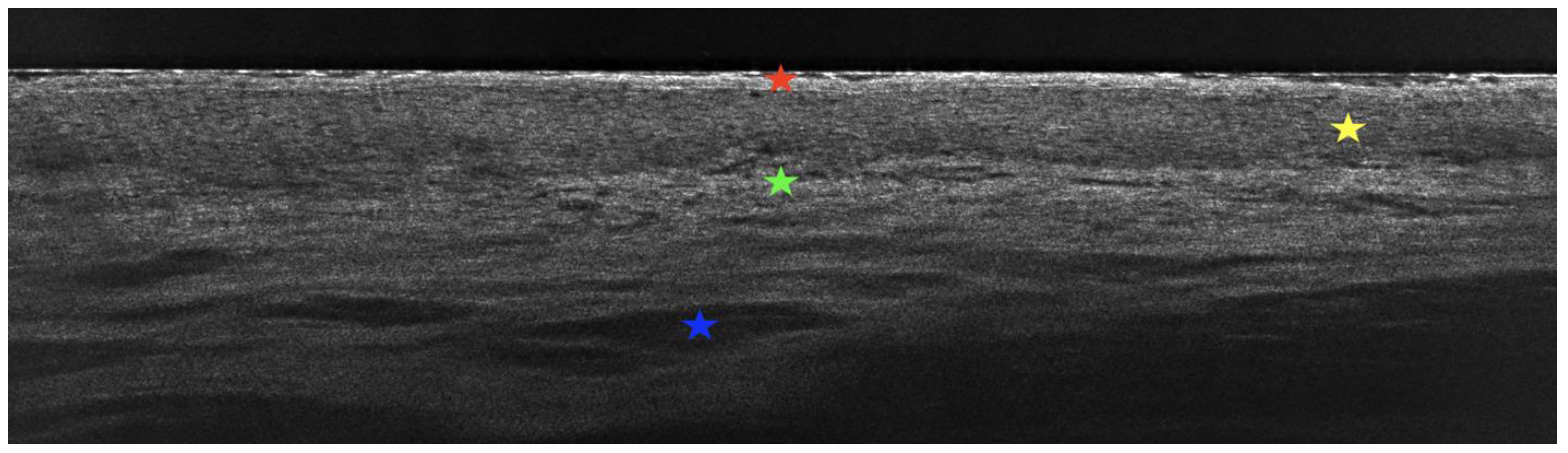
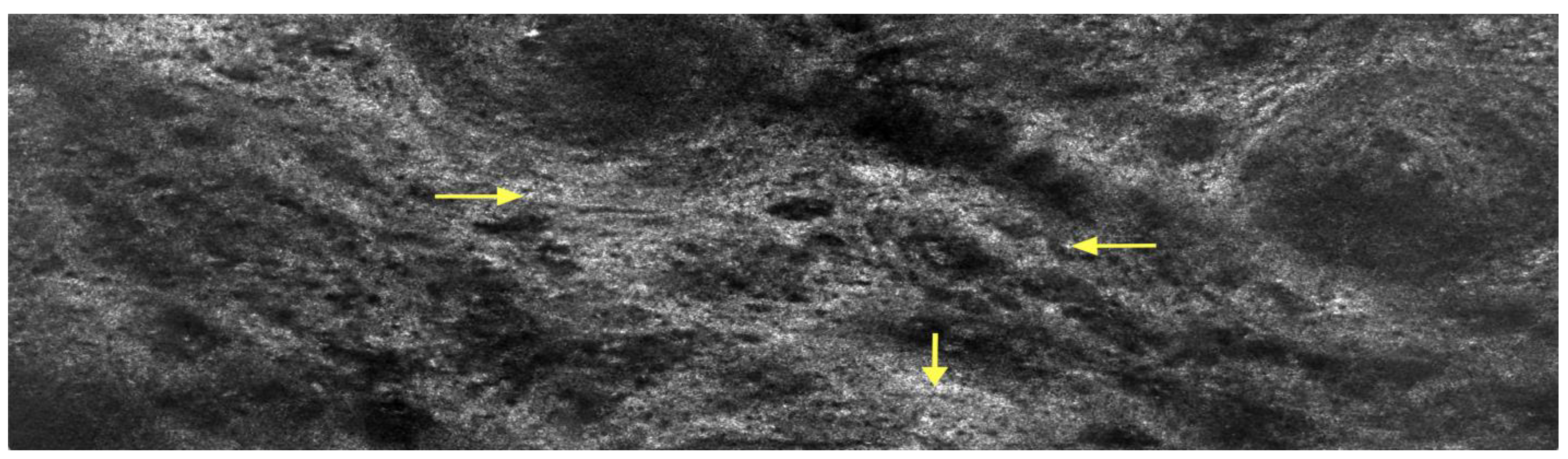
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boch, K.; Langan, E.A.; Kridin, K.; Zillikens, D.; Ludwig, R.J.; Bieber, K. Lichen planus. Front. Med. 2021, 8, 737813. [Google Scholar] [CrossRef]
- Eisen, D. The clinical features, malignant potential, and systemic associations of oral lichen planus: A study of 723 patients. J. Am. Acad. Dermatol. 2002, 46, 207–214. [Google Scholar] [CrossRef]
- Roopashree, M.R.; Gondhalekar, R.V.; Shashikanth, M.C.; George, J.; Thippeswamy, S.H.; Shukla, A. Pathogenesis of oral lichen planus—A review. J. Oral Pathol. Med. 2010, 39, 729–734. [Google Scholar] [CrossRef]
- Elenbaas, A.; Enciso, R.; Al-Eryani, K. Oral lichen planus: A review of clinical features, etiologies, and treatments. Dent. Rev. 2022, 2, 100007. [Google Scholar] [CrossRef]
- Mahon-Smith, A.; Clifford, M.; Batish, A.; Sharp, R.; Panter, C.; Naujoks, C.; Schruf, E.; Compagno, N.; Moreno, S.G. Patient experience of lichen planus: A qualitative exploration of signs, symptoms, and health-related quality of life impacts. Dermatol. Ther. 2023, 13, 2001–2017. [Google Scholar] [CrossRef] [PubMed]
- Walton, K.E.; Bowers, E.V.; Drolet, B.A.; Holland, K.E. Childhood lichen planus: Demographics of a U.S. population. Pediatr. Dermatol. 2010, 27, 34–38. [Google Scholar] [CrossRef]
- Gorouhi, F.; Davari, P.; Fazel, N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci. World J. 2014, 2014, 742826. [Google Scholar] [CrossRef]
- Eisen, D. The evaluation of cutaneous, genital, scalp, nail, esophageal, and ocular involvement in patients with oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1999, 88, 431–436. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Lundqvist, L.; Wahlin, Y.B.; Nylander, E. Mucosal lichen planus, a systemic disease requiring multidisciplinary care: A cross-sectional clinical review from a multidisciplinary perspective. J. Low. Genit. Tract. Dis. 2012, 16, 377–380. [Google Scholar] [CrossRef]
- Decker, A.; Schauer, F.; Lazaro, A.; Monasterio, C.; Schmidt, A.R.; Scmitt-Graeff, A.; Kreisel, W. Esophageal lichen planus: Current knowledge, challenges and future perspectives. World J. Gastroenterol. 2022, 28, 5893–5909. [Google Scholar] [CrossRef] [PubMed]
- Ravi, K.; Codipilly, D.C.; Sunjaya, D.; Fang, H.; Arora, A.S.; Katzka, D.A. Esophageal lichen planus is associated with a significant increase in risk of squamous cell carcinoma. Clin. Gastroenterol. Hepatol. 2019, 17, 1902–1903. [Google Scholar] [CrossRef] [PubMed]
- Müller, S. Oral lichenoid lesions: Distinguishing the benign from the deadly. Mod. Pathol. 2017, 30, 54–67. [Google Scholar] [CrossRef]
- Schlosser, B.J. Lichen planus and lichenoid reactions of the oral mucosa. Dermatol. Ther. 2010, 23, 251–267. [Google Scholar] [CrossRef]
- Latriglia, F.; Ogien, J.; Tavernier, C.; Fischman, S.; Suppa, M.; Perrot, J.L.; Dubois, A. Line-field confocal optical coherence tomography (LC-OCT) for skin imaging in dermatology. Life 2023, 13, 2268. [Google Scholar] [CrossRef]
- Verzi, A.E.; Broggi, G.; Micali, G.; Sorci, F.; Caltabiano, R.; Lacarrubba, F. Line-field confocal optical coherence tomography of psoriasis, eczema and lichen planus: A case series with histopathological correlation. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1884–1889. [Google Scholar] [CrossRef] [PubMed]
- Popa, C.; Sciuca, A.M.; Onofrei, B.A.; Toader, S.; Condurache Hritcu, O.M.; Botoc Colac, C.; Porumb Adrese, E.; Branisteanu, D.E.; Toader, M.P. Integrative approaches for the diagnosis and management of erosive oral lichen planus. Diagnostics 2024, 14, 692. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.K.; Hantash, B.M. Systematic review of current systemic treatment options for erosive lichen planus. Expert. Rev. Dermatol. 2012, 7, 269–282. [Google Scholar] [CrossRef]
- Bray, E.R.; Morrison, B.W. Low-Dose Naltrexone Use in Biopsy-Proven Lichen Planus of the Nails. JAMA Dermatol. 2024, 160, 4098. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Mann, C. Oral erosive lichen planus treated with efalizumab. Arch. Dermatol. 2006, 142, 680–682. [Google Scholar] [CrossRef]
- Damsky, W.; Wang, A.; Olamiju, B.; Peterson, D.; Galan, A.; King, B. Treatment of severe lichen planus with the JAK inhibitor tofacitinib. J. Allergy Clin. Immunol. 2020, 145, 1708–1710. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.L.; Bhamra, R.; Ilankovan, V. Malignant transformation rate of erosive oral lichen planus: A retrospective study. Br. J. Oral Maxillofac. Surg. 2024, 62, 788–793. [Google Scholar] [CrossRef]
- Arora, G.; Bhateja, S.; Bhushan, P.; Solanki, J. A rare case of oral erosive lichen planus in pediatrics. J. Adv. Dent. Res. 2013, 4, 20–23. [Google Scholar] [CrossRef]
| Disease | Differentiating Features |
|---|---|
| Aphthous ulcers |
|
| Behçet’s disease |
|
| Crohn’s disease |
|
| Morsicatio mucosae oris |
|
| Oral lupus erythematosus |
|
| Oropharyngeal candidiasis |
|
| Pemphigus vulgaris |
|
| Paraneoplastic pemphigus |
|
| Oral leukoplakia |
|
| Proliferative verrucous leukoplakia |
|
| Oral squamous cell carcinoma |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szwed, C.; Gudziewski, O.; Sar-Pomian, M.; Olszewska, M.; Rudnicka, L.; Czuwara, J. Diagnostic Difficulties of Erosive Lichen Planus in a Pediatric Patient. Diagnostics 2025, 15, 35. https://doi.org/10.3390/diagnostics15010035
Szwed C, Gudziewski O, Sar-Pomian M, Olszewska M, Rudnicka L, Czuwara J. Diagnostic Difficulties of Erosive Lichen Planus in a Pediatric Patient. Diagnostics. 2025; 15(1):35. https://doi.org/10.3390/diagnostics15010035
Chicago/Turabian StyleSzwed, Carolyn, Olivia Gudziewski, Marta Sar-Pomian, Malgorzata Olszewska, Lidia Rudnicka, and Joanna Czuwara. 2025. "Diagnostic Difficulties of Erosive Lichen Planus in a Pediatric Patient" Diagnostics 15, no. 1: 35. https://doi.org/10.3390/diagnostics15010035
APA StyleSzwed, C., Gudziewski, O., Sar-Pomian, M., Olszewska, M., Rudnicka, L., & Czuwara, J. (2025). Diagnostic Difficulties of Erosive Lichen Planus in a Pediatric Patient. Diagnostics, 15(1), 35. https://doi.org/10.3390/diagnostics15010035







