1. Introduction
Diabetes mellitus (DM) is among the common non-communicable diseases and its incidence is increasing, especially in African countries, including South Africa [
1]. Diabetic foot syndrome is defined by the World Health Organization as a severe complication of DM characterized by foot ulceration associated with neuropathy, varying degrees of ischemia, and infection [
2]. Foot ulceration develops in around 19–34% of patients with DM and may lead to major amputations and/or secondary infection [
3]. Foot infection in patients with DM is referred to as diabetic foot sepsis (DFS), which is more common in male patients with type 2 DM [
4]. The prevalence of DFS in Africa exceeds the global average and leads to comparatively higher rates of major amputations and mortality on the continent [
5,
6,
7,
8,
9].
Most cases of DFS are preceded by a neuropathic, ischaemic, or neuro-ischaemic ulcer. Other factors that contribute to the development of foot ulcerations in patients with DM include poor glycaemic control, host-related elements, and microbial characteristics [
4,
9]. Several species of bacteria and sometimes fungi are involved in DFS, either in isolation or as synergistic infections. It is crucial that, during the investigation of patients with diabetic foot ulcers, an adequate specimen be collected for MC&S to identify causative organisms and their antibiograms [
3,
10,
11,
12]. Empiric antimicrobial therapy should be initiated in a timely manner while awaiting MC&S results to prevent worsening of the already clinically overt infection.
Management of DM and its complications in low- and middle-income countries (LMICs), including South Africa, faces significant challenges, among them poor understanding of the disease; this results in a lot of patients “suffering from” instead of “living with” diabetes. Consequently, majority of patients in LMICs experience inadequate self-care, poor dietary habits, and reduced physical activity, and often manifest suboptimal health-seeking behaviour [
13]. Additionally, the microbial profiles and antimicrobial susceptibility patterns of patients with DFS in LMICs are often different to those in high-income countries [
14]. Compounding these challenges is the high prevalence comorbidities like HIV, which increases risk of DM and the likelihood of severe soft tissue infections [
15]. Limited financial resources in LMICs may also limit access to healthy food, resulting in over-reliance on inexpensive carbohydrate-dense diet, which is diabetogenic [
15].
The frequently isolated organisms in patients with DFS are aerobic gram-positive cocci, with
S. aureus being the most predominant species [
16]. Polymicrobial and chronic infections often involve anaerobic organisms and gram-negative aerobic bacilli such as
P. aeruginosa and
Enterobacteriaceae [
11,
17]. Emergence of antimicrobial resistance is a growing concern. Various strains of bacteria, including extended-spectrum β-lactamase-producing
Enterobacteriaceae, methicillin-resistant
S. aureus (MRSA), specific
P. aeruginosa strains, and vancomycin-resistant enterococci, are frequently isolated in specimens collected for MC&S from patients with DFS [
11,
16,
17].
Obtaining high-quality and representative samples for MC&S followed by targeted antibiotic therapy is crucial for appropriate and effective management of patients with any infection, including DFS [
12,
18]. Collecting specimens for MC&S also provides data on local susceptibility patterns for use as a guide by clinicians during selection of appropriate empirical antimicrobial therapy [
19,
20]. By integrating antibiogram data into clinical practice, clinicians can reduce the misuse of antibiotics and contribute to the effort to curb an increase in the incidence of antimicrobial resistance.
Several methods are used to obtain samples from patients with DFS among them deep tissue biopsy, sampling adjacent bone near the infection site, pus swabs, blood cultures, and fluid aspiration. While the gold standard is deep tissue biopsy, especially when osteomyelitis is suspected, it is acceptable to collect superficial samples if the infection is limited [
21,
22,
23].
Several classification systems are used to grade severity and to guide management of patients with DFS but none of the classification systems is supreme and universally accepted [
24]. The choice of a classification system for grading of DFS depends on a clinician as well as its usability, as some of the classification systems are complex [
24]. It is advisable to use a severity grading system that includes the extent of the severity of infection, degree of ischaemia, causative organisms, and the status of glycaemic control [
24]. Superficial infection is likely to be caused by one species of bacteria whereas deep and severe DFS is likely to be caused by a combination of aerobic and anaerobic organisms. The severity of DFS is also used to guide the duration of antimicrobial therapy [
25].
Ultimately, effective treatment of DFS hinges on a comprehensive understanding of the microbiology and susceptibility patterns of microorganisms commonly associated with DFS. Evaluating antimicrobial utilization and management trends in LMICs is critical for timeous and effective treatment strategies. Resources in the majority of LMICs are limited, patients often present late, and antimicrobial stewardship is not routine. This study aimed to identify causative organisms and their susceptibility profiles in patients presenting with DFS.
2. Patients and Methods
This was a retrospective cross-sectional observational study focusing on patients aged 18 years and older who were admitted to the Department of Surgery at Charlotte Maxeke Johannesburg Academic Hospital (CMJAH) with DFS from 1 January 2017 to 31 December 2019. The study included patients with both primary and recurrent DFS. Data were obtained from weekly morbidity and mortality (M&M) meeting reports stored in the Research Electronic Data Capture (REDCap) database, discharge summaries, and hospital admission files. Data collected included age, sex, race, specimen collected for MC&S and results, antimicrobial(s) prescribed, and overall outcome. The outcome data collected included severity of infection, type of surgical intervention, and the level of amputation.
Descriptive statistical analysis was carried out using STATA SE 17.0 software. Categorical data, such as types of specimens collected, antimicrobials prescribed, and resistance patterns of organisms, were presented as actual counts and percentages. The study also examined resistance to antimicrobials in relation to gender and race, with confidence intervals determined using the exact method. Ethical clearance for the study was obtained from the Human Research Ethics Committee of University of the Witwatersrand (M210943). Consent from individual patients was waived as it was a retrospective study. The study was conducted following guidelines contained in the declaration of Helsinki.
3. Results
Hundred and sixty-eight (168) records met the inclusion criteria. The median age of the included patients was 59 years (IQR 54–67). The median age of male patients was 58.9 years compared to 61 years for females. Eighty-nine (53%) of the patients were black Africans while 26% (44) were White. The majority, 94.6%, of the patients had type II DM. Most cases, 95.8%, presented with wet gangrene of the toes or mid-foot (
Table 1).
The median WCC of male patients was 11 compared to 10.6 for females, but the difference was not statistically significant (
p = 0.438). The median HbA1c in male and female patients were 9.8% and 11.4%, respectively and the difference was not statistically significant (
p = 0.266). One hundred and six (63.1%) had records of the type of specimens collected for MC&S and their results. The collected specimens for MCS included tissue samples in 60.4% (64) of the patients (
Table 2).
Among the top five commonly isolated organisms were
E. faecalis (16%),
P. mirabilis (10.4%),
S. aureus (7.5%),
P. aeruginosa (6.6%), and
K. pneumoniae (4.7%) (
Figure 1).
Of the top 11 identified organisms, K. pneumoniae, Morganella morganii, and A. baumanii exhibited the highest proportion of antimicrobial resistance, with 100% resistance. S. aureus demonstrated resistance rates of 88%, P. mirabilis 80%, and P. aeruginosa 71%. E. faecalis, the most commonly isolated organism, displayed the least resistance at 6.7%.
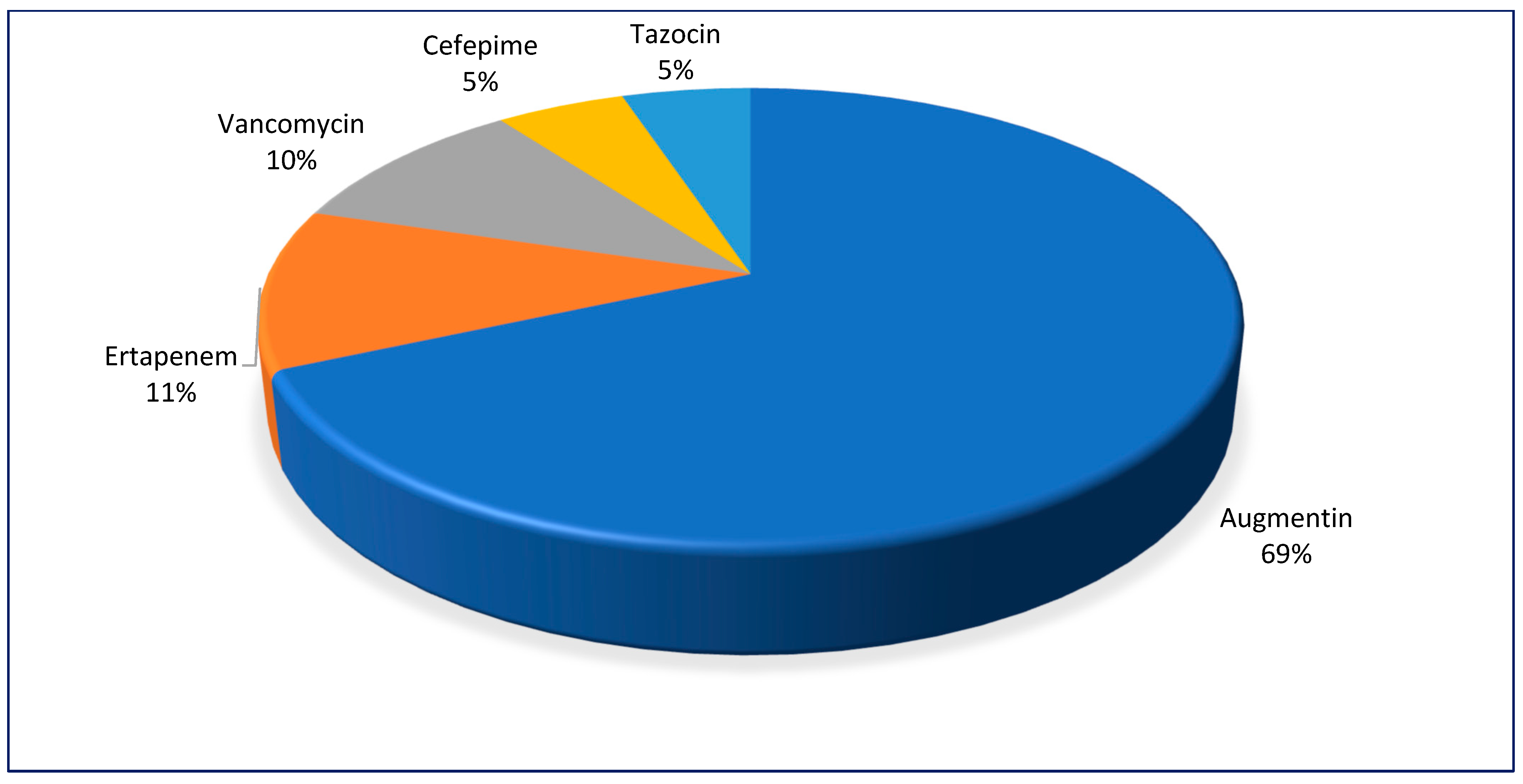
Records of antimicrobial prescriptions were complete in 45.2% (73/168) of the reports and amoxicillin/clavulanic acid was prescribed for 69% (50/73) of the patients with DFS (
Figure 2).
Ertapenem was prescribed in 11% of cases, followed by vancomycin in 10%, and piperacillin/tazobactam and cefepime in 5% each. Other antibiotics and combination therapies were prescribed in less than 2% of cases each. Ampicillin/amoxicillin was tested against the top 11 most cultured organisms and demonstrated 100% resistance against most of them and 8.3% against E. faecalis. All cases of S. marcescens and M. morganii were resistant to amoxicillin-clavulanic acid while K. pneumoniae, P. mirabilis, and E. coli displayed resistance rates of 80%, 50%, and 25%, respectively. Ertapenem, the second most commonly prescribed antibiotic, exhibited resistance in 50% of E. coli and K. pneumoniae. All P. mirabilis and M. morganii species were sensitive to ertapenem. All species of P. mirabilis, P. aeruginosa, E. coli, and A. baumanii were resistant to cefepime compared to 75% K. pneumoniae and 50% of S. marcescens. Resistance to tazobactam-piperacillin was shown in 100% each of K. pneumoniae and A. baumannii. Similarly, 50% each of P. aeruginosa and M. morganii species were resistant to tazobactam-piperacillin (50%). All cases of P. mirabilis were sensitive to tazobactam-piperacillin.
Gram-negative specimens displayed 100% resistance to ampicillin/amoxicillin, gentamycin, ampicillin, ceftriaxone, cefuroxime, cefoxitin, trimethoprim, and trimethoprim/sulfamethoxazole. They also showed some degree of resistance to other common antimicrobials such as amikacin, tobramycin, amoxicillin, amoxicillin/clavulanic acid, piperacillin/tazobactam, cefepime, cefotaxime, ceftriaxone, cefoxitin, ceftazidime, ciprofloxacin, and tigecycline, with at least one bacterial sample demonstrating 100% resistance to these antimicrobials. Ertapenem and imipenem showed promising results, with resistance levels recorded at 50% and 60%, respectively, for gram-negative specimens. Gram-positive bacteria exhibited lower resistance rates per antibiotic. All S. aureus and E. faecalis species cultured showed resistant to ampicillin. None of the S. aureus cultured showed resistance to macrolides, clindamycin, cefotaxime/ceftriaxone, and trimethoprim/sulfamethoxazole.
Polymicrobial infections were recorded in 79% of male patients who had MC&S results compared to 50% in females. The commonly isolated organisms in males were
E. faecalis at 6.9%,
S. aureus at 9.2%,
K. pneumoniae at 7.7%, and
P. mirabilis at 7.7%. In females,
E. faecalis,
P. mirabilis, and
aeruginosa were cultured in 14.3% each, followed by
S. agalactiae at 8.6% and
S. aureus at 5.7%. Notably, no isolates of
S. marcescens were found in females (
Table 3 and
Table 4).
The top three organisms isolated varied based on race. In the Black population, the most common organisms were E. faecalis at 18.5%, P. mirabilis at 9.3%, and K. pneumoniae at 7.4%. Among Caucasians, S. aureus at 20% was the most commonly cultured bacteria, followed by P. mirabilis at 15%, and then 10% each for E. faecalis, P. aeruginosa, and M. morganii. In the Indian population, the most frequently isolated organisms were P. aeruginosa at 18.2%, followed by E. faecalis at 13.6%, and 9% each for P. mirabilis, K. pneumoniae, and E. coli.
Outcomes were analysed based on the microbial profile of ulcers, monomicrobial vs. polymicrobial. Among patients who had MC&S results and had no amputations (8), 75% had polymicrobial while 25% had monomicrobial infections. Among those who underwent amputations (40), 65% had polymicrobial and 35% had monomicrobial infections. The difference in the rate of monomicrobial and polymicrobial infections in patients who had amputation was not statistically significant (p = 0.524).
4. Discussion
The increasing prevalence of DM combined with improved management has led to more people living longer and an elevated risk of complications, including DFS [
21]. The mean age of patients with DFS in the current study was 59 years, falling within the range reported in similar studies conducted in Kenya and the United States [
24,
25]. Notably, a higher proportion of patients were in the 45–64 years age group, aligning with the mean age in this study [
21]. The age of male patients was younger than of females. The earlier onset of complications in males may be attributed to multiple risk factors, including concurrent conditions such as hypertension, smoking, and poor glycaemic control, as well as bad healthcare-seeking behaviours [
21,
26]. However, these factors were not extensively examined in this study.
The prevalence of polymicrobial infections in DFS patients in this study was high, with 77% of cases presenting with infections due to more than one species of bacteria. The high incidence of polymicrobial infections could be due to the high prevalence of severe and deep infections at presentation [
26]. Higher rates of polymicrobial infections observed in our study are consistent with findings in other studies that reported on patients that had deep infections and complex infections [
26,
27,
28]. Close to 96% (95.8%) of patients in the study had wet gangrene. Furthermore, the rate of polymicrobial infections in the current study was higher in male compared to female patients. The observed differences in polymicrobial infections between men and women could have been due to variations in exposure to environmental pathogens and differences in health seeking behaviour, as well as cultural factors [
11,
17]. While external factors like pre-hospital care and hygiene practices might have influenced the diversity of organisms based on race, these were not studied.
The most isolated organism in the study was
E. faecalis, which differs from the findings from a meta-analysis conducted by McDonald et al., where
S. aureus was identified as the predominant isolate in diabetic foot infections [
29]. This variation may be due to patients in the quaternary hospital setting presenting at more advanced stages of the disease being more severely immunocompromised, which could allow unusual commensals like
E. faecalis to predominate [
20].
In our study, all isolated microbial species demonstrated resistance to at least three antimicrobial agents. Among the top three organisms, E. faecalis exhibited the lowest overall resistance to antimicrobial therapies, highlighting its comparatively more favorable susceptibility profile.
Notably, the resistance patterns of
E. faecalis in this study align with results from a previous study also conducted in South Africa by Shobo et al., which showed resistance to vancomycin and little resistance to penicillin [
30]. This study also showed that
Enterococcus spp. are also isolated in patients that require prolonged antibiotic therapies and can be mirrored to those with severe DFS, who often get more than one infection and require prolonged treatment. The data also indicate that combination therapy is more effective, with the resistance rate to ampicillin/amoxicillin being 8.3%. This is particularly significant given the propensity of
E. faecalis to develop resistance [
31]. No
S. aureus were resistant to macrolides, ceftriaxone, cefotaxime, and clindamycin. Resistance patterns between
S. aureus and
E. faecalis sometimes overlap [
32]. However, the sensitivity of
E. faecalis to macrolides and clindamycin was not specifically tested in the current study.
Gram-negative bacteria predominated over gram-positives in infections that presented to our tertiary hospital, which is in keeping with findings from LMICs in Asia countries with severe DFS [
20]. According to global data, diabetic foot sepsis infections are predominantly caused by gram-positive cocci such as
S. aureus, including MRSA) and
β-haemolytic Streptococcus spp. [
9,
10,
33]. Chronic and polymicrobial infections often involve anaerobic and gram-negative aerobic bacilli, including
P. aeruginosa and
Enterobacteriaceae [
33].
Intriguingly, a significant portion of patients in this study did not receive intravenous antibiotic prescriptions. Intravenous antibiotics antimicrobials were only used in patients with deep infections. This prescription trend is in keeping with a general approach to DFS using the WIFi classification and a multidisciplinary team [
34,
35,
36]. Most sepsis cases in the study were localized to the forefoot and midfoot, and the analysis did not include prophylactic antimicrobials. Some DFS cases were chronic wounds with significant biofilm, and thus were managed with local antiseptic measures and dressing, including negative pressure therapy [
37].
It is important to highlight that the top three organisms identified belong to distinct bacterial groups:
Enterococcus spp.,
Streptococcus spp., and
Enterobacteriaceae spp. Due to their varying resistance and susceptibility profiles, the use of a broad-spectrum antibiotics is recommended as an initial treatment option. Broad-spectrum antibiotics, which are effective against a wide range of gram-positive and gram-negative aerobes and anaerobes, have been shown to be efficacious against all three groups. Examples include carbapenems and combination antibiotics such as piperacillin-tazobactam and amoxicillin-clavulanate. However, while these agents are critical in initial management, their use should be restricted to empiric therapy. Prompt de-escalation to targeted antibiotics, guided by resistance profiles obtained through proper specimen collection, should be strongly advocated [
38]. This approach is crucial for preventing the emergence of multidrug-resistant organisms, as it ensures that more effective antibiotics can be utilized for improved source control [
38].
In our research, whether the microbial profile of DFS was monomicrobial or polymicrobial did not have an impact on the likelihood of amputation. This suggests that other factors might have been more influential in determining clinical outcomes in patients with diabetic foot ulcers and may be beyond the scope of this research or better extrapolated from a larger sample size, which would give a better idea of organism susceptibility and resistance patterns that could frame the outcomes better.
This retrospective study presents critical limitations that must be carefully weighed when interpreting the findings. Foremost, the quality and completeness of the data drawn from medical records, discharge summaries, and hospital files may have introduced variations and influenced result robustness. A significant limitation arises from the relatively modest sample size. This limitation raises concerns about the generalizability of the findings, potentially omitting a more comprehensive spectrum of diabetic foot sepsis cases. Furthermore, the non-standardized culture methods employed in the study introduce variability in specimen collection and analysis, potentially impacting result accuracy. This methodological variability may have led to the collection of superficial skin colonizers rather than the specific pathogens associated with diabetic foot sepsis. These limitations underscore the need for future research with more extensive and diverse patient populations, standardized culture methods, and prospective data collection to provide a more comprehensive understanding of diabetic foot sepsis.
5. Conclusions
This study provides valuable insights into the management of diabetic foot sepsis (DFS), highlighting several key findings. A notable gender difference was observed, with DFS being more common in males. Amoxicillin/clavulanic acid was the most frequently prescribed antimicrobial, despite over 47% of patients receiving antibiotics without prior specimen collection for MC&S. This underscores the need for more targeted and evidence-based treatment strategies to mitigate the risk of antimicrobial resistance. The microbiological analysis revealed E. faecalis and P. mirabilis as the most commonly isolated organisms, with more than 67% of the isolates exhibiting resistance to at least one antimicrobial agent. These findings stress the importance of routine specimen collection for MC&S to guide appropriate antibiotic therapy. Moreover, the prevalence of polymicrobial infections and high rates of resistance in this study highlight the need for improved stewardship in antimicrobial use, emphasizing de-escalation strategies after empiric therapy. There was no difference in the rate of amputation in patients with monomicrobial and polymicrobial infection. To better inform clinical practice, future prospective, multicenter studies, particularly in low- to middle-income settings, are essential. Such research can help develop region-specific guidelines and enhance the overall management of DFS, improving patient outcomes while minimizing the threat of multidrug-resistant organisms
Author Contributions
Conceptualization, S.P., E.J., C.G., O.M., A.S., W.N., G.M. and T.E.L.; methodology, S.P., E.J., C.G., O.M., A.S., W.N., G.M. and T.E.L.; formal analysis, S.P., E.J., C.G., O.M., A.S., W.N., G.M., M.S.M. and T.E.L.; investigation, S.P., E.J., C.G., O.M., A.S., W.N., G.M. and T.E.L.; resources, S.P., E.J., C.G., O.M., A.S., W.N., G.M. and T.E.L.; data curation, S.P., M.S.M. and T.E.L.; writing—original draft preparation, S.P., M.S.M. and T.E.L.; writing—review and editing, S.P., E.J., C.G., O.M., A.S., W.N., G.M., M.S.M. and T.E.L.; supervision, T.E.L.; project administration, S.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki. Ethical clearance was obtained from the Human Research Ethics Committee (Medical) of University of the Witwatersrand (M210943 approved on 14 February 2022).
Informed Consent Statement
Patient consent was waived as the study was retrospective and was based on review of already collected records.
Data Availability Statement
Data supporting results in this study will be made available on request.
Acknowledgments
We sincerely appreciate the valuable inputs from the assessors of the Undergraduate Students Research at the Unit of Undergraduate Medical Education of University of the Witwatersrand during protocol development and presentation of the results.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Kamerman, P. Underdiagnosis of hypertension and diabetes mellitus in South Africa. S. Afr. Med. J. 2022, 112, 53–60. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Maida, C.; Pinto, A. Diabetic foot syndrome: Immune-inflammatory features as possible cardiovascular markers in diabetes. World J. Orthop. 2015, 6, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Carro, G.V.; Saurral, R.; Saguez, F.S.; Witman, E.L. Diabetic foot infections: Bacterial isolates from the centers and hospitals of Latin American countries. Int. J. Low. Extrem. Wounds 2020, 21, 562–573. [Google Scholar] [CrossRef] [PubMed]
- Rossboth, S.; Lechleitner, M.; Oberaigner, W. Risk factors for diabetic foot complications in type 2 diabetes-A Systematic review. Endocrinol. Diabetes Metab. 2020, 4, e00175. [Google Scholar] [CrossRef]
- Yu, M.; Lyeles, C.R.; Bent-Shaw, L.A.; Young, B.A. Sex disparities in diabetes process of care measures and self-care in high risk patients. J. Diabetes Res. 2013, 2013, 575814. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lu, J.; Jing, Y.; Tang, S.; Zhu, D.; Bi, Y. Global epidemiology of diabetic foot ulceration: A systematic review and meta-analysis. Ann. Med. 2017, 49, 106–116. [Google Scholar] [CrossRef]
- Abbas, Z.; Gill, G.; Archibald, L. The epidemiology of diabetic limb sepsis: An African perspective. Diabet. Med. 2002, 19, 895–899. [Google Scholar] [CrossRef]
- Hicks, C.; Selvarajah, S.; Mathioudakis, N.; Sherman, R.; Hines, K.; Black, J.; Abularrage, C. Burden of Infected Diabetic Foot Ulcers on Hospital Admissions and Costs. Ann. Vasc. Surg. 2016, 33, 149–158. [Google Scholar] [CrossRef]
- Spichler, A.; Hurwitz, B.; Armstrong, D.; Lipsky, B. Microbiology of diabetic foot infections: From Louis Pasteur to ‘crime scene investigation’. BMC Med. 2015, 13, 2. [Google Scholar] [CrossRef]
- Richard, J.; Lavigne, J.; Sotto, A. Diabetes and foot infection: More than double trouble. Diabetes Metab. Res. Rev. 2012, 28, 46–53. [Google Scholar] [CrossRef]
- Lipsky, B.; Berendt, A.; Cornia, P.; Pile, J.; Peters, E.; Armstrong, D.; Deery, G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic Foot Infections. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef] [PubMed]
- Law, T.; Chibabhai, V.; Nana, T. Analysis and comparison of cumulative antibiograms for the Charlotte Maxeke Johannesburg Academic Hospital adult intensive care and high-care units, 2013 and 2017. S. Afr. Med. J. 2019, 110, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Mogre, V.; Johnson, N.A.; Tzelepis, F.; Paul, C. Barriers to diabetic self-care: A qualitative study of patients’ and healthcare providers’ perspectives. J. Clin. Nurs. 2019, 28, 2296–2308. [Google Scholar] [CrossRef] [PubMed]
- Wada, F.W.; Mekonnen, M.F.; Sawiso, E.D.; Kolato, S.; Woldegiorgis, L.; Kera, G.K.; El-Khatib, Z.; Ashuro, A.A.; Biru, M.; Boltena, M.T. Bacterial profile and antimicrobial resistance patterns of infected diabetic foot ulcers in sub-Saharan africa: A systematic review and meta-analysis. Sci. Rep. 2023, 13, 14655. [Google Scholar] [CrossRef] [PubMed]
- Goedecke, J.H.; Mendham, A.E. Pathophysiology of type 2 diabetes in sub-Saharan Africans. Diabetologia 2022, 65, 1967–1980. [Google Scholar] [CrossRef]
- Nicolau, D.; Stein, G. Therapeutic Options for Diabetic Foot Infections; a review with emphasis on tissue penetration characteristics. J. Am. Podiatr. Med. Assoc. 2010, 100, 52–63. [Google Scholar] [CrossRef]
- Lipsky, B. Diabetic Foot Infections: Microbiology Made Modern? Array of hope. Diabetes Care 2007, 30, 2171–2172. [Google Scholar] [CrossRef][Green Version]
- Bhardwaj, S.; Mehra, P.; Dhanjal, D.S.; Sharma, P.; Sharma, V.; Singh, R.; Nepovimova, E.; Chopra, C.; Kuca, K. Antibiotics and antibiotics resistance-Flip sides of the same coin. Curr. Pharm. Des. 2022, 28, 2312–2329. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Raudonis, R.; Glick, B.R.; Lin, T.J.; Cheng, Z. Antibiotic resistance in Pseudomonas aeruginosa: Mechanism and alternative therapeutic strategies. Biotechnol. Adv. 2019, 37, 177–192. [Google Scholar] [CrossRef]
- Sultana, R.; Ahmed, I.; Saima, S.; Salam, M.T.; Sultana, S. Diabetic foot ulcer-a systematic review on relevant microbial etiology and antibiotic resistance in Asian countries. Diabetes Metab. Syndr. Clin. Res. Rev. 2023, 17, 102783. [Google Scholar] [CrossRef]
- Gregg, E.W.; Sattar, N.; Ali, M.K. The changing face of diabetes complications. Lancet Diabetes Endocrinol. 2016, 4, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Laakso, M.; Kiiski, J.; Karppelin, M.; Helminen, M.; Kaartinen, I. Pathogens causing diabetic foot infection and the reliability of the superficial culture. Surg. Infect. 2021, 22, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Nyamu, P.N.; Otieno, C.F.; Amayo, E.O.; McLigeyo, S.O. Risk factors and prevalence of diabetic foot ulcers at Kenyatta National Hospital, Nairobi. East. Afr. Med. J. 2003, 80, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Ghotaslou, R.; Memar, M.Y.; Alizadeh, N. Classification, microbiology and treatment of diabetic foot infections. J. Wound Care 2018, 27, 434–441. [Google Scholar] [CrossRef]
- McNeely, M.J.; Boyko, E.J.; Ahroni, J.H.; Stensel, V.L.; Reiber, G.E.; Smith, D.G.; Pecoraro, R.F. The independent contributions of diabetic neuropathy and vasculopathy in foot ulceration: How great are the risks? Diabetes Care 1995, 18, 216–219. [Google Scholar] [CrossRef]
- Henig, O.; Pogue, J.M.; Cha, R.; Kilgore, P.E.; Hayat, U.; Ja’ara, M.; Ali, R.M.; Mahboob, S.; Pansare, R.; Deeds, K.; et al. Epidemiology of Diabetic Foot Infection in the Metro-Detroit Area With a Focus on Independent Predictors for Pathogens Resistant to Recommended Empiric Antimicrobial Therapy. Open Forum. Infect. Dis. 2018, 5, ofy245. [Google Scholar] [CrossRef]
- Usman, Y.; Bakari, A.; Abdullahi, I.N.; Ahmad, A.; Sani-Bello, F.; Sagay, A.; Olayinka, A.T. Phenotypic profile and antibiogram of biofilm-producing bacteria isolates from diabetic foot ulcers in Zaria, Nigeria. Niger. Postgrad. Med. J. 2021, 28, 233–239. [Google Scholar] [CrossRef]
- Sasikumar, K.; Vijayakumar, C.; Jagdish, S.; Kadambari, D.; Kumar, N.R.; Biswas, R.; Parija, S.C. Clinico-microbiological profile of septic diabetic foot with special reference to anaerobic infection. Cureus 2018, 10, e2252. [Google Scholar] [CrossRef]
- Macdonald, K.E.; Boeckh, S.; Stacey, H.J.; Jones, J.D. The microbiology of diabetic foot infections: A meta-analysis. BMC Infect. Dis. 2021, 21, 770. [Google Scholar] [CrossRef]
- Shobo, C.O.; Essack, S.Y.; Bester, L.A. Enterococcal contamination of hospital environment in Kwazulu-Natal South Africa. J. Appl. Microbiol. 2022, 132, 654–664. [Google Scholar] [CrossRef]
- Garcia-Solache, M.; Rice, L.B. The Enterococcus: A Model of Adaptability to Its Environment. Clin. Microbiol. Rev. 2019, 32, e00058. [Google Scholar] [CrossRef] [PubMed]
- Osei Sekyere, J.; Mensah, E. Molecular epidemiology and mechanisms of antibiotic resistance in Enterococcus spp., Staphylococcus spp., and Streptococcus spp. in Africa: A systematic review from a one health perspective. Ann. N. Y. Acad. Sci. 2019, 1465, 29–58. [Google Scholar] [CrossRef] [PubMed]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; de Franciscis, S. Chronic wound infections: The role of pseudomonas aeruginosa and Staphylococcus aureus. Expert. Rev. Anti Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Darling, J.D.; McCallum, J.C.; Soden, P.A.; Guzman, R.J.; Wyers, M.C.; Hamdan, A.D.; Verhagen, H.J.; Schermerhorn, M.L. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system after first-time lower extremity revascularization. J. Vasc. Surg. 2017, 65, 695–704. [Google Scholar] [CrossRef]
- Cull, D.L.; Manos, G.; Hartley, M.C.; Taylor, S.M.; Langan, E.M.; Eidt, J.F.; Johnson, B.L. An early validation of the Society for Vascular Surgery Lower Extremity Threatened Limb Classification System. J. Vasc. Surg. 2014, 60, 1535–1541. [Google Scholar] [CrossRef]
- Darling, J.D.; McCallum, J.C.; Soden, P.A.; Meng, Y.; Wyers, M.C.; Hamdan, A.D.; Verhagen, H.J.; Schermerhorn, M.L. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) classification system following infrapopliteal endovascular interventions for critical limb ischaemia. J. Vasc. Surg. 2016, 64, 616–622. [Google Scholar] [CrossRef]
- Snyder, R.J.; Bohn, G.; Hanft, J.; Harkless, L.; Kim, P.; Lavery, L.; Schultz, G.; Wolcott, R. Wound biofilm: Current perspectives and strategies on Biofilm disruption and treatment. Wounds 2017, 29, S1–S17. [Google Scholar]
- Kollef, M.H.; Shorr, A.F.; Bassetti, M.; Timsit, J.-F.; Micek, S.T.; Michelson, A.P.; Garnacho-Montero, J. Timing of antibiotic therapy in the ICU. Crit. Care 2021, 25, 360. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).