Predicting Adverse Neurodevelopmental Outcomes in Premature Neonates with Intrauterine Growth Restriction Using a Three-Layered Neural Network
Abstract
1. Introduction
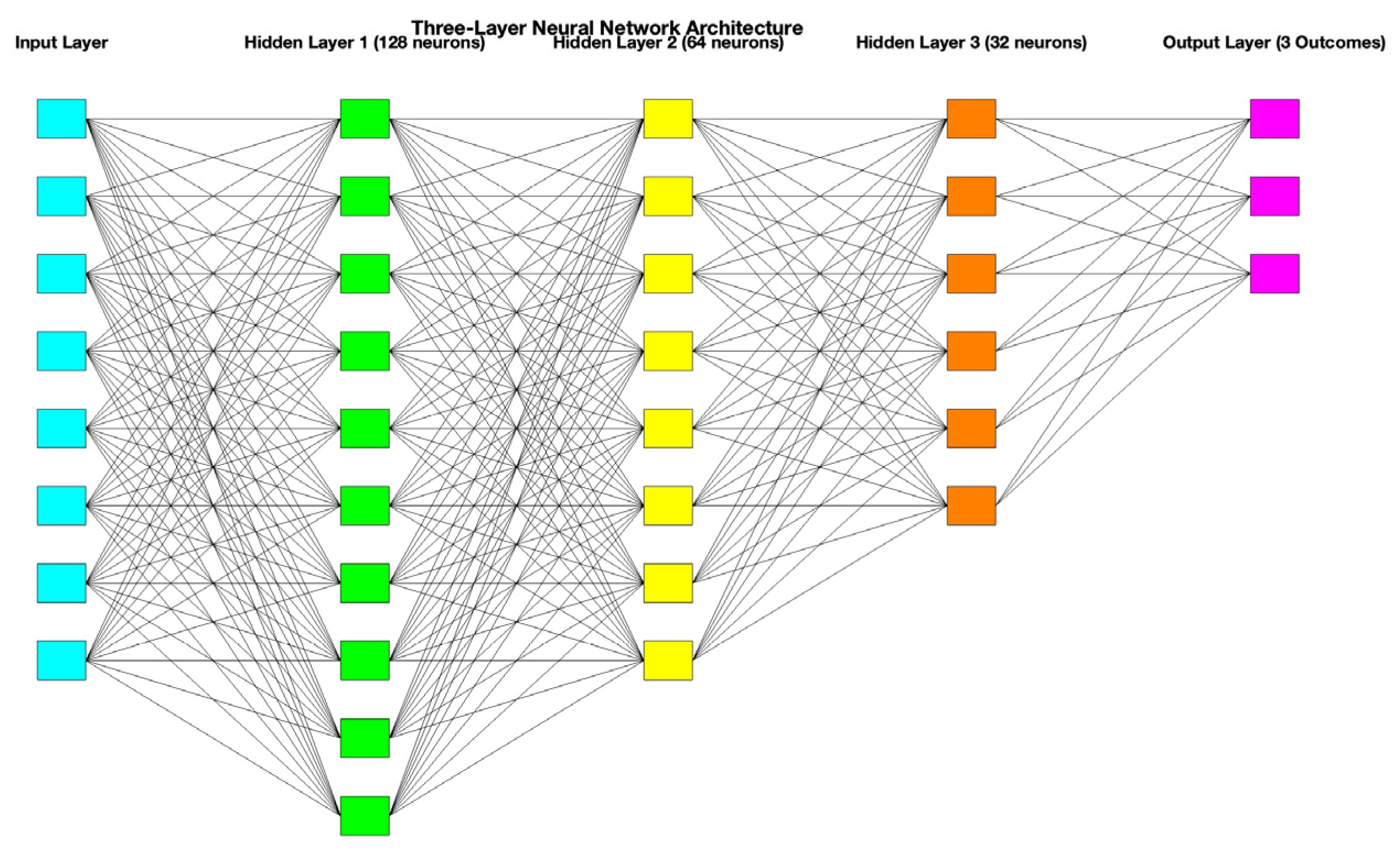
2. Materials and Methods
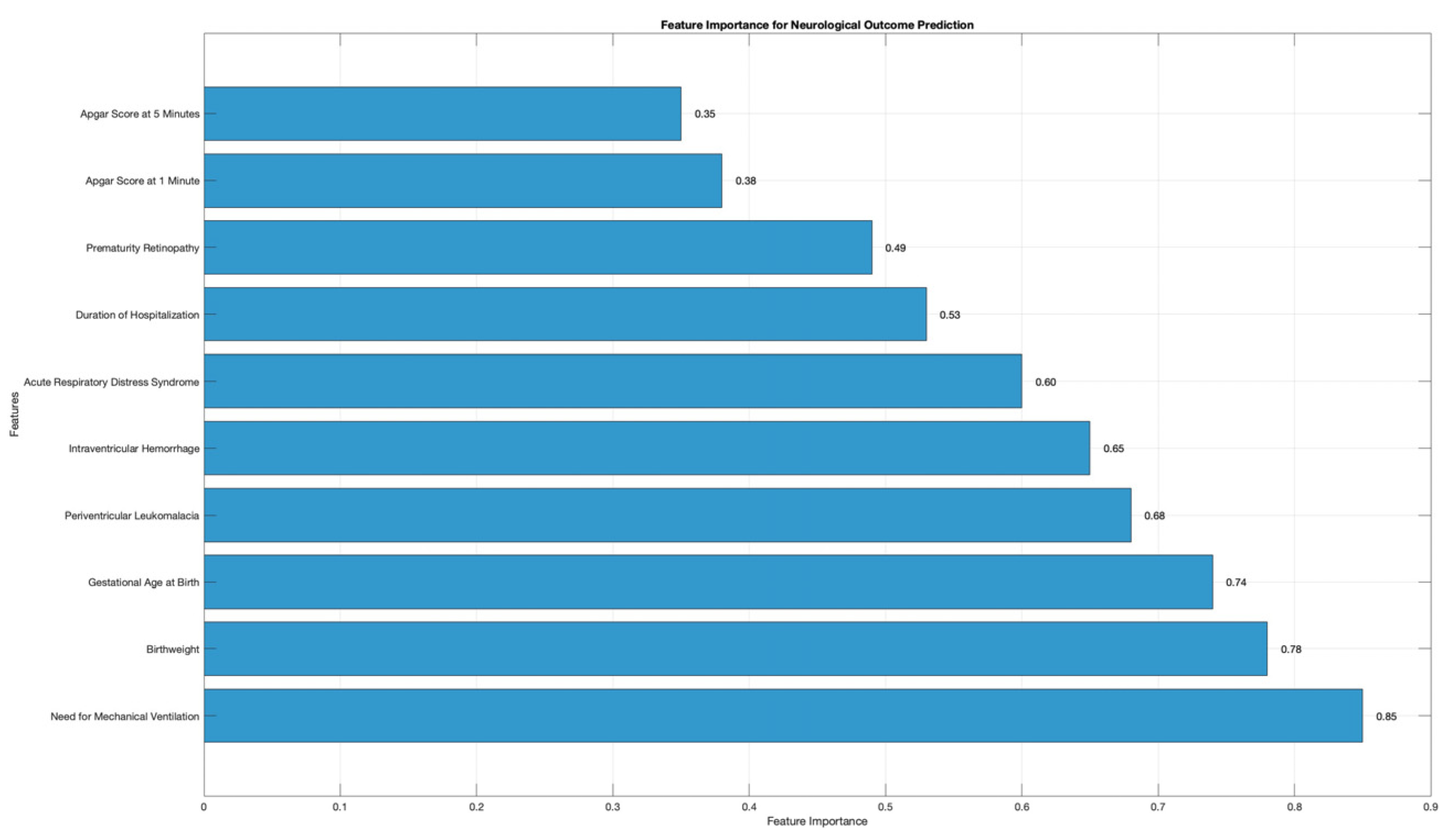
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gaccioli, F.; Aye, I.; Sovio, U.; Charnock-Jones, D.S.; Smith, G.C.S. Screening for fetal growth restriction using fetal biometry combined with maternal biomarkers. Am. J. Obstet. Gynecol. 2018, 218, S725–S737. [Google Scholar] [CrossRef]
- Kesavan, K.; Devaskar, S.U. Intrauterine Growth Restriction: Postnatal Monitoring and Outcomes. Pediatr. Clin. N. Am. 2019, 66, 403–423. [Google Scholar] [CrossRef]
- Sacchi, C.; Marino, C.; Nosarti, C.; Vieno, A.; Visentin, S.; Simonelli, A. Association of Intrauterine Growth Restriction and Small for Gestational Age Status With Childhood Cognitive Outcomes: A Systematic Review and Meta-analysis. JAMA Pediatr. 2020, 174, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Mierzynski, R.; Dluski, D.; Darmochwal-Kolarz, D.; Poniedziałek-Czajkowska, E.; Leszczynska-Gorzelak, B.; Kimber-Trojnar, Z.; Agnieszka, W.; Oleszczuk, J. Intra-uterine Growth Retardation as a Risk Factor of Postnatal Metabolic Disorders. Curr. Pharm. Biotechnol. 2016, 17, 587–596. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Luo, K.; Chen, P. Mechanisms Underlying Neurologic Injury in Intrauterine Growth Restriction. J. Child. Neurol. 2021, 36, 776–784. [Google Scholar] [CrossRef]
- Monteith, C.; Flood, K.; Pinnamaneni, R.; Levine, T.A.; Alderdice, F.A.; Unterscheider, J.; McAuliffe, F.M.; Dicker, P.; Tully, E.C.; Malone, F.D.; et al. An abnormal cerebroplacental ratio (CPR) is predictive of early childhood delayed neurodevelopment in the setting of fetal growth restriction. Am. J. Obstet. Gynecol. 2019, 221, 273.e271–273.e279. [Google Scholar] [CrossRef]
- Albers, C.A.; Grieve, A.J. Test Review: Bayley, N. Bayley Scales of Infant and Toddler Development–Third Edition. San Antonio, TX: Harcourt Assessment. J. Psychoeduc. Assess. 2007, 25, 180–190. [Google Scholar] [CrossRef]
- Mendonça, B.; Sargent, B.; Fetters, L. Cross-cultural validity of standardized motor development screening and assessment tools: A systematic review. Dev. Med. Child. Neurol. 2016, 58, 1213–1222. [Google Scholar] [CrossRef] [PubMed]
- McLester-Davis, L.W.Y.; Shankar, A.; Kataria, L.A.; Hidalgo, A.G.; van Eer, E.D.; Koendjbiharie, A.P.; Ramjatan, R.; Hatch, V.I.; Middleton, M.A.; Zijlmans, C.W.R.; et al. Validity, reliability, and transcultural adaptations of the Bayley Scales of Infant and Toddler Development (BSID-III-NL) for children in Suriname. Early Hum. Dev. 2021, 160, 105416. [Google Scholar] [CrossRef]
- McHenry, M.S.; Oyungu, E.; Yang, Z.; Hines, A.C.; Ombitsa, A.R.; Vreeman, R.C.; Abubakar, A.; Monahan, P.O. Cultural adaptation of the Bayley Scales of Infant and Toddler Development, 3rd Edition for use in Kenyan children aged 18–36 months: A psychometric study. Res. Dev. Disabil. 2021, 110, 103837. [Google Scholar] [CrossRef]
- Gardella, B.; Dominoni, M.; Caporali, C.; Cesari, S.; Fiandrino, G.; Longo, S.; De Vito, G.B.; Naboni, C.; Tonduti, D.; Perotti, G.; et al. Placental features of fetal vascular malperfusion and infant neurodevelopmental outcomes at 2 years of age in severe fetal growth restriction. Am. J. Obstet. Gynecol. 2021, 225, 413.e411. [Google Scholar] [CrossRef]
- Leite, D.F.B.; Cecatti, J.G. Fetal Growth Restriction Prediction: How to Move beyond. Sci. World J. 2019, 2019, 1519048. [Google Scholar] [CrossRef]
- Leavitt, K.; Odibo, L.; Nwosu, O.; Odibo, A.O. Comparing the cerebro-placental to umbilico-cerebral Doppler ratios for the prediction of adverse neonatal outcomes in pregnancies complicated by fetal growth restriction. J. Matern. Fetal Neonatal Med. 2022, 35, 5904–5908. [Google Scholar] [CrossRef] [PubMed]
- Monaghan, C.; Kalafat, E.; Binder, J.; Thilaganathan, B.; Khalil, A. Prediction of adverse pregnancy outcome in monochorionic diamniotic twin pregnancy complicated by selective fetal growth restriction. Ultrasound Obstet. Gynecol. 2019, 53, 200–207. [Google Scholar] [CrossRef]
- Harabor, V.; Mogos, R.; Nechita, A.; Adam, A.-M.; Adam, G.; Melinte-Popescu, A.-S.; Melinte-Popescu, M.; Stuparu-Cretu, M.; Vasilache, I.-A.; Mihalceanu, E. Machine Learning Approaches for the Prediction of Hepatitis B and C Seropositivity. Int. J. Environ. Res. Public Health 2023, 20, 2380. [Google Scholar] [CrossRef] [PubMed]
- Melinte-Popescu, M.; Vasilache, I.A.; Socolov, D.; Melinte-Popescu, A.S. Prediction of HELLP Syndrome Severity Using Machine Learning Algorithms-Results from a Retrospective Study. Diagnostics 2023, 13, 287. [Google Scholar] [CrossRef] [PubMed]
- Vicoveanu, P.; Vasilache, I.A.; Scripcariu, I.S.; Nemescu, D.; Carauleanu, A.; Vicoveanu, D.; Covali, A.R.; Filip, C.; Socolov, D. Use of a Feed-Forward Back Propagation Network for the Prediction of Small for Gestational Age Newborns in a Cohort of Pregnant Patients with Thrombophilia. Diagnostics 2022, 12, 1009. [Google Scholar] [CrossRef] [PubMed]
- Amiel-Tison, C. Update of the Amiel-Tison neurologic assessment for the term neonate or at 40 weeks corrected age. Pediatr. Neurol. 2002, 27, 196–212. [Google Scholar] [CrossRef]
- Villar, J.; Cavoretto, P.I.; Barros, F.C.; Romero, R.; Papageorghiou, A.T.; Kennedy, S.H. Etiologically Based Functional Taxonomy of the Preterm Birth Syndrome. Clin Perinatol 2024, 51, 475–495. [Google Scholar] [CrossRef]
- Morales-Prieto, D.M.; Fuentes-Zacarías, P.; Murrieta-Coxca, J.M.; Gutierrez-Samudio, R.N.; Favaro, R.R.; Fitzgerald, J.S.; Markert, U.R. Smoking for two- effects of tobacco consumption on placenta. Mol. Asp. Med. 2022, 87, 101023. [Google Scholar] [CrossRef]
- Härkönen, J.; Lindberg, M.; Karlsson, L.; Karlsson, H.; Scheinin, N.M. Education is the strongest socio-economic predictor of smoking in pregnancy. Addiction 2018, 113, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Lange, S.; Probst, C.; Rehm, J.; Popova, S. National, regional, and global prevalence of smoking during pregnancy in the general population: A systematic review and meta-analysis. Lancet Glob. Health 2018, 6, e769–e776. [Google Scholar] [CrossRef]
- Cornish, E.F.; McDonnell, T.; Williams, D.J. Chronic Inflammatory Placental Disorders Associated With Recurrent Adverse Pregnancy Outcome. Front. Immunol. 2022, 13, 825075. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Groom, K.M.; Oyston, C.; Chamley, L.W.; Clark, A.R.; James, J.L. The placenta in fetal growth restriction: What is going wrong? Placenta 2020, 96, 10–18. [Google Scholar] [CrossRef]
- Miglioli, C.; Canini, M.; Vignotto, E.; Pecco, N.; Pozzoni, M.; Victoria-Feser, M.P.; Guerrier, S.; Candiani, M.; Falini, A.; Baldoli, C.; et al. The maternal-fetal neurodevelopmental groundings of preterm birth risk. Heliyon 2024, 10, e28825. [Google Scholar] [CrossRef]
- Chu, A.; Dhindsa, Y.; Sim, M.S.; Altendahl, M.; Tsui, I. Prenatal intrauterine growth restriction and risk of retinopathy of prematurity. Sci. Rep. 2020, 10, 17591. [Google Scholar] [CrossRef]
- Misan, N.; Michalak, S.; Kapska, K.; Osztynowicz, K.; Ropacka-Lesiak, M. Blood-Brain Barrier Disintegration in Growth-Restricted Fetuses with Brain Sparing Effect. Int. J. Mol. Sci. 2022, 23, 12349. [Google Scholar] [CrossRef] [PubMed]
- Khazardoost, S.; Ghotbizadeh, F.; Sahebdel, B.; Nasiri Amiri, F.; Shafaat, M.; Akbarian-Rad, Z.; Pahlavan, Z. Predictors of Cranial Ultrasound Abnormalities in Intrauterine Growth-Restricted Fetuses Born between 28 and 34 Weeks of Gestation: A Prospective Cohort Study. Fetal Diagn. Ther. 2019, 45, 238–247. [Google Scholar] [CrossRef]
- Kodric, J.; Sustersic, B.; Paro-Panjan, D. Psychosocial functioning in adolescents: Results according to Amiel-Tison neurological assessment in a group of preterm infants. Dev. Neurorehabil. 2019, 22, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.T.; Hsieh, W.S.; Hsu, C.H.; Chen, L.C.; Lee, W.T.; Chiu, N.C.; Wu, Y.C.; Jeng, S.F. A psychometric study of the Bayley Scales of Infant and Toddler Development–3rd Edition for term and preterm Taiwanese infants. Res. Dev. Disabil. 2013, 34, 3875–3883. [Google Scholar] [CrossRef] [PubMed]
- Ballot, D.E.; Ramdin, T.; Rakotsoane, D.; Agaba, F.; Davies, V.A.; Chirwa, T.; Cooper, P.A. Use of the Bayley Scales of Infant and Toddler Development, Third Edition, to Assess Developmental Outcome in Infants and Young Children in an Urban Setting in South Africa. Int. Sch. Res. Not. 2017, 2017, 1631760. [Google Scholar] [CrossRef] [PubMed]
- Salah El-Din, E.M.; Monir, Z.M.; Shehata, M.A.; Abouelnaga, M.W.; Abushady, M.M.; Youssef, M.M.; Megahed, H.S.; Salem, S.M.E.; Metwally, A.M. A comparison of the performance of normal middle social class Egyptian infants and toddlers with the reference norms of the Bayley Scales—Third edition (Bayley III): A pilot study. PLoS ONE 2021, 16, e0260138. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristics | IUGR Group (n = 27 Patients) | Control Group (n = 179 Patients) | p Value |
|---|---|---|---|
| Age, years (mean ± SD) | 24.15 ± 4.36 | 26.16 ± 3.28 | 0.34 |
| BMI, kg/m2, (mean and standard deviation) | 23.36 ± 2.61 | 23.48 ± 3.18 | 0.76 |
| Level of education (n/%) | Primary school (≤4 years of study)-2 (7.4%) Pre-high school (5–8 years of study)-7 (25.93%) High-school (9–12 years of study)-12 (44.44%) ≥Bachelor degree-6 (22.22%) | Primary school (≤4 years of study)-20 (11.7%) Pre-high school (5–8 years of study)-62 (34.64%) High-school (9–12 years of study)-63 (35.20%) ≥Bachelor degree-34 (18.99%) | 0.67 |
| Smoking habit (n/%) | Yes =11 (40.7%) | Yes = 16 (8.93%) | <0.001 |
| Vaginal infections (n/%) | Yes = 3 (11.11%) | Yes = 41 (22.9%) | 0.16 |
| Chorioamnionitis (n/%) | Yes = 1 (3.7%) | Yes = 9 (5.02%) | 0.76 |
| Prolonged rupture of membranes (n/%) | Yes = 1 (3.7%) | Yes = 10 (5.58%) | 0.68 |
| Diabetes (n/%) | Yes = 2 (7.4%) | Yes = 5 (2.79%) | 0.21 |
| Preeclampsia (n/%) | Yes = 15 (55.55%) | Yes = 14 (7.8%) | < 0.001 |
| Abruptio placentae (n/%) | Yes = 2 (7.4%) | Yes = 4 (2.23%) | 0.13 |
| HELLP (Hemolysis, Elevated Liver enzymes, and Low Platelets) syndrome (n/%) | Yes = 1 (3.7%) | Yes = 3 (1.67%) | 0.47 |
| Maternal thrombophilia (n/%) | Yes = 1 (3.7%) | Yes = 5 (2.79%) | 0.79 |
| History of adverse pregnancy outcomes (n/%) | Yes = 5 (18.5%) | Yes = 10 (5.58%) | 0.01 |
| Neonatal Outcome | IUGR Group (n = 27 Patients) | Control Group (n = 179 Patients) | p Value |
|---|---|---|---|
| Gestational age at birth, weeks (median and IQR) | 31 (30–32) | 30 (28–32) | 0.06 |
| Birthweight, g (median and IQR) | 1300 (1050–1400) | 1400 (1020–1750) | 0.04 |
| Apgar score at 1 min (median and IQR) | 5 (4–7) | 5.5 (4–7) | 0.92 |
| Apgar score at 5 min (median and IQR) | 7 (5–7) | 7 (5–7) | 0.95 |
| ARDS (n/%) | Yes = 24 (88.8%) | Yes = 170 (94.97%) | 0.20 |
| Need for mechanical ventilation (n/%) | Yes = 16 (59.25%) | Yes = 53 (29.6%) | 0.005 |
| ROP (n/%) | Stage I-2 (7.41%) Stage II-1 (3.7%) Stage III-0 (0%) | Stage I-17 (9.5%) Stage II-17 (9.5%) Stage III-1 (0.55%) | 0.35 |
| IVF (n/%) | Grade I-5 (18.52%) Grade II-4 (14.81%) Grade III-0 (0%) Grade IV-1 (3.7%) | Grade I-29 (16.20%) Grade II-17 (9.5%) Grade III-4 (2.23%) Grade IV-0 (0%) | 0.21 |
| PVL (n/%) | Grade I-2 (7.41%) Grade II-1 (3.7%) Grade III-1 (3.7%) Grade IV-1 (3.7%) | Grade I-6 (16.20%) Grade II-0 (0%) Grade III-1 (0.55%) Grade IV-0 (0%) | 0.06 |
| Duration of hospitalization, days (mean ± SD) | 46.25 ± 20.30 | 49.77 ± 29.30 | 0.27 |
| Neonatal Outcome | IUGR Group (n = 27 Patients) | Control Group (n = 179 Patients) | p Value |
|---|---|---|---|
| Amiel Tison scale at discharge (n/%) | Mild-6 (22.22%) Moderate-16 (59.25%) Severe-5 (18.51%) | Mild-34 (18.99%) Moderate-111 (14.81%) Severe-34 (62.01%) | 0.92 |
| Bailey-III scale evaluation at 24 months considering CC score | Mild-4 (14.81%) Moderate-2 (3.7%) Severe-1 (3.7%) | Mild-20 (11.17%) Moderate-14 (7.82%) Severe-1 (0.55%) | 0.42 |
| Bailey-III scale evaluation at 24 months considering LC score | Mild-1 (3.7%) Moderate-4 (14.81%) Severe-3 (11.11%) | Mild-5 (2.79%) Moderate-54 (30.16%) Severe-26 (14.52%) | 0.30 |
| Bailey-III scale evaluation at 24 months considering MC score | Mild-7 (25.92%) Moderate-3 (11.11%) Severe-1 (3.7%) | Mild-32 (17.87%) Moderate-13 (7.26%) Severe-2 (1.11%) | 0.38 |
| Bailey-III scale evaluation at 24 months considering mixed delays | Mild-1 (3.7%) Moderate-1 (3.7%) Severe-0 (0%) | Mild-7 (3.91%) Moderate-5 (2.79%) Severe-0 (0%) | 0.47 |
| Neurodevelopmental Outcome | Grade | Se (%) | Sp (%) | FPR (%) | Matthews Coefficient | Accuracy (%) | Precision | F1 Score | MeanSe | MeanSp | MeanAcc |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cognitive delay | Mild | 75 | 66.6 | 33.3 | 0.41 | 71.4 | 0.75 | 0.75 | 79.89 | 77.18 | 80.54 |
| Moderate | 66.6 | 94 | 5 | 0.73 | 85.7 | 0.85 | 0.73 | 84.55 | 81.42 | 85.29 | |
| Severe | 50 | 100 | 0 | 0.63 | 83.3 | 1 | 0.66 | 62.76 | 80.51 | 73.30 | |
| Motor delay | Mild | 75 | 90 | 10 | 0.66 | 83.3 | 0.85 | 0.8 | 85.03 | 82.14 | 85.72 |
| Moderate | 40 | 88.8 | 11 | 0.33 | 71.4 | 0.66 | 0.5 | 68.06 | 66.36 | 68.46 | |
| Severe | 20 | 87.5 | 12.5 | 0.10 | 61.5 | 0.5 | 0.28 | 57.73 | 55.94 | 58.16 | |
| Language delay | Mild | 62.3 | 87.5 | 12.5 | 0.37 | 80 | 0.5 | 0.5 | 78.50 | 76.07 | 79.08 |
| Moderate | 60 | 85.7 | 14.2 | 0.47 | 75 | 0.75 | 0.66 | 74.80 | 73.06 | 75.95 | |
| Severe | 40 | 83.3 | 16.6 | 0.26 | 63.6 | 0.66 | 0.5 | 63.85 | 61.87 | 64.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bivoleanu, A.; Gheorghe, L.; Doroftei, B.; Scripcariu, I.-S.; Vasilache, I.-A.; Harabor, V.; Adam, A.-M.; Adam, G.; Munteanu, I.V.; Susanu, C.; et al. Predicting Adverse Neurodevelopmental Outcomes in Premature Neonates with Intrauterine Growth Restriction Using a Three-Layered Neural Network. Diagnostics 2025, 15, 111. https://doi.org/10.3390/diagnostics15010111
Bivoleanu A, Gheorghe L, Doroftei B, Scripcariu I-S, Vasilache I-A, Harabor V, Adam A-M, Adam G, Munteanu IV, Susanu C, et al. Predicting Adverse Neurodevelopmental Outcomes in Premature Neonates with Intrauterine Growth Restriction Using a Three-Layered Neural Network. Diagnostics. 2025; 15(1):111. https://doi.org/10.3390/diagnostics15010111
Chicago/Turabian StyleBivoleanu, Anca, Liliana Gheorghe, Bogdan Doroftei, Ioana-Sadiye Scripcariu, Ingrid-Andrada Vasilache, Valeriu Harabor, Ana-Maria Adam, Gigi Adam, Iulian Valentin Munteanu, Carolina Susanu, and et al. 2025. "Predicting Adverse Neurodevelopmental Outcomes in Premature Neonates with Intrauterine Growth Restriction Using a Three-Layered Neural Network" Diagnostics 15, no. 1: 111. https://doi.org/10.3390/diagnostics15010111
APA StyleBivoleanu, A., Gheorghe, L., Doroftei, B., Scripcariu, I.-S., Vasilache, I.-A., Harabor, V., Adam, A.-M., Adam, G., Munteanu, I. V., Susanu, C., Solomon-Condriuc, I., & Harabor, A. (2025). Predicting Adverse Neurodevelopmental Outcomes in Premature Neonates with Intrauterine Growth Restriction Using a Three-Layered Neural Network. Diagnostics, 15(1), 111. https://doi.org/10.3390/diagnostics15010111







