Abstract
Bone metastasis has been reported in up to 70% of patients with advanced breast cancer. A total of 55.76% of skeletal metastases in women were derived from breast cancer. However, patients with bone metastasis from an occult primary breast cancer are a rare subset of patients. Here, we present the case of a 38-year-old woman who had sternum pain for 4 months. A whole-body PET-CT scan revealed that the FDG uptake of both the sternum and internal mammary node was significantly increased. The final diagnosis of occult breast cancer was established by immunohistochemical (IHC) staining, which is of great significance for identifying the origin of a metastatic tumor despite no visualized lesions of mammary glands.
Occult breast cancer (OBC) is defined as an undetectable primary breast tumor presenting with clinically recognizable metastatic cancer [1]. It accounts for 0.3–1% of all breast cancers [2], and axillary and cervical lymph node metastasis is the common first clinical presentation [3]. Bone metastasis has been reported in up to 70% of patients with advanced breast cancer [4]. However, patients with bone metastasis from an occult primary breast cancer are a rare subset of patients, especially those with sternum metastasis [5]. Here, we present the case of a 38-year-old woman who had sternum pain for 4 months.
The patient also had an occasional cough. Physical examination revealed normal vital signs and tenderness in the sternum. A plain CT of her chest showed bone destruction at the level of sternal notches 2–4, with soft tissue mass (Figure 1A,B). A subsequent whole-body PET-CT scan revealed that the FDG uptake of the sternum and internal mammary node was significantly increased and other lesions with abnormal FDG uptake were not found (Figure 1C,D). To rule out multiple myeloma, serum-free light chain analysis displayed that serum-free light chain kappa was slightly increased (concentration 23.3 mg/L). Urine protein electrophoresis demonstrated a slight increase in urine protein (0.16 g/24 h). The other results of serum and urine protein electrophoresis and immunofixation, serum β2 microglobulin and serum concentration of common tumor markers were normal. In order to further determine the characteristics of her sternum lesions, sternum biopsy was performed. The histopathology showed that the tumor cells were infiltrated in the form of nests and cords in the bone tissue and skeletal muscle.
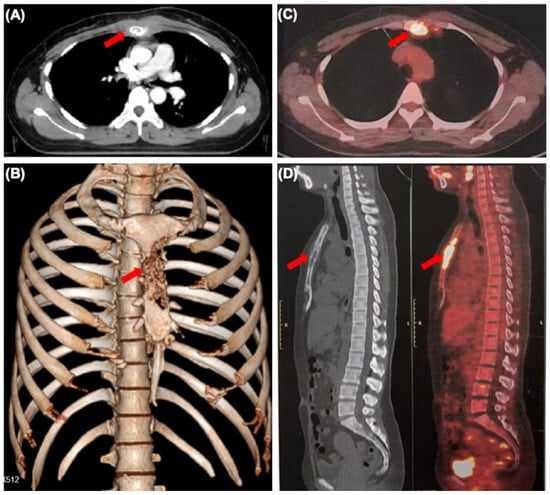
Figure 1.
Sternum metastasis with soft tissue mass from occult breast cancer. (A) CT scan showing lytic lesions in the sternum (arrow). (B) Three-dimensional reconstruction of CT scan showing sternum lesion (arrow). (C,D) The whole-body PET-CT scan showing increased sternum FDG uptake (arrow).
IHC staining showed that tumor cells were diffuse positive for cytokeratin and breast-specific markers GATA3 and GCDFP-15. The tumor cells expressed estrogen receptor (ER) at 60% (Figure 2B), progesterone receptor (PR) at 5% (Figure 2C), and androgen receptor (AR) at 80%, a Her-2/neu score of 1+ (Figure 2E) and negative for CK5/6 (Figure 2F). The proliferation index of Ki-67 was up to 80% (Figure 2D). However, the tumor cells were negative for Vimintin, TTF1, PAX8, P40, and CgA. Therefore, the histopathological and IHC evaluation of sternum lesions was consistent with metastatic breast carcinoma, luminal type B, despite no significant abnormality being observed in the breast MRI (Figure 3).
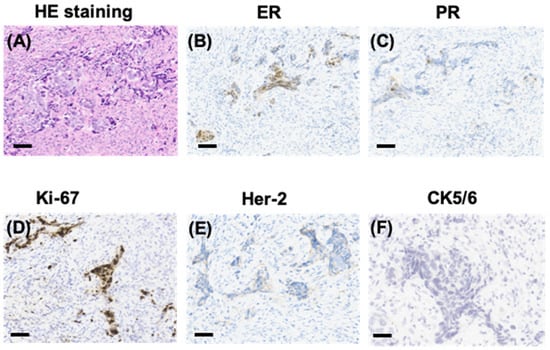
Figure 2.
Pathological manifestations of the sternum lesions. HE staining showing sternum lesions (A) and immunohistochemical (IHC) staining showing positivity for ER (B), PR (C), and Ki-67 (D), and a Her-2/neu score of 1+ (E), and negativity for CK5/6 (F) in sternum tissue (scale bars represent 200 µm).
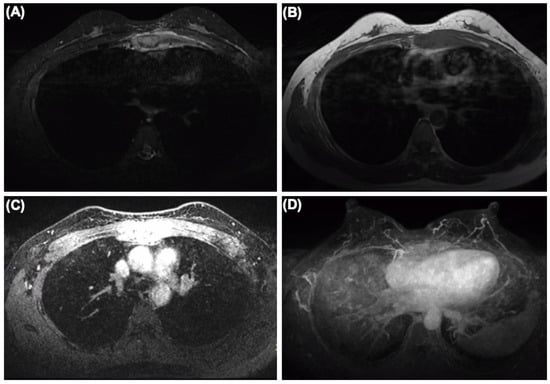
Figure 3.
The MRI of the breast showing sternum and internal mammary node lesions without visualized lesions of both mammary glands and bilateral axillary lymph nodes. (A) SPIR (Spectral Presaturaton with Inversion Recovery) T2-weighted MR image. (B) T1-weighted MR image. (C) SPIR T1-weighted MR image. (D) Maximum density projection reconstruction.
After diagnosis, the patient received systemic therapy with endocrine therapy and a CDK4/6 inhibitor, and local radiotherapy (RT) to sternum and internal mammary node lesions (Figure 4A,B). After local RT, a CT scan was performed to evaluate their therapeutic effect. The sternum and internal mammary node lesions were significantly receded after RT (Figure 5A,B).
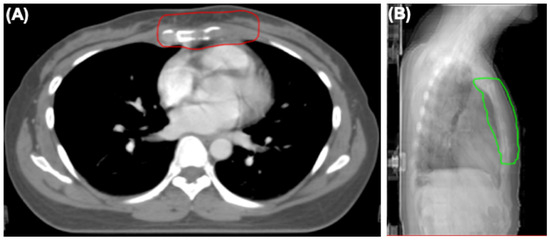
Figure 4.
The radiotherapy field of sternum and internal mammary node lesions. (A) Axial view showing clinical target (CTV) of sternum and internal mammary node lesions (red line). (B) Lateral view showing planning target volume (PTV) of sternum and internal mammary node lesions (green line).
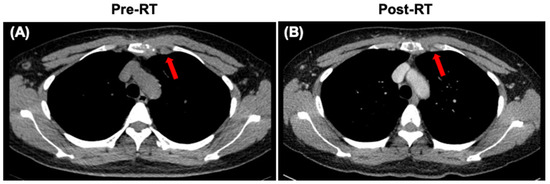
Figure 5.
CT images of the sternum and internal mammary node lesions (arrows). (A) CT scan showing the sternum and internal mammary node lesions at diagnosis. (B) CT scan showing the sternum and internal mammary node lesions after local radiotherapy (RT).
Recently, ER+/HER2+ patients have been reported as more likely to have metastases to bone compared to ER−/HER2+ patients in a nationwide autopsy study using artificial intelligence [6]. In this case, the patient was diagnosed as luminal type B of occult breast carcinoma. According to the above study, the molecular type of this patient has a higher possibility of bone metastasis, which might support the finding of the sternum as the first metastatic site. However, another recent study demonstrated that the majority of distant metastases in advanced breast cancer putatively arise from occult regional nodal disease [7]. An interesting feature in this case was that the internal mammary node was also involved in soft tissue mass around sternum lesions, while the FDG uptake of axillary and supraclavicular lymph nodes, with a higher possibility of metastasis, was normal. Therefore, it was difficult to determine whether the sternum was the first metastatic site to invade the internal mammary lymph nodes or vice versa.
Occult breast cancer presenting as sternum pain is an uncommon condition. Any non-specific symptoms, such as bone pain as in the present case, should require high vigilance for occult breast cancer. The role of whole-body PET-CT in assessing possible systemic involvement and the potential primary tumor site is important. IHC is of great significance to identify the origin of a metastatic tumor despite no positive signs in mammary glands.
Author Contributions
D.W. provided the patient’s history and documents, and drafted the manuscript. T.Z., F.Q. and Q.W. performed the treatment process. S.G., B.Z. and F.H. reviewed the pathological slides and took slide photographs. W.Q. reviewed the radiographic images. All authors contributed in the management of the patient, reviewing the images, pathologic and IHC slides, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant from the National Natural Science Foundation of China (82073142, DW; 82173089, TZ; and 82073332, QW), and Immunoradiotherapy Research Fund of Chinese Society of Radiation Oncology (Z-2017-24-2108, DW).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
We declare no competing interests.
References
- Halsted, W.S. The Results of Radical Operations for the Cure of Carcinoma of the Breast. Ann. Surg. 1907, 46, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Walker, G.V.; Smith, G.L.; Perkins, G.H.; Oh, J.L.; Woodward, W.; Yu, T.K.; Hunt, K.K.; Hoffman, K.; Strom, E.A.; Buchholz, T.A. Population-based analysis of occult primary breast cancer with axillary lymph node metastasis. Cancer 2010, 116, 4000–4006. [Google Scholar] [CrossRef] [PubMed]
- Toss, A.; Moscetti, L.; Cascinu, S. Occult breast cancer: The uncommon presentation of a common disease. Chin. Clin. Oncol. 2019, 8, S10. [Google Scholar] [CrossRef]
- Buijs, J.T.; van der Pluijm, G. Osteotropic cancers: From primary tumor to bone. Cancer Lett. 2009, 273, 177–193. [Google Scholar] [CrossRef]
- Carsote, M.; Terzea, D.; Vasilescu, F.; Cucu, A.P.; Ciuche, A.; Nistor, C. Sternum Metastases: From Case-Identifying Strategy to Multidisciplinary Management. Diagnostics 2023, 13, 2698. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh, F.; Snoek, J.A.A.; Voorham, Q.J.; van Oijen, M.G.H.; Hugen, N.; Nagtegaal, I.D. Association of metastatic pattern in breast cancer with tumor and patient-specific factors: A nationwide autopsy study using artificial intelligence. Breast Cancer 2024, 31, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Bitencourt, A.; Rossi Saccarelli, C.; Morris, E.A.; Flynn, J.; Zhang, Z.; Khan, A.; Gillespie, E.; Cahlon, O.; Mueller, B.; Cuaron, J.J.; et al. Regional Lymph Node Involvement Among Patients With De Novo Metastatic Breast Cancer. JAMA Netw. Open 2020, 3, e2018790. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).