PD-L1 as a Urine Biomarker in Renal Cell Carcinoma—A Case Series and Proof-of-Concept Study
Abstract
1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Urine Samples and PD-L1 Analysis
2.3. Immunoblot Analysis of Exosomes
2.4. Targeted Next Generation Sequencing (NGS)
2.5. Statistical Analysis
3. Results
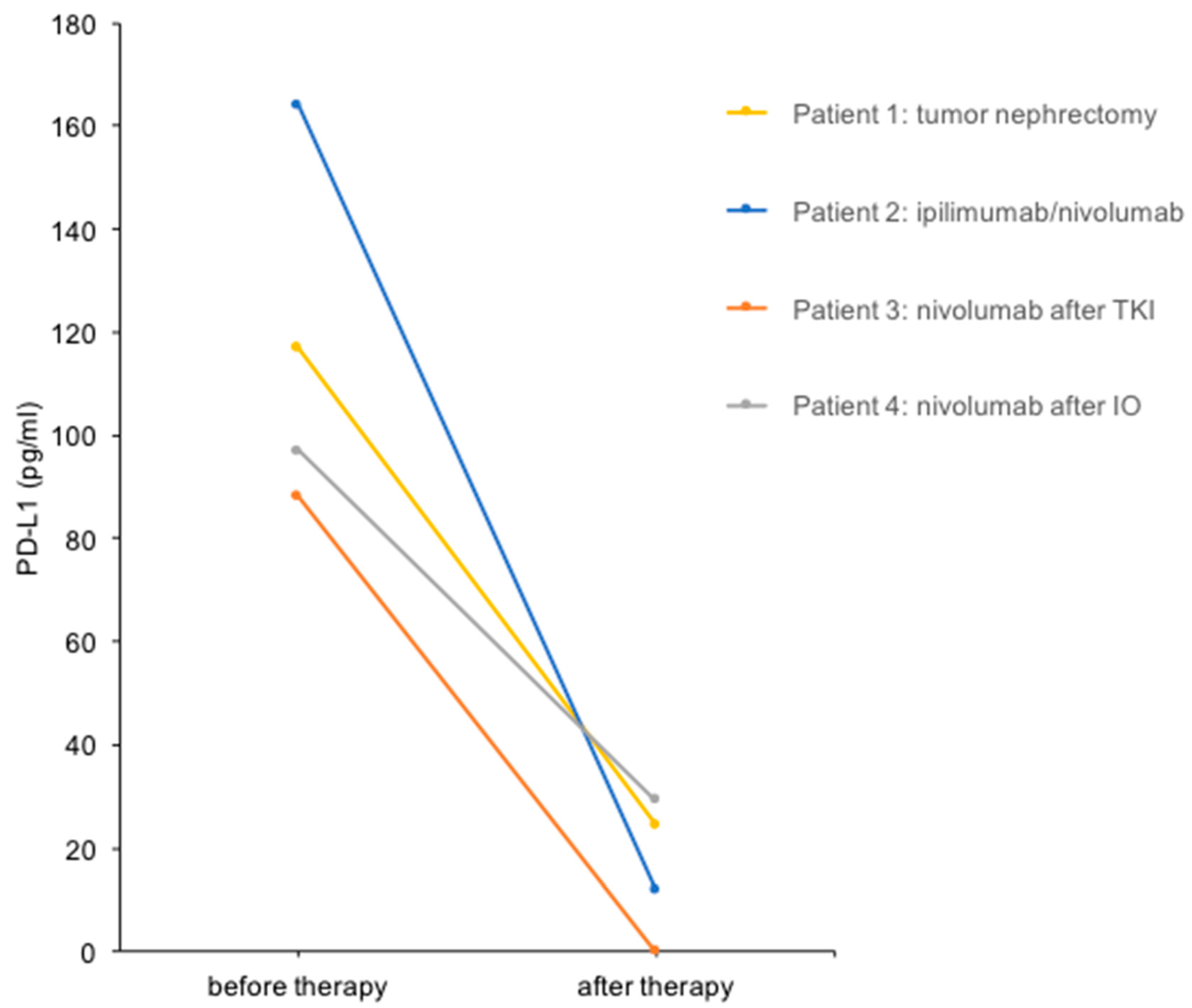
3.1. Urine PD-L1 Levels Decrease in Patients with mRCC after Cytoreductive Nephrectomy or Immune Checkpoint Blockade
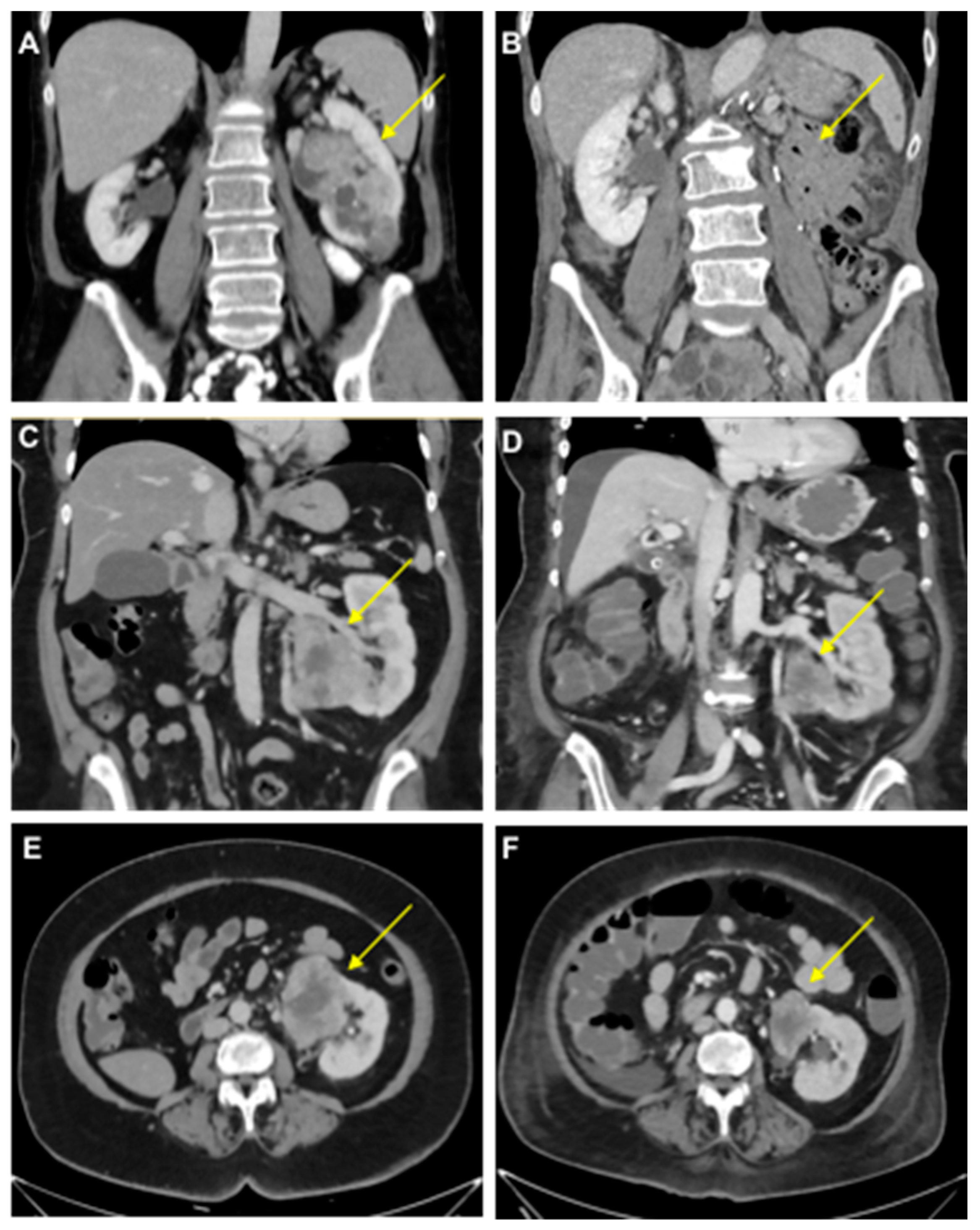
- Case study 1:
- Case study 2:
- Case study 3:
- Case study 4:
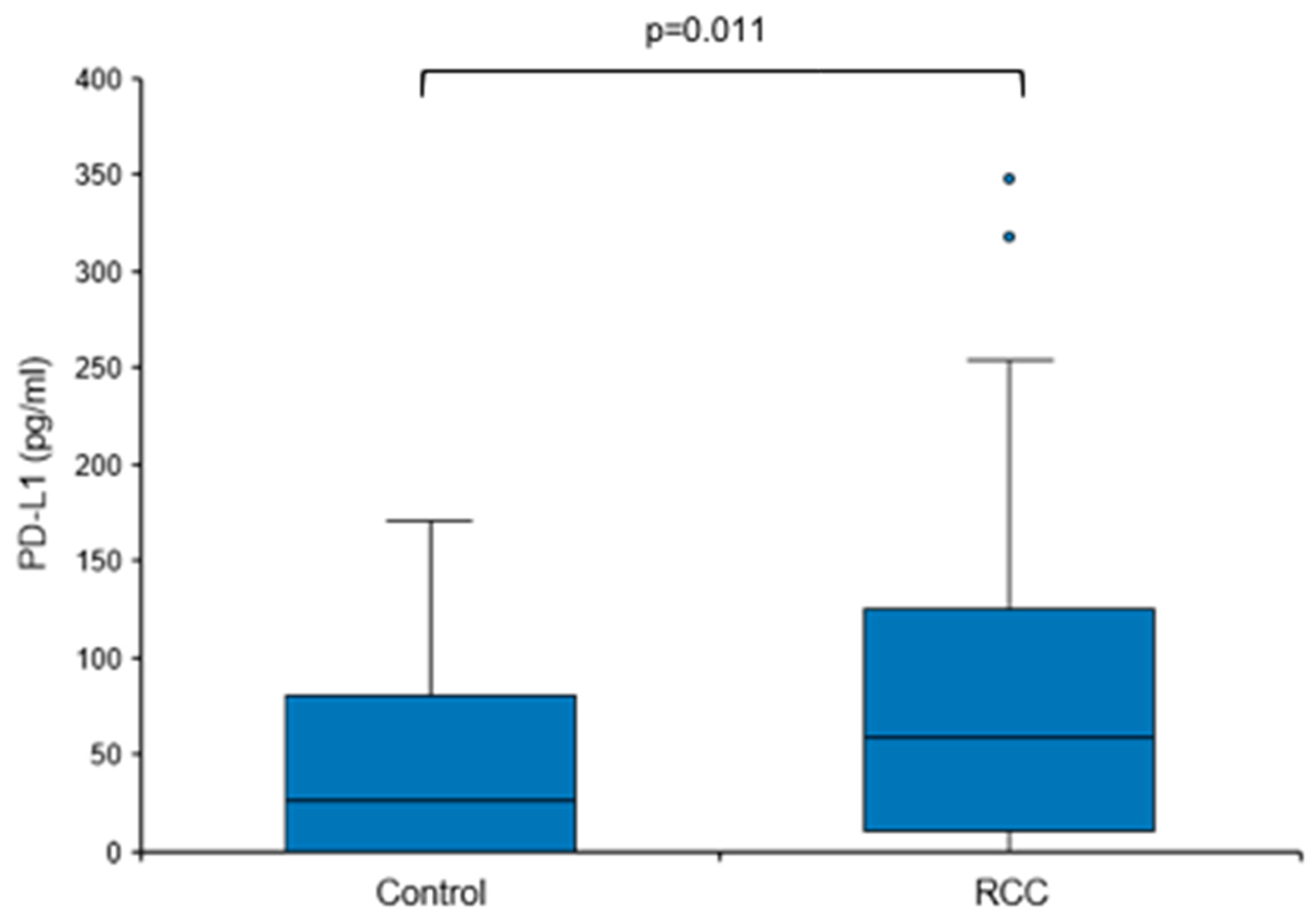
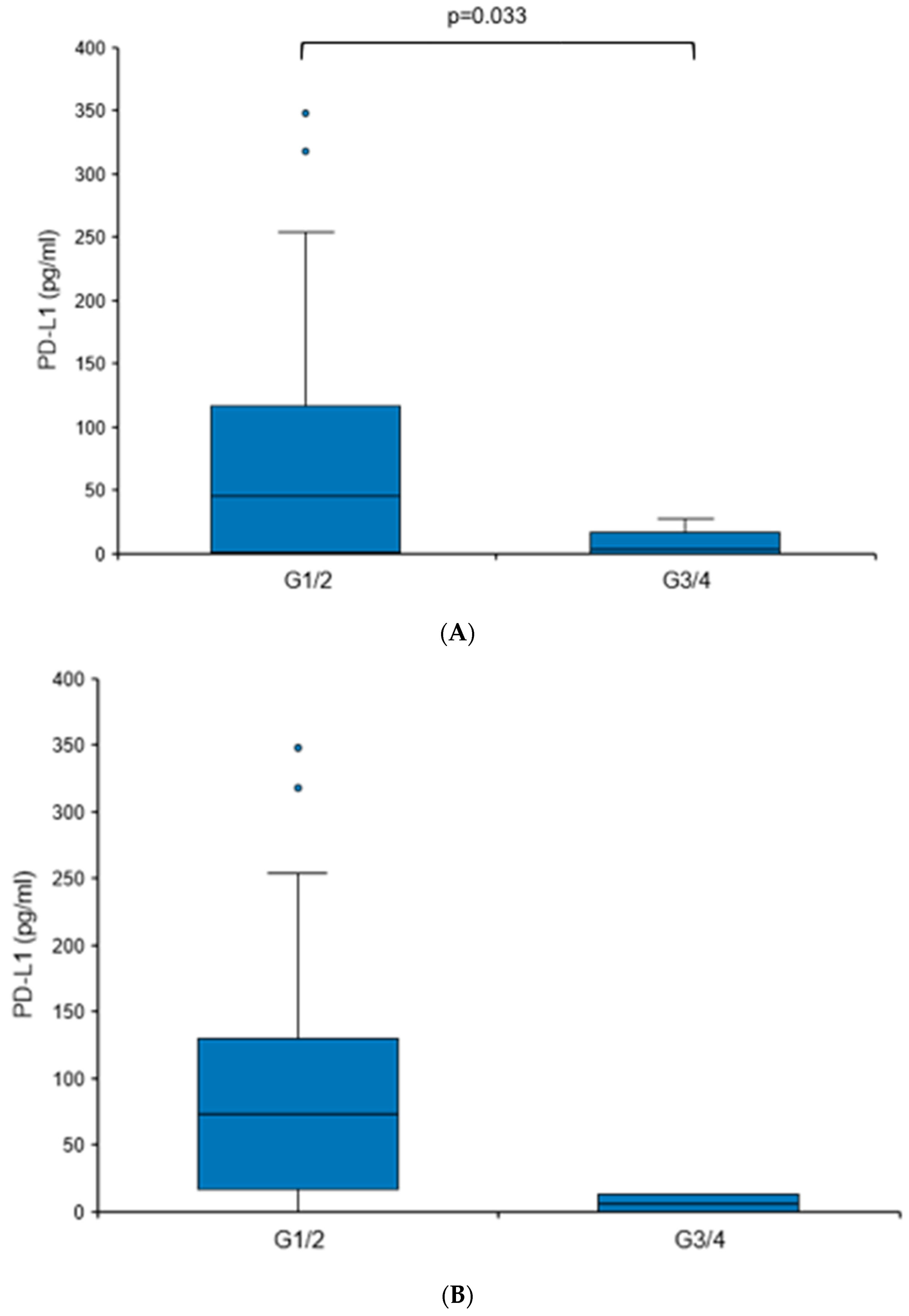
3.2. Elevated Urine PD-L1 Levels in Treatment-Naïve Patients with RCC
3.3. Exosomes May Be the Source of Urine PD-L1 in a Subset of Patients
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Motzer, R.J.; Jonasch, E.; Agarwal, N.; Bhayani, S.; Bro, W.P.; Chang, S.S.; Choueiri, T.K.; Costello, B.A.; Derweesh, I.H.; Fishman, M.; et al. Kidney Cancer, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 804–834. [Google Scholar] [CrossRef] [PubMed]
- Leading Sites of New Cancer Cases and Deaths—2021 Estimates: Cancer Facts & Figures. 2021. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/leading-sites-of-new-cancer-cases-and-deaths.pdf (accessed on 20 October 2021).
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Wehle, M.J.; Thiel, D.D.; Petrou, S.P.; Young, P.R.; Frank, I.; Karsteadt, N. Conservative management of incidental contrast-enhancing renal masses as safe alternative to invasive therapy. Urology 2004, 64, 49–52. [Google Scholar] [CrossRef]
- Krabbe, L.-M.; Bagrodia, A.; Margulis, V.; Wood, C.G. Surgical management of renal cell carcinoma. Semin. Interv. Radiol. 2014, 31, 27–32. [Google Scholar] [CrossRef]
- Bedke, J.; Gauler, T.; Grünwald, V.; Hegele, A.; Herrmann, E.; Hinz, S.; Janssen, J.; Schmitz, S.; Schostak, M.; Tesch, H.; et al. Systemic therapy in metastatic renal cell carcinoma. World J. Urol. 2017, 35, 179–188. [Google Scholar] [CrossRef]
- Rini, B.I.; Battle, D.; Figlin, R.A.; George, D.J.; Hammers, H.; Hutson, T.; Jonasch, E.; Joseph, R.W.; McDermott, D.F.; Motzer, R.J.; et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of advanced renal cell carcinoma (RCC). J. Immunother. Cancer 2019, 7, 354. [Google Scholar] [CrossRef] [PubMed]
- Ljungberg, B.; Albiges, L.; Bensalah, K.; Bex, A.; Giles, R.H.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU Guidelines: Edn. presented at the EAU Annual Congress Milan; EAU Guidelines Office: Arnhem, The Netherlands, 2021; Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 14 February 2024).
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7116507/ (accessed on 7 November 2023). [CrossRef]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Eckstein, M.; Erben, P.; Kriegmair, M.C.; Worst, T.S.; Weiß, C.-A.; Wirtz, R.M.; Wach, S.; Stoehr, R.; Sikic, D.; Geppert, C.I.; et al. Performance of the Food and Drug Administration/EMA-approved programmed cell death ligand-1 assays in urothelial carcinoma with emphasis on therapy stratification for first-line use of atezolizumab and pembrolizumab. Eur. J. Cancer 2019, 106, 234–243. [Google Scholar] [CrossRef]
- Eckstein, M.; Gupta, S. New insights in predictive determinants of the tumor immune microenvironment for immune checkpoint inhibition: A never ending story? Ann. Transl. Med. 2019, 7, S135. [Google Scholar] [CrossRef] [PubMed]
- Carretero-González, A.; Lora, D.; Sobrino, I.M.; Sanz, I.S.; Bourlon, M.T.; Herranz, U.A.; Chanzá, N.M.; Castellano, D.; de Velasco, G. The Value of PD-L1 Expression as Predictive Biomarker in Metastatic Renal Cell Carcinoma Patients: A Meta-Analysis of Randomized Clinical Trials. Cancers 2020, 12, 1945. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Zhao, B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: Meta-analysis. BMJ 2018, 362, k3529. [Google Scholar] [CrossRef] [PubMed]
- Tosev, G.; Wahafu, W.; Reimold, P.; Damgov, I.; Schwab, C.; Aksoy, C.; Kaczorowski, A.; Stenzinger, A.; Nyarangi-Dix, J.; Hohenfellner, M.; et al. Detection of PD-L1 in the urine of patients with urothelial carcinoma of the bladder. Sci. Rep. 2021, 11, 14244. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Qiu, X.; Zhang, Z.; Zhang, S.; Zhang, Y.; Liang, Y.; Guo, J.; Peng, H.; Chen, M.; Fu, Y.-X.; et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat. Commun. 2020, 11, 4835. Available online: https://www.nature.com/articles/s41467-020-18570-x (accessed on 7 November 2023). [CrossRef] [PubMed]
- Oh, S.A.; Wu, D.-C.; Cheung, J.; Navarro, A.; Xiong, H.; Cubas, R.; Totpal, K.; Chiu, H.; Wu, Y.; Comps-Agrar, L.; et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat. Cancer 2020, 1, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Mayoux, M.; Roller, A.; Pulko, V.; Sammicheli, S.; Chen, S.; Sum, E.; Jost, C.; Fransen, M.F.; Buser, R.B.; Kowanetz, M.; et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci. Transl. Med. 2020, 12, eaav7431. [Google Scholar] [CrossRef] [PubMed]
- Kazdal, D.; Endris, V.; Allgäuer, M.; Kriegsmann, M.; Leichsenring, J.; Volckmar, A.L.; Harms, A.; Kirchner, M.; Kriegsmann, K.; Neumann, O.; et al. Spatial and Temporal Heterogeneity of Panel-Based Tumor Mutational Burden in Pulmonary Adenocarcinoma: Separating Biology From Technical Artifacts. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 1935–1947. [Google Scholar] [CrossRef]
- Friedhoff, J.; Schneider, F.; Jurcic, C.; Endris, V.; Kirchner, M.; Sun, A.; Bolnavu, I.; Pohl, L.; Teroerde, M.; Kippenberger, M.; et al. BAP1 and PTEN mutations shape the immunological landscape of clear cell renal cell carcinoma and reveal the intertumoral heterogeneity of T cell suppression: A proof-of-concept study. Cancer Immunol. Immunother. 2023, 72, 1603–1618. [Google Scholar] [CrossRef]
- Denize, T.; Hou, Y.; Pignon, J.-C.; Walton, E.; West, D.J.; Freeman, G.J.; Braun, D.A.; Wu, C.J.; Gupta, S.; Motzer, R.J.; et al. Transcriptomic Correlates of Tumor Cell PD-L1 Expression and Response to Nivolumab Monotherapy in Metastatic Clear Cell Renal Cell Carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 4045–4055. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9481706/ (accessed on 7 November 2023). [CrossRef]
- Mori, K.; Abufaraj, M.; Mostafaei, H.; Quhal, F.; Fajkovic, H.; Remzi, M.; Karakiewicz, P.I.; Egawa, S.; Schmidinger, M.; Shariat, S.F.; et al. The Predictive Value of Programmed Death Ligand 1 in Patients with Metastatic Renal Cell Carcinoma Treated with Immune-checkpoint Inhibitors: A Systematic Review and Meta-analysis. Eur. Urol. 2021, 79, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Frigola, X.; Inman, B.A.; Lohse, C.M.; Krco, C.J.; Cheville, J.C.; Thompson, R.H.; Leibovich, B.; Blute, M.L.; Dong, H.; Kwon, E.D. Identification of a soluble form of B7-H1 that retains immunosuppressive activity and is associated with aggressive renal cell carcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2011, 17, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Gershtein, E.S.; Morozov, A.A.; Goryacheva, I.O.; Filipenko, M.L.; Alferov, A.A.; Bezhanova, S.D.; Bazaev, V.V.; Kazantseva, I.A. Soluble Ligand of the Immune Checkpoint Receptor (sPD-L1) in Blood Serum of Patients with Renal Cell Carcinoma. Bull. Exp. Biol. Med. 2019, 166, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Kamai, T.; Masuda, A.; Nukui, A.; Abe, H.; Arai, K.; Yoshida, K.I. Higher preoperative serum levels of PD-L1 and B7-H4 are associated with invasive and metastatic potential and predictable for poor response to VEGF-targeted therapy and unfavorable prognosis of renal cell carcinoma. Cancer Med. 2016, 5, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Larrinaga, G.; Solano-Iturri, J.D.; Errarte, P.; Unda, M.; Loizaga-Iriarte, A.; Pérez-Fernández, A.; Echevarría, E.; Asumendi, A.; Manini, C.; Angulo, J.C.; et al. Soluble PD-L1 Is an Independent Prognostic Factor in Clear Cell Renal Cell Carcinoma. Cancers 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J.; Tu, H.; Liang, D.; Chang, D.W.; Ye, Y.; Wu, X. Soluble immune checkpoint-related proteins as predictors of tumor recurrence, survival, and T cell phenotypes in clear cell renal cell carcinoma patients. J. Immunother. Cancer 2019, 7, 334. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, S.; Huotilainen, S.; Carlsson, J.; Sundqvist, P. Soluble Levels of CD163, PD-L1, and IL-10 in Renal Cell Carcinoma Patients. Diagnostics 2022, 12, 336. Available online: https://pubmed.ncbi.nlm.nih.gov/35204426/ (accessed on 7 November 2023). [CrossRef] [PubMed]
- Thompson, R.H.; Kuntz, S.M.; Leibovich, B.C.; Dong, H.; Lohse, C.M.; Webster, W.S.; Sengupta, S.; Frank, I.; Parker, A.S.; Zincke, H.; et al. Tumor B7-H1 is associated with poor prognosis in renal cell carcinoma patients with long-term follow-up. Cancer Res. 2006, 66, 3381–3385. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, T.; Ishiguro, Y.; Ohtake, S.; Kato, I.; Ito, Y.; Ito, H.; Makiyama, K.; Kondo, K.; Miyoshi, Y.; Yumura, Y.; et al. PD-1 and PD-L1 are more highly expressed in high-grade bladder cancer than in low-grade cases: PD-L1 might function as a mediator of stage progression in bladder cancer. BMC Urol. 2018, 18, 97. [Google Scholar] [CrossRef]
- Martin, A.M.; Bell, W.R.; Yuan, M.; Harris, L.; Poore, B.; Arnold, A.; Engle, E.L.; Asnaghi, L.; Lim, M.; Raabe, E.H.; et al. PD-L1 Expression in Pediatric Low-Grade Gliomas Is Independent of BRAF V600E Mutational Status. J. Neuropathol. Exp. Neurol. 2020, 79, 74–85. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, X.; Jiang, Y.; Jia, H.; Shi, X.; Han, Y.; Li, Q.; Li, W. Soluble Programmed Cell Death Protein 1 and Its Ligand: Potential Biomarkers to Predict Acute Kidney Injury After Surgery in Critically Ill Patients. J. Inflamm. Res. 2022, 15, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Afaneh, C.; Muthukumar, T.; Lubetzky, M.; Ding, R.; Snopkowski, C.; Sharma, V.K.; Seshan, S.; Dadhania, D.; Schwartz, J.E.; Suthanthiran, M. Urinary cell levels of mRNA for OX40, OX40L, PD-1, PD-L1, or PD-L2 and acute rejection of human renal allografts. Transplantation 2010, 90, 1381–1387. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Chen, F.; Qin, L.; He, R.; Yuan, J. sPD-1 and sPD-L1 Levels in Serum and Urine of Patients with Primary Nephrotic Syndrome and their Clinical Significance. Clin. Lab. 2021, 67, 2169. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.F.; Schneider, A.K.; Cesson, V.; Dartiguenave, F.; Lucca, I.; Jichlinski, P.; Nardelli-Haefliger, D.; Derré, L. Conventional and PD-L1-expressing Regulatory T Cells are Enriched During BCG Therapy and may Limit its Efficacy. Eur. Urol. 2018, 74, 540–544. Available online: https://pubmed.ncbi.nlm.nih.gov/30033046/ (accessed on 7 November 2023). [CrossRef] [PubMed]
- Morrissey, S.M.; Yan, J. Exosomal PD-L1: Roles in Tumor Progression and Immunotherapy. Trends Cancer 2020, 6, 550–558. [Google Scholar] [CrossRef]
- Vikerfors, A.; Davidsson, S.; Frey, J.; Jerlström, T.; Carlsson, J. Soluble PD-L1 in Serum and Urine in Urinary Bladder Cancer Patients. Cancers 2021, 13, 5841. [Google Scholar] [CrossRef]

| Variable | RCC (n = 49) | Control (n = 31) |
|---|---|---|
| Age (years) | ||
| Median | 64 | 61 |
| IQR | 57–72 | 42–69 |
| Sex, n (%) | ||
| Female | 16 (32.7) | 11 (35.5) |
| Male | 33 (67.3) | 20 (64.5) |
| Histology, n (%) | ||
| Clear cell | 35 (71.4) | n.a. |
| Papillary | 7 (14.3) | n.a. |
| Chromophobe | 4 (8.2) | n.a. |
| Other | 3 (6.1) | n.a. |
| T stage, n (%) | ||
| T1 | 28 (57.2) | n.a. |
| T2 | 3 (6.1) | n.a. |
| T3 | 14 (28.5) | n.a. |
| T4 | 4 (8.2) | n.a. |
| N stage, n (%) | ||
| N0 | 19 (38.8) | n.a. |
| Nx | 24 (49.0) | n.a. |
| N1 | 6 (12.2) | n.a. |
| M stage, n (%) | ||
| M0 | 9 (18.4) | n.a. |
| Mx | 33 (67.3) | n.a. |
| M1 | 7 (14.3) | |
| Grade, n (%) | ||
| G1 | 5 (10.2) | n.a. |
| G2 | 33 (67.4) | n.a. |
| G3 | 1 (2.0) | n.a. |
| G4 | 2 (4.1) | n.a. |
| Gx | 8 (16.3) | n.a. |
| Diagnosis | n (%) |
|---|---|
| Angiomyolipoma | 4 (12.9) |
| Appendicitis | 1 (3.2) |
| Bosniak II cyst | 1 (3.2) |
| Prostate hyperplasia | 2 (6.5) |
| Cystitis | 5 (16.1) |
| Epididymitis | 1 (3.2) |
| Urolithiasis | 5 (16.1) |
| Oncocytoma | 7 (22.6) |
| Phimosis | 1 (3.2) |
| Prostatitis | 1 (3.2) |
| Pyelonephritis | 2 (6.5) |
| Urethral stricture | 1 (3.2) |
| Variable | RCC (n = 29) |
|---|---|
| Age (years) | |
| Median | 68 |
| IQR | 62–77 |
| Sex, n (%) | |
| Female | 5 (17.2) |
| Male | 24 (82.8) |
| Histology, n (%) | |
| Clear cell | 19 (65.5) |
| Papillary | 6 (20.7) |
| Chromophobe | 2 (6.9) |
| Other | 2 (6.9) |
| T stage, n (%) | |
| T1 | 22 (75.9) |
| T2 | 2 (6.9) |
| T3 | 5 (17.2) |
| T4 | 0 (0) |
| N stage, n (%) | |
| N0 | 18 (62.1) |
| Nx | 10 (34.5) |
| N1 | 1 (3.4) |
| M stage, n (%) | |
| M0 | 18 (62.1) |
| Mx | 8 (27.6) |
| M1 | 3 (10.3) |
| Grade, n (%) | |
| G1 | 6 (20.7) |
| G2 | 13 (44.8) |
| G3 | 4 (13.8) |
| G4 | 2 (6.9) |
| Gx | 4 (13.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reimold, P.; Tosev, G.; Kaczorowski, A.; Friedhoff, J.; Schwab, C.; Schütz, V.; Görtz, M.; Panzer, N.; Heller, M.; Aksoy, C.; et al. PD-L1 as a Urine Biomarker in Renal Cell Carcinoma—A Case Series and Proof-of-Concept Study. Diagnostics 2024, 14, 741. https://doi.org/10.3390/diagnostics14070741
Reimold P, Tosev G, Kaczorowski A, Friedhoff J, Schwab C, Schütz V, Görtz M, Panzer N, Heller M, Aksoy C, et al. PD-L1 as a Urine Biomarker in Renal Cell Carcinoma—A Case Series and Proof-of-Concept Study. Diagnostics. 2024; 14(7):741. https://doi.org/10.3390/diagnostics14070741
Chicago/Turabian StyleReimold, Philipp, Georgi Tosev, Adam Kaczorowski, Jana Friedhoff, Constantin Schwab, Viktoria Schütz, Magdalena Görtz, Niklas Panzer, Martina Heller, Cem Aksoy, and et al. 2024. "PD-L1 as a Urine Biomarker in Renal Cell Carcinoma—A Case Series and Proof-of-Concept Study" Diagnostics 14, no. 7: 741. https://doi.org/10.3390/diagnostics14070741
APA StyleReimold, P., Tosev, G., Kaczorowski, A., Friedhoff, J., Schwab, C., Schütz, V., Görtz, M., Panzer, N., Heller, M., Aksoy, C., Himmelsbach, R., Walle, T., Zschäbitz, S., Jäger, D., Duensing, A., Stenzinger, A., Hohenfellner, M., & Duensing, S. (2024). PD-L1 as a Urine Biomarker in Renal Cell Carcinoma—A Case Series and Proof-of-Concept Study. Diagnostics, 14(7), 741. https://doi.org/10.3390/diagnostics14070741







