Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serum MCP-1, Activin-A, Clusterin, and Standard Biochemical Measurements
2.3. Statistical Analysis
3. Results
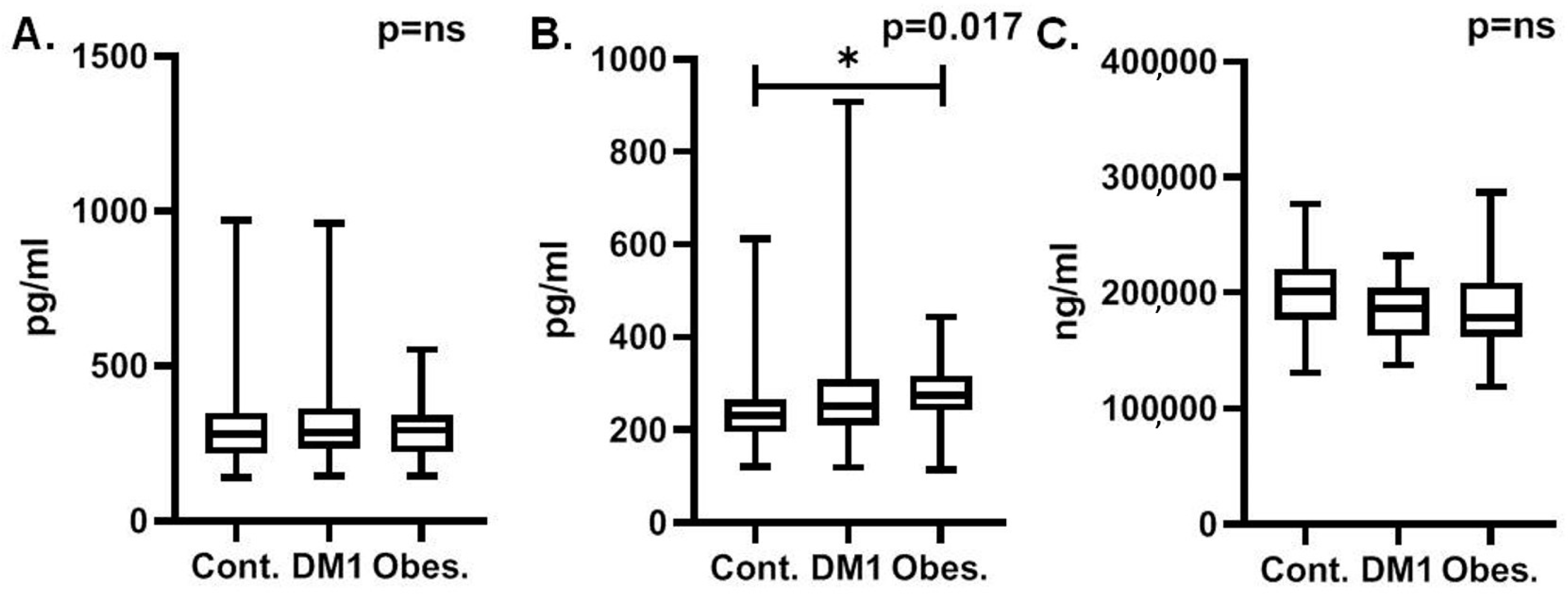
3.1. MCP-1 Outcomes
3.2. Activin-A Outcomes
3.3. Clusterin Outcomes
4. Discussion
4.1. MCP-1 and Obesity
4.2. MCP-1 and Type-2 Diabetes
4.3. MCP-1 and Type-1 Diabetes
4.4. Activin-A and Obesity
4.5. Activin-A and Type-2 Diabetes
4.6. Activin-A and Type-1 Diabetes
4.7. Clusterin and Obesity
4.8. Clusterin and Type-2 Diabetes
5. Limitations and Strengths
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lister, N.B.; Baur, L.A.; Felix, J.F.; Hill, A.J.; Marcus, C.; Reinehr, T.; Summerbell, C.; Wabitsch, M. Child and Adolescent Obesity. Nat. Rev. Dis. Primers 2023, 9, 24. [Google Scholar] [CrossRef]
- Umano, G.R.; Shabanova, V.; Pierpont, B.; Mata, M.; Nouws, J.; Tricò, D.; Galderisi, A.; Santoro, N.; Caprio, S. A Low Visceral Fat Proportion, Independent of Total Body Fat Mass, Protects Obese Adolescent Girls against Fatty Liver and Glucose Dysregulation: A Longitudinal Study. Int. J. Obes. 2019, 43, 673–682. [Google Scholar] [CrossRef]
- Kaess, B.M.; Pedley, A.; Massaro, J.M.; Murabito, J.; Hoffmann, U.; Fox, C.S. The Ratio of Visceral to Subcutaneous Fat, a Metric of Body Fat Distribution, Is a Unique Correlate of Cardiometabolic Risk. Diabetologia 2012, 55, 2622–2630. [Google Scholar] [CrossRef]
- Preis, S.R.; Massaro, J.M.; Robins, S.J.; Hoffmann, U.; Vasan, R.S.; Irlbeck, T.; Meigs, J.B.; Sutherland, P.; D’Agostino, R.B.; O’Donnell, C.J.; et al. Abdominal Subcutaneous and Visceral Adipose Tissue and Insulin Resistance in the Framingham Heart Study. Obesity 2010, 18, 2191–2198. [Google Scholar] [CrossRef]
- Atkinson, M.A.; Eisenbarth, G.S.; Michels, A.W. Type 1 Diabetes. Lancet 2014, 383, 69–82. [Google Scholar] [CrossRef]
- Willcox, A.; Richardson, S.J.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. Analysis of Islet Inflammation in Human Type 1 Diabetes. Clin. Exp. Immunol. 2009, 155, 173–181. [Google Scholar] [CrossRef]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte Chemoattractant Protein 1 Acts as a T-Lymphocyte Chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef]
- Huber, J.; Kiefer, F.W.; Zeyda, M.; Ludvik, B.; Silberhumer, G.R.; Prager, G.; Zlabinger, G.J.; Stulnig, T.M. CC Chemokine and CC Chemokine Receptor Profiles in Visceral and Subcutaneous Adipose Tissue Are Altered in Human Obesity. J. Clin. Endocrinol. Metab. 2008, 93, 3215–3221. [Google Scholar] [CrossRef]
- Breslin, W.L.; Johnston, C.A.; Strohacker, K.; Carpenter, K.C.; Davidson, T.R.; Moreno, J.P.; Foreyt, J.P.; McFarlin, B.K. Obese Mexican American Children Have Elevated MCP-1, TNF-α, Monocyte Concentration, and Dyslipidemia. Pediatrics 2012, 129, e1180-6. [Google Scholar] [CrossRef]
- Catalán, V.; Gómez-Ambrosi, J.; Ramirez, B.; Rotellar, F.; Pastor, C.; Silva, C.; Rodríguez, A.; Gil, M.J.; Cienfuegos, J.A.; Frühbeck, G. Proinflammatory Cytokines in Obesity: Impact of Type 2 Diabetes Mellitus and Gastric Bypass. Obes. Surg. 2007, 17, 1464–1474. [Google Scholar] [CrossRef]
- Kim, C.S.; Park, H.S.; Kawada, T.; Kim, J.H.; Lim, D.; Hubbard, N.E.; Kwon, B.S.; Erickson, K.L.; Yu, R. Circulating Levels of MCP-1 and IL-8 Are Elevated in Human Obese Subjects and Associated with Obesity-Related Parameters. Int. J. Obes. 2006, 30, 1347–1355. [Google Scholar] [CrossRef]
- Cancello, R.; Henegar, C.; Viguerie, N.; Taleb, S.; Poitou, C.; Rouault, C.; Coupaye, M.; Pelloux, V.; Hugol, D.; Bouillot, J.L.; et al. Reduction of Macrophage Infiltration and Chemoattractant Gene Expression Changes in White Adipose Tissue of Morbidly Obese Subjects after Surgery-Induced Weight Loss. Diabetes 2005, 54, 2277–2286. [Google Scholar] [CrossRef]
- Zietz, B.; Büchler, C.; Herfarth, H.; Müller-Ladner, U.; Spiegel, D.; Schölmerich, J.; Schäffler, A. Caucasian Patients with Type 2 Diabetes Mellitus Have Elevated Levels of Monocyte Chemoattractant Protein-1 That Are Not Influenced by the -2518 A-->G Promoter Polymorphism. Diabetes Obes. Metab. 2005, 7, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Piemonti, L.; Calori, G.; Lattuada, G.; Mercalli, A.; Ragogna, F.; Garancini, M.P.; Ruotolo, G.; Luzi, L.; Perseghin, G. Association between Plasma Monocyte Chemoattractant Protein-1 Concentration and Cardiovascular Disease Mortality in Middle-Aged Diabetic and Nondiabetic Individuals. Diabetes Care 2009, 32, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Simeoni, E.; Hoffmann, M.M.; Winkelmann, B.R.; Ruiz, J.; Fleury, S.; Boehm, B.O.; März, W.; Vassalli, G. Association between the A-2518G Polymorphism in the Monocyte Chemoattractant Protein-1 Gene and Insulin Resistance and Type 2 Diabetes Mellitus. Diabetologia 2004, 47, 1574–1580. [Google Scholar] [CrossRef]
- Phillips, D.J. Activins, Inhibins and Follistatins in the Large Domestic Species. Domest. Anim. Endocrinol. 2005, 28, 1–16. [Google Scholar] [CrossRef]
- Zaragosi, L.E.; Wdziekonski, B.; Villageois, P.; Keophiphath, M.; Maumus, M.; Tchkonia, T.; Bourlier, V.; Mohsen-Kanson, T.; Ladoux, A.; Elabd, C.; et al. Activin a Plays a Critical Role in Proliferation and Differentiation of Human Adipose Progenitors. Diabetes 2010, 59, 2513–2521. [Google Scholar] [CrossRef]
- Andersen, G.; Ueland, T.; Knudsen, E.C.; Scholz, H.; Yndestad, A.; Sahraoui, A.; Smith, C.; Lekva, T.; Otterdal, K.; Halvorsen, B.; et al. Activin A Levels Are Associated with Abnormal Glucose Regulation in Patients with Myocardial Infarction: Potential Counteracting Effects of Activin A on Inflammation. Diabetes 2011, 60, 1544–1551. [Google Scholar] [CrossRef]
- Park, S.; Mathis, K.W.; Lee, I.K. The Physiological Roles of Apolipoprotein J/Clusterin in Metabolic and Cardiovascular Diseases. Rev. Endocr. Metab. Disord. 2014, 15, 45–53. [Google Scholar] [CrossRef]
- Daimon, M.; Oizumi, T.; Karasawa, S.; Kaino, W.; Takase, K.; Tada, K.; Jimbu, Y.; Wada, K.; Kameda, W.; Susa, S.; et al. Association of the Clusterin Gene Polymorphisms with Type 2 Diabetes Mellitus. Metabolism 2011, 60, 815–822. [Google Scholar] [CrossRef]
- Freedman, D.S.; Berenson, G.S. Tracking of BMI z Scores for Severe Obesity. Pediatrics 2017, 140, e20171072. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.L.; Pavkov, M.E.; Magliano, D.J.; Shaw, J.E.; Gregg, E.W. Global Trends in Diabetes Complications: A Review of Current Evidence. Diabetologia 2019, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Samaan, M.C.; Obeid, J.; Nguyen, T.; Thabane, L.; Timmons, B.W. Chemokine (C-C Motif) Ligand 2 Is a Potential Biomarker of Inflammation & Physical Fitness in Obese Children: A Cross-Sectional Study. BMC Pediatr. 2013, 13, 47. [Google Scholar] [CrossRef]
- Kanda, H.; Tateya, S.; Tamori, Y.; Kotani, K.; Hiasa, K.I.; Kitazawa, R.; Kitazawa, S.; Miyachi, H.; Maeda, S.; Egashira, K.; et al. MCP-1 Contributes to Macrophage Infiltration into Adipose Tissue, Insulin Resistance, and Hepatic Steatosis in Obesity. J. Clin. Investig. 2006, 116, 1494–1505. [Google Scholar] [CrossRef] [PubMed]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory Mechanisms in Obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory Links between Obesity and Metabolic Disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Genoni, G.; Prodam, F.; Marolda, A.; Giglione, E.; Demarchi, I.; Bellone, S.; Bona, G. Obesity and Infection: Two Sides of One Coin. Eur. J. Pediatr. 2014, 173, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Na, H.N.; Nam, J.H. Adenovirus 36 as an Obesity Agent Maintains the Obesity State by Increasing MCP-1 and Inducing Inflammation. J. Infect. Dis. 2012, 205, 914–922. [Google Scholar] [CrossRef]
- Kocazeybek, B.; Dinc, H.O.; Ergin, S.; Saribas, S.; Ozcabi, B.T.; Cizmecigil, U.; Altan, E.; Atalik, K.; Yüksel, P.; Taner, Z.; et al. Evaluation of Adenovirus-36 (Ad-36) Antibody Seropositivity and Adipokine Levels in Obese Children. Microb. Pathog. 2017, 108, 27–31. [Google Scholar] [CrossRef]
- De Lemos, J.A.; Morrow, D.A.; Sabatine, M.S.; Murphy, S.A.; Gibson, C.M.; Antman, E.M.; McCabe, C.H.; Cannon, C.P.; Braunwald, E. Association between Plasma Levels of Monocyte Chemoattractant Protein-1 and Long-Term Clinical Outcomes in Patients with Acute Coronary Syndromes. Circulation 2003, 107, 690–695. [Google Scholar] [CrossRef]
- Deo, R.; Khera, A.; McGuire, D.K.; Murphy, S.A.; Meo Neto, J.D.P.; Morrow, D.A.; De Lemos, J.A. Association among Plasma Levels of Monocyte Chemoattractant Protein-1, Traditional Cardiovascular Risk Factors, and Subclinical Atherosclerosis. J. Am. Coll. Cardiol. 2004, 44, 1812–1818. [Google Scholar] [CrossRef]
- Miller, T.L.; Somarriba, G.; John Orav, E.; Mendez, A.J.; Neri, D.; Schaefer, N.; Forster, L.; Goldberg, R.; Scott, G.B.; Lipshultz, S.E. Biomarkers of vascular dysfunction in children infected with human immunodeficiency virus-1. J. Acquir. Immune Defic. Syndr. 2010, 55, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Selvaraju, V.; Babu, J.R.; Geetha, T. Association of salivary C-reactive protein with the obesity measures and markers in children. Diabetes Metab. Syndr. Obes. 2019, 23, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Yueniwati, Y.; Yurina, V.; Indra, M.R. Thicker Carotid Intima Media Thickness in Children with Monocyte Chemoattractant Protein-1: A-2138T and A-2464G Mutation. Neurol. Res. Int. 2014, 2014, 176535. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ylä-Herttuala, S.; Lipton, B.A.; Rosenfeld, M.E.; Särkioja, T.; Yoshimura, T.; Leonard, E.J.; Witztum, J.L.; Steinberg, D. Expression of Monocyte Chemoattractant Protein 1 in Macrophage-Rich Areas of Human and Rabbit Atherosclerotic Lesions. Proc. Natl. Acad. Sci. USA 1991, 88, 5252–5256. [Google Scholar] [CrossRef] [PubMed]
- Arakelyan, A.; Petrkova, J.; Hermanova, Z.; Boyajyan, A.; Lukl, J.; Petrek, M. Serum Levels of the MCP-1 Chemokine in Patients with Ischemic Stroke and Myocardial Infarction. Mediat. Inflamm. 2005, 2005, 175–179. [Google Scholar] [CrossRef]
- Tretjakovs, P.; Jurka, A.; Bormane, I.; Mackevics, V.; Mikelsone, I.; Balode, L.; Reihmane, D.; Stukena, I.; Bahs, G.; Aivars, J.I.; et al. Relation of Inflammatory Chemokines to Insulin Resistance and Hypoadiponectinemia in Coronary Artery Disease Patients. Eur. J. Intern. Med. 2009, 20, 712–717. [Google Scholar] [CrossRef]
- Martynowicz, H.; Janus, A.; Nowacki, D.; Mazur, G. The Role of Chemokines in Hypertension. Adv. Clin. Exp. Med. 2014, 23, 319–325. [Google Scholar] [CrossRef]
- Lameire, N. Diabetes and Diabetic Nephropathy—A Worldwide Problem. Acta Diabetol. 2004, 41 (Suppl. 1), S3–S5. [Google Scholar] [CrossRef]
- Chow, F.; Ozols, E.; Nikolic-Paterson, D.J.; Atkins, R.C.; Tesch, G.H. Macrophages in Mouse Type 2 Diabetic Nephropathy: Correlation with Diabetic State and Progressive Renal Injury. Kidney Int. 2004, 65, 116–128. [Google Scholar] [CrossRef]
- Banba, N.; Nakamura, T.; Matsumura, M.; Kuroda, H.; Hattori, Y.; Kasai, K. Possible Relationship of Monocyte Chemoattractant Protein-1 with Diabetic Nephropathy. Kidney Int. 2000, 58, 684–690. [Google Scholar] [CrossRef]
- Wada, T.; Furuichi, K.; Sakai, N.; Iwata, Y.; Yoshimoto, K.; Shimizu, M.; Takeda, S.I.; Takasawa, K.; Yoshimura, M.; Kida, H.; et al. Up-Regulation of Monocyte Chemoattractant Protein-1 in Tubulointerstitial Lesions of Human Diabetic Nephropathy. Kidney Int. 2000, 58, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Nadkarni, G.N.; Rao, V.; Ismail-Beigi, F.; Fonseca, V.A.; Shah, S.V.; Simonson, M.S.; Cantley, L.; Devarajan, P.; Parikh, C.R.; Coca, S.G. Association of Urinary Biomarkers of Inflammation, Injury, and Fibrosis with Renal Function Decline: The ACCORD Trial. Clin. J. Am. Soc. Nephrol. 2016, 11, 1343–1352. [Google Scholar] [CrossRef] [PubMed]
- Tam, F.W.K.; Riser, B.L.; Meeran, K.; Rambow, J.A.; Pusey, C.D.; Frankel, A.H. Urinary Monocyte Chemoattractant Protein-1 (MCP-1) and Connective Tissue Growth Factor (CCN2) as Prognostic Markers for Progression of Diabetic Nephropathy. Cytokine 2009, 47, 37–42. [Google Scholar] [CrossRef]
- Chen, M.C.; Proost, P.; Gysemans, C.; Mathieu, C.; Eizirik, D.L. Monocyte Chemoattractant Protein-1 Is Expressed in Pancreatic Islets from Prediabetic NOD Mice and in Interleukin-1 Beta-Exposed Human and Rat Islet Cells. Diabetologia 2001, 44, 325–332. [Google Scholar] [CrossRef]
- Reddy, S.; Bai, Y.; Robinson, E.; Ross, J. Immunolocalization of Monocyte Chemoattractant Protein-1 in Islets of NOD Mice during Cyclophosphamide Administration. Ann. N. Y. Acad. Sci. 2006, 1079, 103–108. [Google Scholar] [CrossRef]
- Böni-Schnetzler, M.; Ehses, J.A.; Faulenbach, M.; Donath, M.Y. Insulitis in Type 2 Diabetes. Diabetes Obes. Metab. 2008, 10 (Suppl. 4), 201–204. [Google Scholar] [CrossRef] [PubMed]
- Chiarelli, F.; Cipollone, F.; Mohn, A.; Marini, M.; Iezzi, A.; Fazia, M.; Tumini, S.; De Cesare, D.; Pomilio, M.; Pierdomenico, S.D.; et al. Circulating Monocyte Chemoattractant Protein-1 and Early Development of Nephropathy in Type 1 Diabetes. Diabetes Care 2002, 25, 1829–1834. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Takahashi, M.; Ogata, M. Relationship between Glycoxidation and Cytokines in the Vitreous of Eyes with Diabetic Retinopathy. Jpn. J. Ophthalmol. 2002, 46, 406–412. [Google Scholar] [CrossRef]
- Elner, S.G.; Elner, V.M.; Jaffe, G.J.; Stuart, A.; Kunkel, S.L.; Strieter, R.M. Cytokines in Proliferative Diabetic Retinopathy and Proliferative Vitreoretinopathy. Curr. Eye Res. 1995, 14, 1045–1053. [Google Scholar] [CrossRef]
- Spalding, K.L.; Arner, E.; Westermark, P.O.; Bernard, S.; Buchholz, B.A.; Bergmann, O.; Blomqvist, L.; Hoffstedt, J.; Näslund, E.; Britton, T.; et al. Dynamics of Fat Cell Turnover in Humans. Nature 2008, 453, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Permana, P.A.; Nair, S.; Lee, Y.H.; Luczy-Bachman, G.; Vozarova De Courten, B.; Tataranni, P.A. Subcutaneous Abdominal Preadipocyte Differentiation In Vitro Inversely Correlates with Central Obesity. Am. J. Physiol. Endocrinol. Metab. 2004, 286, E958–E962. [Google Scholar] [CrossRef] [PubMed]
- Isakson, P.; Hammarstedt, A.; Gustafson, B.; Smith, U. Impaired Preadipocyte Differentiation in Human Abdominal Obesity: Role of Wnt, Tumor Necrosis Factor-Alpha, and Inflammation. Diabetes 2009, 58, 1550–1557. [Google Scholar] [CrossRef]
- Dani, C. Activins in Adipogenesis and Obesity. Int. J. Obes. 2013, 37, 163–166. [Google Scholar] [CrossRef]
- Zeller, J.; Krüger, C.; Lamounier-Zepter, V.; Sag, S.; Strack, C.; Mohr, M.; Loew, T.; Schmitz, G.; Maier, L.; Fischer, M.; et al. The Adipo-Fibrokine Activin A Is Associated with Metabolic Abnormalities and Left Ventricular Diastolic Dysfunction in Obese Patients. ESC Heart Fail. 2019, 6, 362–370. [Google Scholar] [CrossRef]
- Hu, J.; Wang, X.; Wei, S.M.; Tang, Y.H.; Zhou, Q.; Huang, C.X. Activin A Stimulates the Proliferation and Differentiation of Cardiac Fibroblasts via the ERK1/2 and P38-MAPK Pathways. Eur. J. Pharmacol. 2016, 789, 319–327. [Google Scholar] [CrossRef]
- Chauhan, A.; Gupta, A.; Goyal, P.; Kumar, T. Serum Levels of Activin A: Marker of Insulin Resistance and Cardiovascular Risk in Prediabetics. J. Family Med. Prim. Care 2022, 11, 5983. [Google Scholar] [CrossRef]
- Brown, M.L.; Ungerleider, N.; Bonomi, L.; Andrzejewski, D.; Burnside, A.; Schneyer, A. Effects of Activin A on Survival, Function and Gene Expression of Pancreatic Islets from Non-Diabetic and Diabetic Human Donors. Islets 2014, 6, e1017226. [Google Scholar] [CrossRef][Green Version]
- Bradley, D.; Blaszczak, A.; Yin, Z.; Liu, J.; Joseph, J.J.; Wright, V.; Anandani, K.; Needleman, B.; Noria, S.; Renton, D.; et al. Clusterin Impairs Hepatic Insulin Sensitivity and Adipocyte Clusterin Associates with Cardiometabolic Risk. Diabetes Care 2019, 42, 466–475. [Google Scholar] [CrossRef]
- Aronis, K.N.; Vamvini, M.T.; Chamberland, J.P.; Mantzoros, C.S. Circulating Clusterin (Apolipoprotein J) Levels Do Not Have Any Day/Night Variability and Are Positively Associated with Total and LDL Cholesterol Levels in Young Healthy Individuals. J. Clin. Endocrinol. Metab. 2011, 96, E1871–E1875. [Google Scholar] [CrossRef] [PubMed]
- Kloucková, J.; Lacinová, Z.; Kaválková, P.; Trachta, P.; Kasalickỳ, M.; Haluzíková, D.; Mráz, M.; Haluzík, M. Plasma Concentrations and Subcutaneous Adipose Tissue MRNA Expression of Clusterin in Obesity and Type 2 Diabetes Mellitus: The Effect of Short-Term Hyperinsulinemia, Very-Low-Calorie Diet and Bariatric Surgery. Physiol. Res. 2016, 65, 481–492. [Google Scholar] [CrossRef]
- Kim, S.S.; Song, S.H.; Kim, J.H.; Jeon, Y.K.; Kim, B.H.; Kang, M.C.; Chun, S.W.; Hong, S.H.; Chung, M.; Kim, Y.K.; et al. Urine Clusterin/Apolipoprotein J Is Linked to Tubular Damage and Renal Outcomes in Patients with Type 2 Diabetes Mellitus. Clin. Endocrinol. 2017, 87, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, S.; Wei, M.; Ishimura, E.; Kakehashi, A.; Mori, K.; Nishizawa, Y.; Inaba, M.; Wanibuchi, H. Proteome Analysis of Laser Microdissected Glomeruli from Formalin-Fixed Paraffin-Embedded Kidneys of Autopsies of Diabetic Patients: Nephronectin Is Associated with the Development of Diabetic Glomerulosclerosis. Nephrol. Dial. Transplant. 2012, 27, 1889–1897. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rastaldi, M.P.; Candiano, G.; Musante, L.; Bruschi, M.; Armelloni, S.; Rimoldi, L.; Tardanico, R.; Cherchi, S.S.; Ferrario, F.; Montinaro, V.; et al. Glomerular Clusterin Is Associated with PKC-Alpha/Beta Regulation and Good Outcome of Membranous Glomerulonephritis in Humans. Kidney Int. 2006, 70, 477–485. [Google Scholar] [CrossRef]
- He, J.; Dijkstra, K.L.; Bakker, K.; Bus, P.; Bruijn, J.A.; Scharpfenecker, M.; Baelde, H.J. Glomerular Clusterin Expression Is Increased in Diabetic Nephropathy and Protects against Oxidative Stress-Induced Apoptosis in Podocytes. Sci. Rep. 2020, 10, 14888. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Guan, Q.; Kwan, C.C.H.; Chen, H.; Gleave, M.E.; Nguan, C.Y.C.; Du, C. Loss of Clusterin Expression Worsens Renal Ischemia-Reperfusion Injury. Am. J. Physiol. Renal Physiol. 2010, 298, F568–F578. [Google Scholar] [CrossRef]
| Controls (n = 43) | T1DM (n = 43) | Obesity (n = 43) | p-Value (ANOVA for Quantitative Variables) | |
|---|---|---|---|---|
| Age (years) | 12.3 ± 4.3 | 12.6 ± 3.8 | 12.4 ± 3.8 | ns |
| Gender (males/females) | 12/31 | 20/23 | 18/25 | ns |
| BMI z-score | 0.61 ± 0.73 | 0.59 ± 0.89 | 2.17 ± 0.45 | <0.001 |
| Serum creatinine (mg/dL) | 0.66 ± 0.13 | 0.7 ± 0.12 | 0.65 ± 0.13 | ns |
| eGFR (mL/min/1.73 m2) | 95.4 ± 17.3 | 92.6 ± 21.5 | 97.6 ± 15.4 | ns |
| TSH (mIU/L) | 2 ± 0.86 | 1.9 ± 0.79 | 2.45 ± 1.1 | 0.015 |
| FT4 (ng/dL) | 1.27 ± 0.18 | 1.25 ± 0.17 | 1.24 ± 0.21 | ns |
| Cholesterol (mg/dL) | 161 ± 26.4 | 167 ± 29.7 | 163 ± 37.8 | ns |
| LDL (mg/dL) | 93 ± 28.7 | 94 ± 23.9 | 95 ± 36.3 | 0.04 |
| HDL (mg/dL) | 61 ± 13.1 | 60 ± 11.2 | 50 ± 12.7 | <0.001 |
| Triglycerides (mg/dL) | 63 ± 21.6 | 66 ± 26.8 | 89 ± 56.4 | 0.003 |
| Controls | T1DM | Obesity | p-Value (ANOVA for Clusterin and Kruskal–Wallis Test for MCP-1 and Activin-A) | |
|---|---|---|---|---|
| MCP-1 (pg/mL) | 305.5 ±143.4 | 326.8 ± 164.2 | 297.6 ± 99.35 | ns |
| Activin-A (pg/mL) | 244.5 ± 89.85 | 278.1 ± 125.4 | 278.2 ± 68.27 | 0.0168 |
| Clusterin (ng/mL) | 200,023 ± 34,656 | 186,222 ± 25,739 | 186,679 ± 39,289 | ns |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostopoulou, E.; Kalavrizioti, D.; Davoulou, P.; Papachristou, E.; Sinopidis, X.; Fouzas, S.; Dassios, T.; Gkentzi, D.; Kyriakou, S.I.; Karatza, A.; et al. Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus. Diagnostics 2024, 14, 450. https://doi.org/10.3390/diagnostics14040450
Kostopoulou E, Kalavrizioti D, Davoulou P, Papachristou E, Sinopidis X, Fouzas S, Dassios T, Gkentzi D, Kyriakou SI, Karatza A, et al. Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus. Diagnostics. 2024; 14(4):450. https://doi.org/10.3390/diagnostics14040450
Chicago/Turabian StyleKostopoulou, Eirini, Dimitra Kalavrizioti, Panagiota Davoulou, Evangelos Papachristou, Xenophon Sinopidis, Sotirios Fouzas, Theodore Dassios, Despoina Gkentzi, Stavroula Ioanna Kyriakou, Ageliki Karatza, and et al. 2024. "Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus" Diagnostics 14, no. 4: 450. https://doi.org/10.3390/diagnostics14040450
APA StyleKostopoulou, E., Kalavrizioti, D., Davoulou, P., Papachristou, E., Sinopidis, X., Fouzas, S., Dassios, T., Gkentzi, D., Kyriakou, S. I., Karatza, A., Dimitriou, G., Goumenos, D., Spiliotis, B. E., Plotas, P., & Papasotiriou, M. (2024). Monocyte Chemoattractant Protein-1 (MCP-1), Activin-A and Clusterin in Children and Adolescents with Obesity or Type-1 Diabetes Mellitus. Diagnostics, 14(4), 450. https://doi.org/10.3390/diagnostics14040450









