Segregated Dynamical Networks for Biological Motion Perception in the Mu and Beta Range Underlie Social Deficits in Autism
Abstract
1. Introduction
1.1. BMP and MNS
1.2. Connectivity in Individuals with ASD
1.3. Aim of the Present Study
Hypotheses
- (1)
- Behavior: In individuals with ASD, we predicted more difficulties in distinguishing human from random motion. We also explored brain–behavior correlations
- (2)
- EEG: Further, we postulated a weaker desynchronization in the mu and beta rhythms in response to BMP
- (3)
- Source Analysis: In addition, we anticipated altered network characteristics in individuals with ASD, possibly with distinct sub-networks for form and for motion
- (4)
- tPDC: We expected the associated coherence and directional connectivity values for mu and low-beta rhythms to be weaker and more local in individuals with ASD, with distinct directionalities between form, motion, and MNS
- (5)
- SVM: Finally, we anticipated that the behavioral and electrophysiological parameters can separate both groups (TDC, ASD) using classification algorithms.
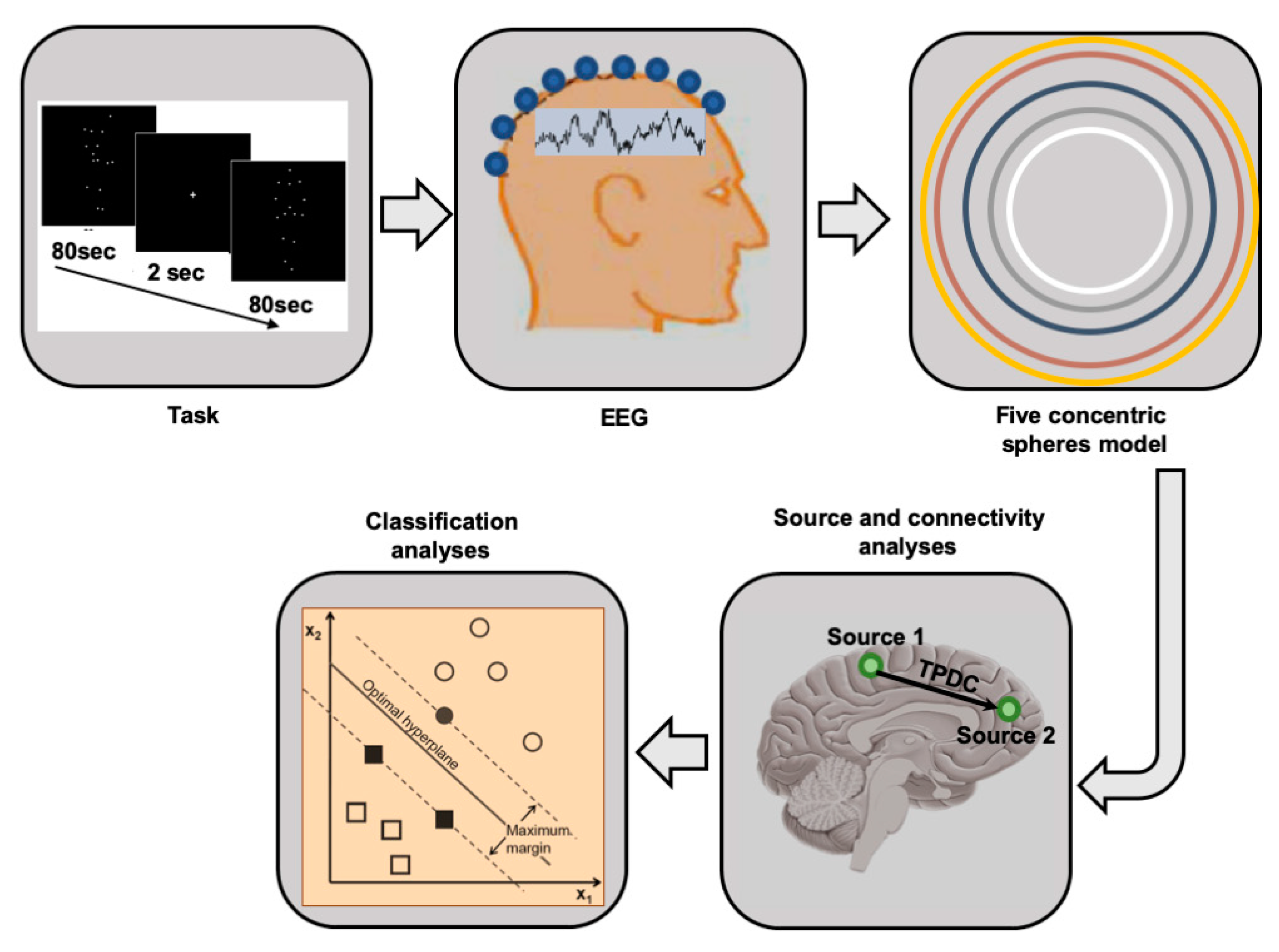
2. Methods
2.1. Subjects
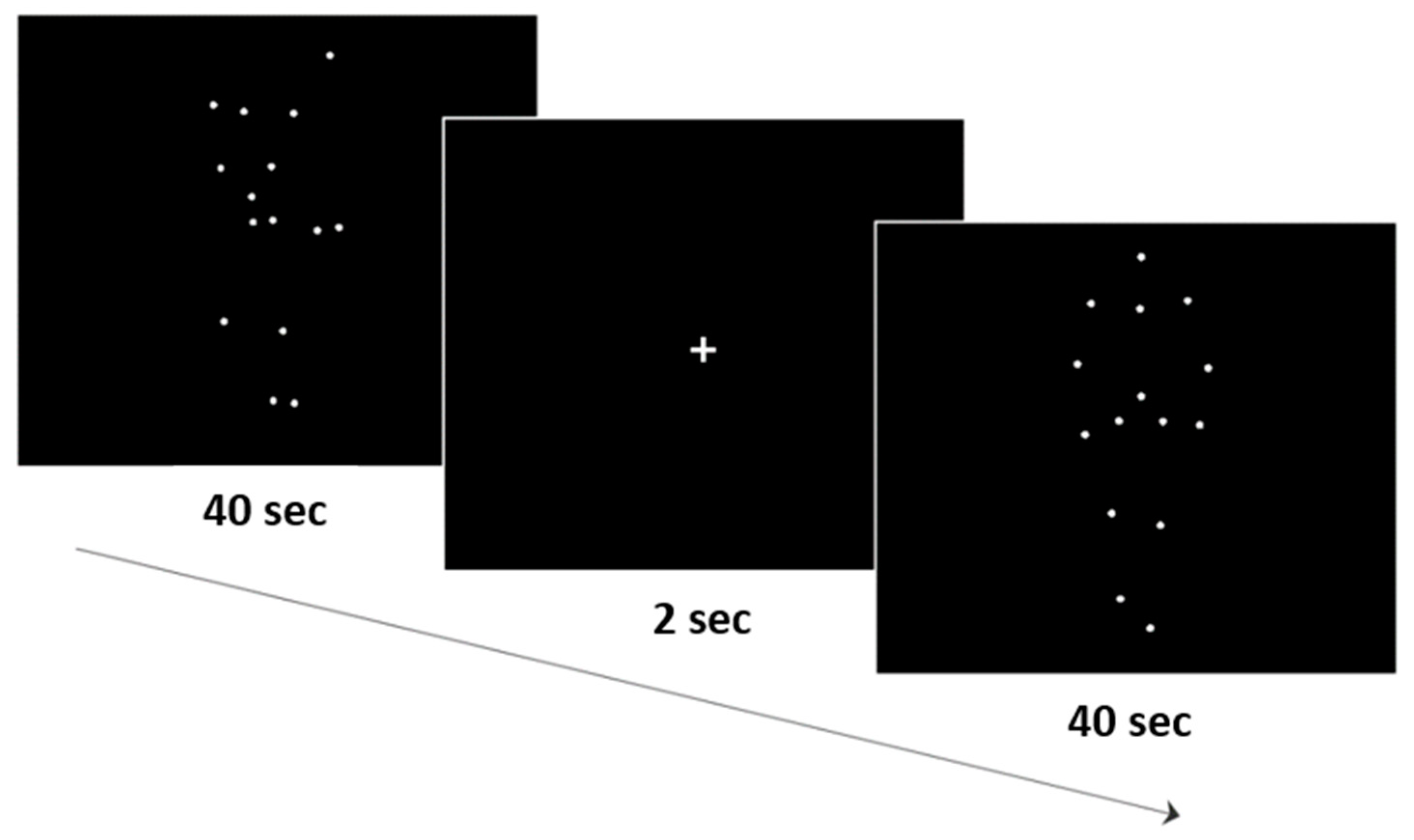
2.2. Experimental Design, Procedure, and Stimuli Presentation
2.3. EEG Recordings
2.4. Signal Pre-Processing
2.5. EEG Synchrony
2.6. Source Analysis
2.7. Directionality Analysis
2.8. Statistical Analysis
2.9. SVM Analyses
3. Results
3.1. Sample Characteristics
3.2. Behavioral Data
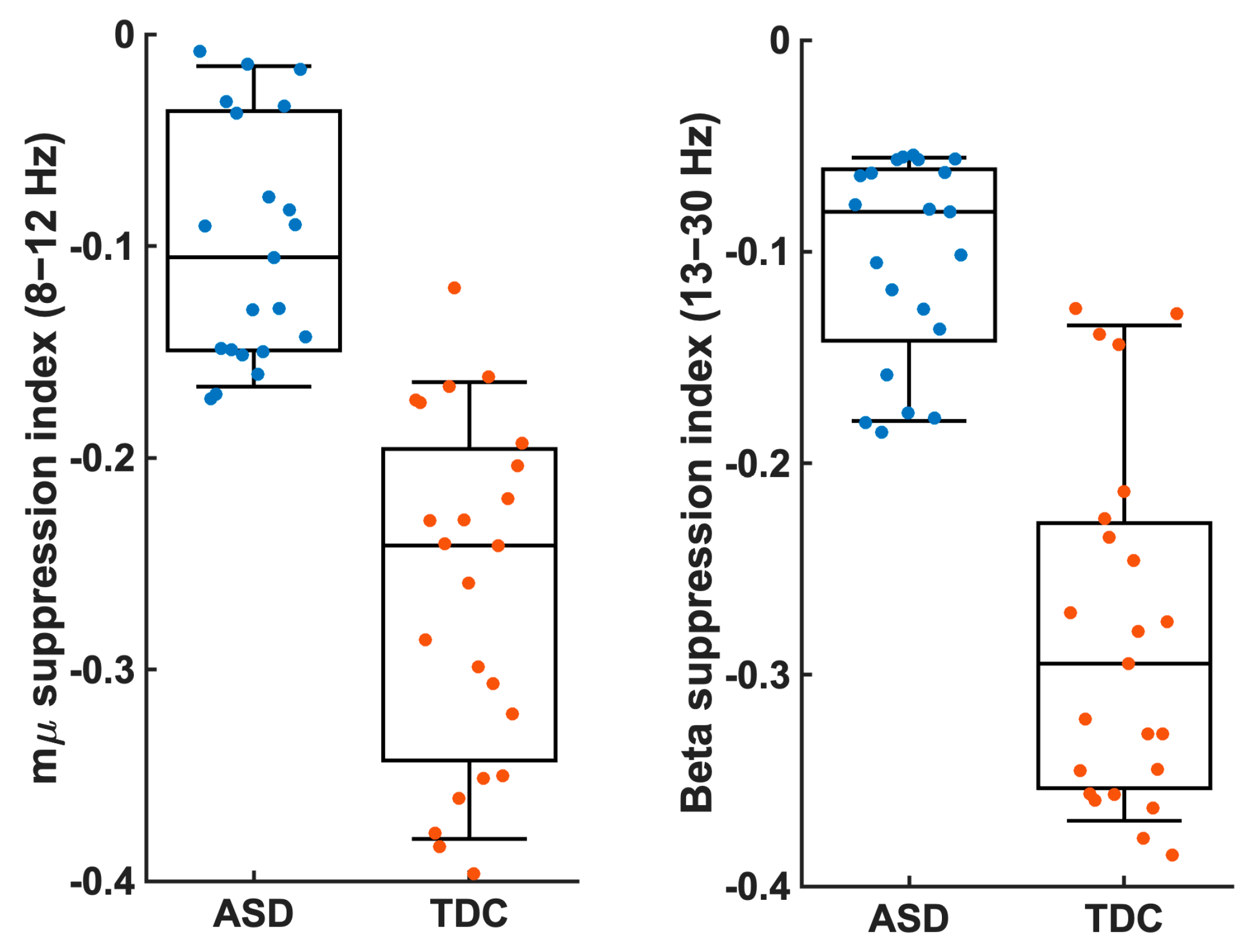
3.3. EEG Synchrony
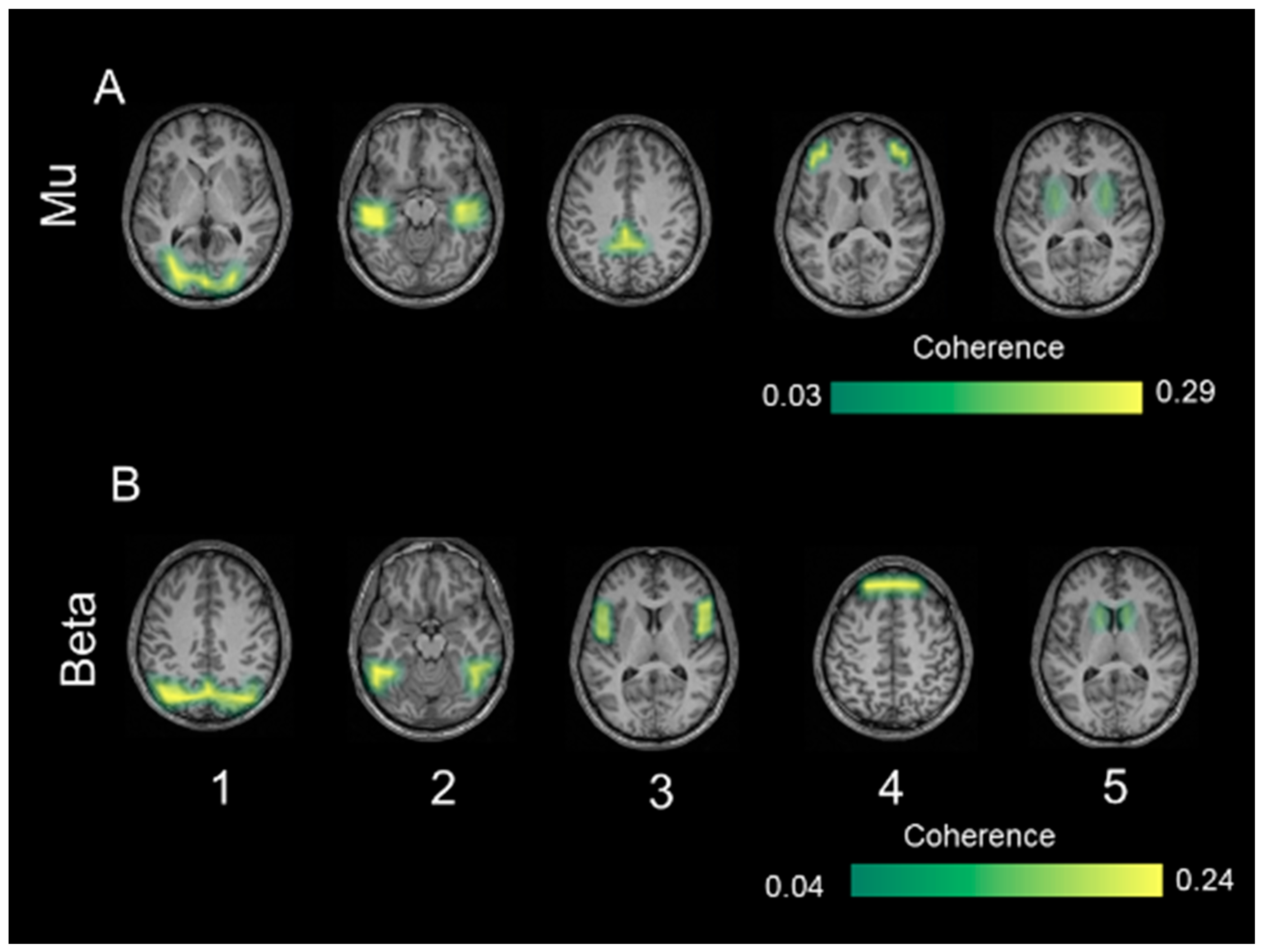
3.4. Coherent Sources
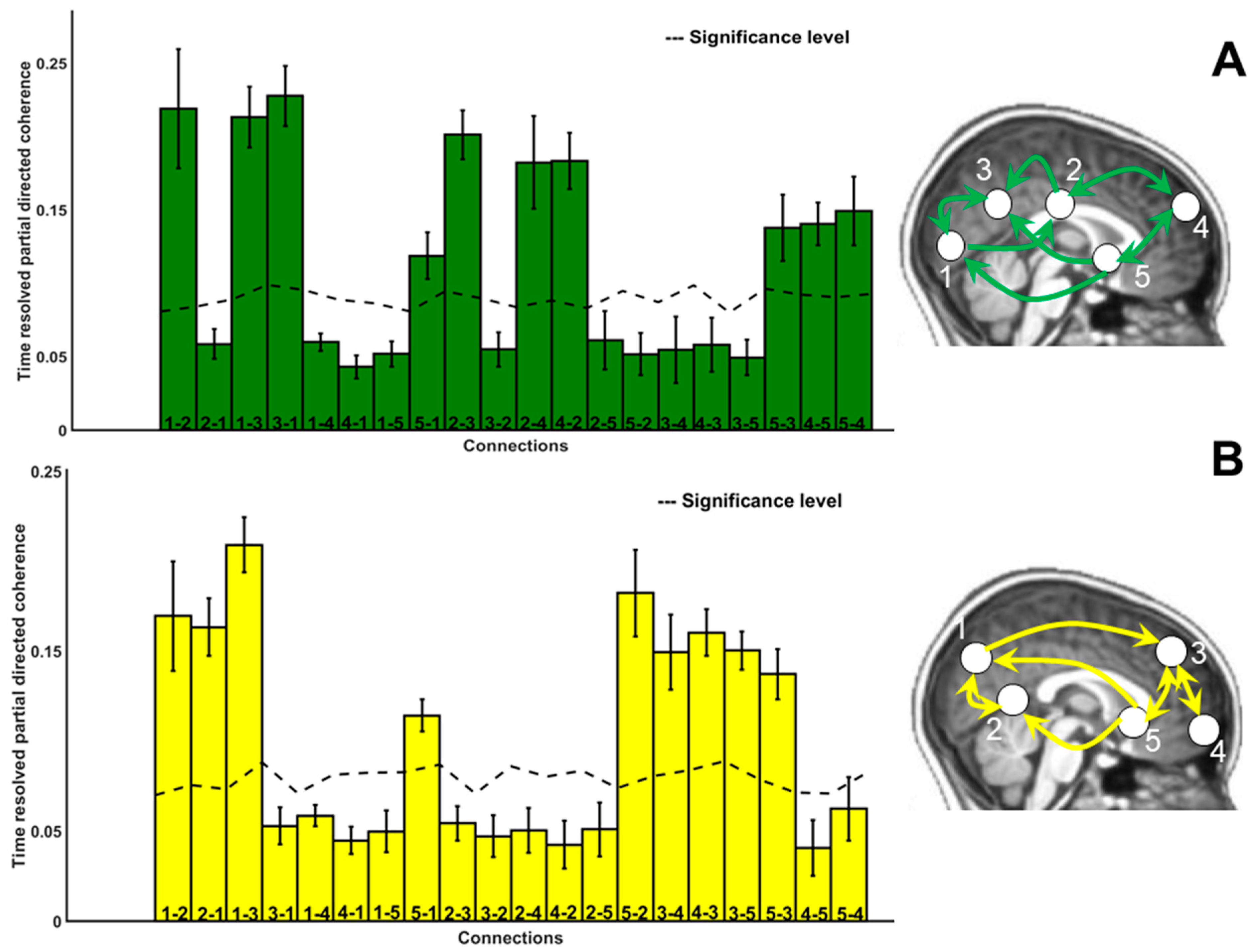
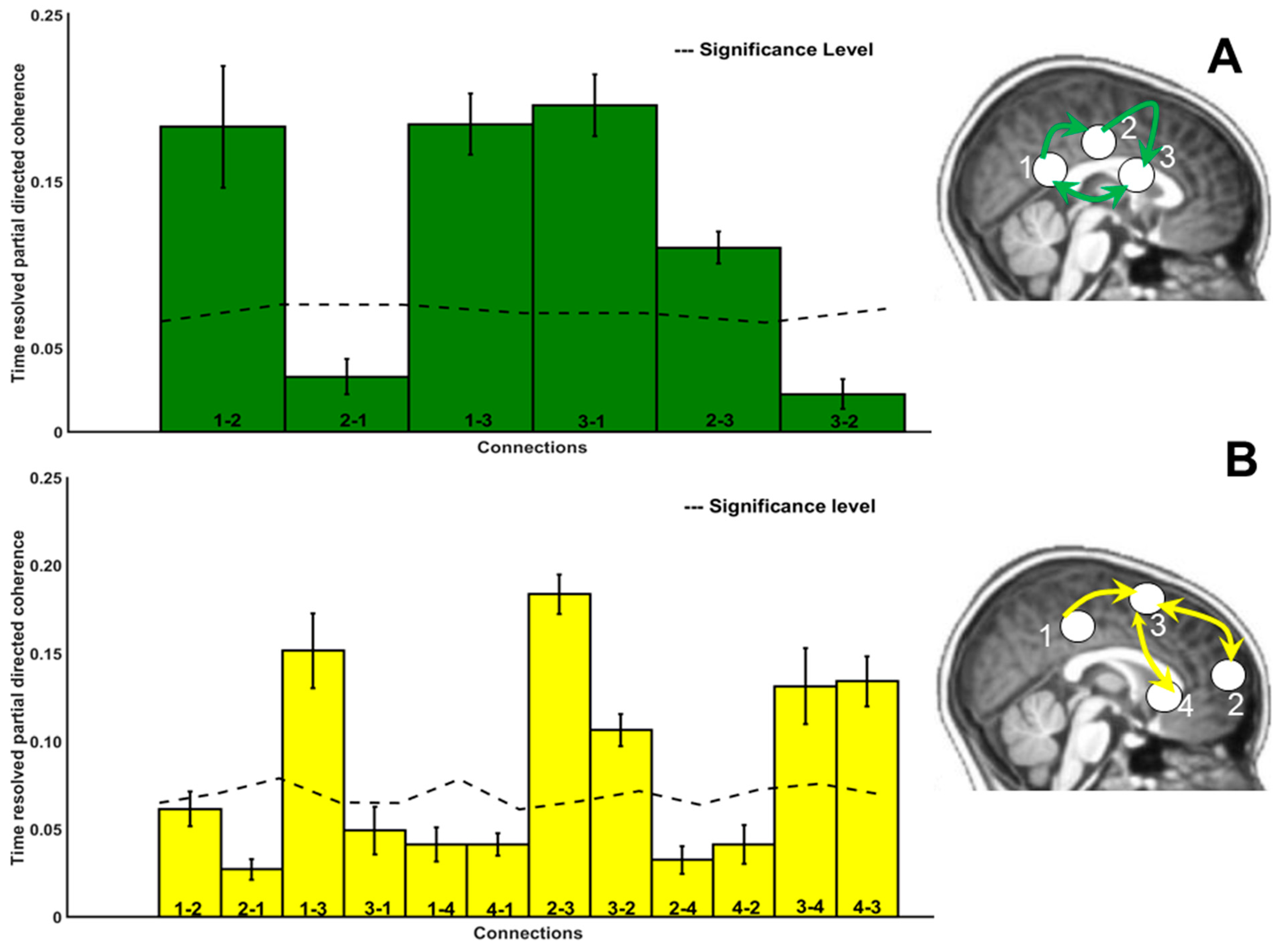
3.5. Directional Connectivity
3.6. Brain–Behavior Correlation
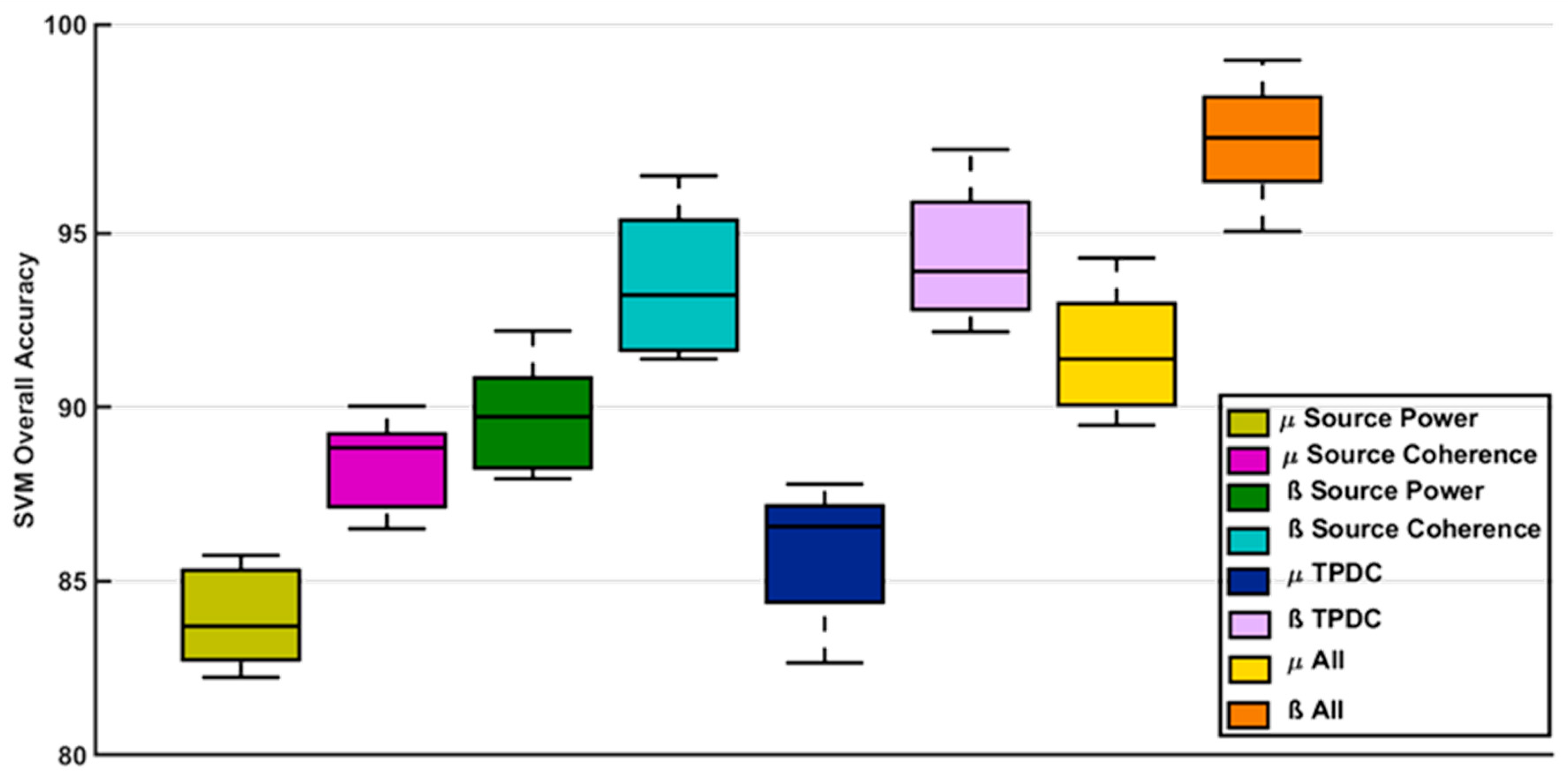
3.7. Classification Results
4. Discussion
4.1. Behavioral Data
4.2. EEG
4.3. Coherent Sources
4.3.1. Connectivity: Paths
4.3.2. Connectivity: Range
4.3.3. Connectivity: Strength
4.4. SVM Classifier
5. Conclusions
6. Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | Autism Spectrum Disorder |
| BMP | Biological Motion Perception |
| DICS | Dynamic Imaging of Coherent Sources |
| EEG | Electroencephalography |
| MNS | Mirror Neuron System |
| PLW | Point-like Walkers |
| SVM | Support Vector Machine |
| TDC | Typically Developing Controls |
| ToM | Theory of Mind |
| tPDC | time-resolved Partial Directed Coherence |
| TRT | time reversal technique |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th ed.; American Psychiatric Association: Washington, DC, USA, 2010. [Google Scholar]
- Williams, J.H.G.; Whiten, A.; Singh, T. A Systematic Review of Action Imitation in Autistic Spectrum Disorder. J. Autism Dev. Disord. 2004, 34, 285–299. [Google Scholar] [CrossRef]
- Bertollo, J.R.; Strang, J.F.; Anthony, L.G.; Kenworthy, L.; Wallace, G.L.; Yerys, B.E. Adaptive Behavior in Youth with Autism Spectrum Disorder: The Role of Flexibility. J. Autism Dev. Disord. 2019, 50, 42–50. [Google Scholar] [CrossRef]
- Stone, W.L.; Yoder, P.J. Predicting Spoken Language Level in Children with Autism Spectrum Disorders. Autism 2001, 5, 341–361. [Google Scholar] [CrossRef]
- Freitag, C.M.; Konrad, C.; Häberlen, M.; Kleser, C.; von Gontard, A.; Reith, W.; Troje, N.F.; Krick, C. Perception of biological motion in autism spectrum disorders. Neuropsychologia 2008, 46, 1480–1494. [Google Scholar] [CrossRef] [PubMed]
- Jack, A.; Keifer, C.M.; Pelphrey, K.A. Cerebellar contributions to biological motion perception in autism and typical development. Hum. Brain Mapp. 2017, 38, 1914. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, M.D.; Pelphrey, K.A. Disrupted action perception in autism: Behavioral evidence, neuroendophenotypes, and diagnostic utility. Dev. Cogn. Neurosci. 2012, 2, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Zaidel, A.; Goin-Kochel, R.P.; Angelaki, D.E. Self-motion perception in autism is compromised by visual noise but integrated optimally across multiple senses. Proc. Natl. Acad. Sci. USA 2015, 112, 6461–6466. [Google Scholar] [CrossRef] [PubMed]
- Johansson, G. Visual perception of biological motion and a model for its analysis. Percept. Psychophys. 1973, 14, 201–211. [Google Scholar] [CrossRef]
- Simion, F.; Regolin, L.; Bulf, H. A predisposition for biological motion in the newborn baby. Proc. Natl. Acad. Sci. USA 2008, 105, 809–813. [Google Scholar] [CrossRef] [PubMed]
- Todorova, G.K.; Hatton, R.E.M.; Pollick, F.E. Biological motion perception in autism spectrum disorder: A meta-analysis. Mol. Autism 2019, 10, 49. [Google Scholar] [CrossRef] [PubMed]
- McKay, L.S.; Simmons, D.R.; McAleer, P.; Marjoram, D.; Piggot, J.; Pollick, F.E. Do distinct atypical cortical networks process biological motion information in adults with Autism Spectrum Disorders? NeuroImage 2012, 59, 1524–1533. [Google Scholar] [CrossRef]
- Saffin, J.M.; Tohid, H. Walk like me, talk like me. Neurosciences 2016, 21, 108–119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strauß, B.; Schumacher, J. Elternfragebogen über das Verhalten von Kindern und Jugendlichen. In Klinische Interviewsund Ratingskalen; Hogrefe Verlag: Göttingen, Germany, 2004. [Google Scholar]
- Fraiman, D.; Saunier, G.; Martins, E.F.; Vargas, C.D. Biological Motion Coding in the Brain: Analysis of Visually Driven EEG Functional Networks. PLoS ONE 2014, 9, e84612. [Google Scholar] [CrossRef] [PubMed]
- Puzzo, I.; Cooper, N.R.; Cantarella, S.; Russo, R. Measuring the effects of manipulating stimulus presentation time on sensorimotor alpha and low beta reactivity during hand movement observation. NeuroImage 2011, 57, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Kröger, A.; Bletsch, A.; Krick, C.; Siniatchkin, M.; Jarczok, T.A.; Freitag, C.M.; Bender, S. Visual event-related potentials to biological motion stimuli in autism spectrum disorders. Soc. Cogn. Affect. Neurosci. 2014, 9, 1214–1222. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.E.; Cunnington, R. More than an imitation game: Top-down modulation of the human mirror system. Neurosci. Biobehav. Rev. 2017, 75, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, M.; Guerreschi, M.; Lutzenberger, W.; Krägeloh-Mann, I. Social Interaction Revealed by Motion: Dynamics of Neuromagnetic Gamma Activity. Cereb. Cortex 2010, 20, 2361–2367. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, V.; Rizzolatti, G.; Wicker, B.; Keysers, C. The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. NeuroImage 2007, 35, 1674–1684. [Google Scholar] [CrossRef]
- Chaminade, T.; Rosset, D.; Da Fonseca, D.; Hodgins, J.K.; Deruelle, C. Anthropomorphic bias found in typically developing children is not found in children with autistic spectrum disorder. Autism 2013, 19, 248–251. [Google Scholar] [CrossRef]
- Oberman, L.M.; Ramachandran, V.S.; Pineda, J.A. Modulation of mu suppression in children with autism spectrum disorders in response to familiar or unfamiliar stimuli: The mirror neuron hypothesis. Neuropsychologia 2008, 46, 1558–1565. [Google Scholar] [CrossRef]
- Arbib, M. The Mirror System Hypothesis. In Action to Language via the Mirror Neuron System; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Perry, A.; Troje, N.F.; Bentin, S. Exploring motor system contributions to the perception of social information: Evidence from EEG activity in the mu/alpha frequency range. Soc. Neurosci. 2010, 5, 272–284. [Google Scholar] [CrossRef]
- Cheon, K.-A.; Kim, Y.-S.; Oh, S.-H.; Park, S.-Y.; Yoon, H.-W.; Herrington, J.; Nair, A.; Koh, Y.-J.; Jang, D.-P.; Leventhal, B.L.; et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A Diffusion Tensor Imaging study. Brain Res. 2011, 1417, 77–86. [Google Scholar] [CrossRef]
- Just, M.A.; Keller, T.A.; Malave, V.L.; Kana, R.K.; Varma, S. Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neurosci. Biobehav. Rev. 2012, 36, 1292–1313. [Google Scholar] [CrossRef]
- Rane, P.; Cochran, D.; Hodge, S.M.; Haselgrove, C.; Kennedy, D.; Frazier, J.A. Connectivity in Autism: A review of MRI connectivity studies. Harv. Rev. Psychiatry 2016, 23, 223. [Google Scholar] [CrossRef]
- Solso, S.; Xu, R.; Proudfoot, J.; Hagler, D.J.; Campbell, K.; Venkatraman, V.; Barnes, C.C.; Ahrens-Barbeau, C.; Pierce, K.; Dale, A.; et al. Diffusion Tensor Imaging Provides Evidence of Possible Axonal Overconnectivity in Frontal Lobes in Autism Spectrum Disorder Toddlers. Biol. Psychiatry 2016, 79, 676–684. [Google Scholar] [CrossRef]
- Weinstein, M.; Ben-Sira, L.; Levy, Y.; Zachor, D.A.; Ben Itzhak, E.; Artzi, M.; Tarrasch, R.; Eksteine, P.M.; Hendler, T.; Ben Bashat, D. Abnormal white matter integrity in young children with autism. Hum. Brain Mapp. 2011, 32, 534–543. [Google Scholar] [CrossRef]
- Courchesne, E.; Pierce, K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Ameis, S.H.; Fan, J.; Rockel, C.; Voineskos, A.N.; Lobaugh, N.J.; Soorya, L.; Wang, A.T.; Hollander, E.; Anagnostou, E. Impaired Structural Connectivity of Socio-Emotional Circuits in Autism Spectrum Disorders: A Diffusion Tensor Imaging Study. PLoS ONE 2011, 6, e28044. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Chua, S.; Cheung, V.; Khong, P.; Tai, K.; Wong, T.; Ho, T.; McAlonan, G. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J. Child Psychol. Psychiatry 2009, 50, 1102–1112. [Google Scholar] [CrossRef] [PubMed]
- Koldewyn, K.; Whitney, D.; Rivera, S.M. Neural correlates of coherent and biological motion perception in autism. Dev. Sci. 2011, 14, 1075–1088. [Google Scholar] [CrossRef] [PubMed]
- Alaerts, K.; Swinnen, S.P.; Wenderoth, N. Neural processing of biological motion in autism: An investigation of brain activity and effective connectivity. Sci. Rep. 2017, 7, 5612. [Google Scholar] [CrossRef]
- Shih, P.; Keehn, B.; Oram, J.K.; Leyden, K.M.; Keown, C.L.; Müller, R.-A. Functional Differentiation of Posterior Superior Temporal Sulcus in Autism: A Functional Connectivity Magnetic Resonance Imaging Study. Biol. Psychiatry 2011, 70, 270–277. [Google Scholar] [CrossRef]
- Barttfeld, P.; Amoruso, L.; Ais, J.; Cukier, S.; Bavassi, L.; Tomio, A.; Manes, F.; Ibanez, A.; Sigman, M. Organization of brain networks governed by long-range connections index autistic traits in the general population. J. Neurodev. Disord. 2013, 5, 16. [Google Scholar] [CrossRef]
- Nyström, P.; Jones, E.; Darki, F.; Bölte, S.; Falck-Ytter, T. Atypical Topographical Organization of Global Form and Motion Processing in 5-Month-Old Infants at Risk for Autism. J. Autism Dev. Disord. 2021, 51, 364–370. [Google Scholar] [CrossRef] [PubMed]
- Annaz, D.; Remington, A.; Milne, E.; Coleman, M.; Campbell, R.; Thomas, M.S.C.; Swettenham, J. Development of motion processing in children with autism. Dev. Sci. 2010, 13, 826–838. [Google Scholar] [CrossRef] [PubMed]
- Bakroon, A.; Lakshminarayanan, V. Do different experimental tasks affect psychophysical measurements of motion perception in autism-spectrum disorder? An analysis. Clin. Optom. 2018, 10, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Decety, J.; Yang, C.; Liu, J.; Cheng, Y. Unbroken mirror neurons in autism spectrum disorders. J. Child Psychol. Psychiatry 2010, 51, 981–988. [Google Scholar] [CrossRef] [PubMed]
- Raymaekers, R.; Wiersema, J.R.; Roeyers, H. EEG study of the mirror neuron system in children with high functioning autism. Brain Res. 2009, 1304, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ruysschaert, L.; Warreyn, P.; Wiersema, J.R.; Oostra, A.; Roeyers, H. Exploring the Role of Neural Mirroring in Children with Autism Spectrum Disorder. Autism Res. 2014, 7, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Cleary, L.; Looney, K.; Brady, N.; Fitzgerald, M. Inversion effects in the perception of the moving human form: A comparison of adolescents with autism spectrum disorder and typically developing adolescents. Autism 2014, 18, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Sotoodeh, M.S.; Taheri-Torbati, H.; Sohrabi, M.; Ghoshuni, M. Perception of biological motions is preserved in people with autism spectrum disorder: Electrophysiological and behavioural evidences. J. Intellect. Disabil. Res. 2019, 63, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Sotoodeh, M.S.; Taheri-Torbati, H.; Hadjikhani, N.; Lassalle, A. Preserved action recognition in children with autism spectrum disorders: Evidence from an EEG and eye-tracking study. Psychophysiology 2021, 58, e13740. [Google Scholar] [CrossRef] [PubMed]
- Cusack, J.P.; Williams, J.H.; Neri, P. Action Perception Is Intact in Autism Spectrum Disorder. J. Neurosci. 2015, 35, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Hubert, B.; Wicker, B.; Moore, D.G.; Monfardini, E.; Duverger, H.; Da Fonséca, D.; Deruelle, C. Brief Report: Recognition of Emotional and Non-emotional Biological Motion in Individuals with Autistic Spectrum Disorders. J. Autism Dev. Disord. 2007, 37, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Haykin, S. Kalman Filtering and Neural Networks; Adaptive and Learning Systems for Signal Processing, Communications and Control Series; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Fiebelkorn, I.C.; Kastner, S. Functional Specialization in the Attention Network. Annu. Rev. Psychol. 2020, 71, 221–249. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.A.; Nelson, A.T. Dual Extended Kalman Filter Methods. In Kalman Filtering and Neural Networks; John Wiley & Sons: Hoboken, NJ, USA, 2001. [Google Scholar]
- Federici, A.; Parma, V.; Vicovaro, M.; Radassao, L.; Casartelli, L.; Ronconi, L. Anomalous Perception of Biological Motion in Autism: A Conceptual Review and Meta-Analysis. Sci. Rep. 2020, 10, 4576. [Google Scholar] [CrossRef] [PubMed]
- Vanvuchelen, M.; Van Schuerbeeck, L.; Roeyers, H.; De Weerdt, W. Understanding the mechanisms behind deficits in imitation: Do individuals with autism know ‘what’ to imitate and do they know ‘how’ to imitate? Res. Dev. Disabil. 2013, 34, 538–545. [Google Scholar] [CrossRef][Green Version]
- Ulloa, E.R.; Pineda, J.A. Recognition of point-light biological motion: Mu rhythms and mirror neuron activity. Behav. Brain Res. 2007, 183, 188–194. [Google Scholar] [CrossRef]
- Japaridze, N.; Siniatchkin, M.; Muthuraman, M.; Raethjen, J.; Stephani, U.; Moeller, F. Dynamic imaging of coherent sources in absences and generalized photoparoxysmal responses—A comparison with EEG-FMRI studies. Z. Für Epileptol. 2013, 1, 19–24. [Google Scholar] [CrossRef]
- Gross, J.; Kujala, J.; Hämäläinen, M.; Timmermann, L.; Schnitzler, A.; Salmelin, R. Dynamic imaging of coherent sources: Studying neural interactions in the human brain. Proc. Natl. Acad. Sci. USA 2001, 98, 694–699. [Google Scholar] [CrossRef]
- Liljeström, M.; Kujala, J.; Jensen, O.; Salmelin, R. Neuromagnetic localization of rhythmic activity in the human brain: A comparison of three methods. NeuroImage 2005, 25, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Muthuraman, M.; Raethjen, J.; Koirala, N.; Anwar, A.R.; Mideksa, K.G.; Elble, R.; Groppa, S.; Deuschl, G. Cerebello-cortical network fingerprints differ between essential, Parkinson’s and mimicked tremors. Brain 2018, 141, 1770–1781. [Google Scholar] [CrossRef] [PubMed]
- Blinowska, K.J. Review of the methods of determination of directed connectivity from multichannel data. Med. Biol. Eng. Comput. 2011, 49, 521–529. [Google Scholar] [CrossRef] [PubMed]
- WHO. International Statistical Classification of Diseases and Related Health Problems 10; World Health Organization: Geneva, Switzerland, 2004. [Google Scholar]
- Rühl, D.; Bölte, S.; Feineis-Matthews, S.; Poustka, F. (Eds.) Diagnostische Beobachtungsskala für Autistische Störungen. ADOS.; Manual; Deutsche Fassung der Autism Diagnostic Observation Schedule von Catherine Lord; Huber: Bern, Switzerland, 2004. [Google Scholar]
- Raven, J.C.; Raven, J.; Court, J.H.; Bulheller, S.; Häcker, H. Coloured Progressive Matrices: Mit der Parallelform des Tests und der Puzzle-Form; Harcourt Test Services: Frankfurt, Germany, 2006. [Google Scholar]
- Troje, N.F. Decomposing biological motion: A framework for analysis and synthesis of human gait patterns. J. Vis. 2002, 2, 2. [Google Scholar] [CrossRef]
- Klimesch, W.; Russegger, H.; Doppelmayr, M.; Pachinger, T. A method for the calculation of induced band power: Implications for the significance of brain oscillations. Electroencephalogr. Clin. Neurophysiol. Potentials Sect. 1998, 108, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef]
- Oostenveld, R.; Fries, P.; Maris, E.; Schoffelen, J.-M. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput. Intell. Neurosci. 2011, 2011, 156869. [Google Scholar] [CrossRef]
- Van Veen, B.D.; Van Drongelen, W.; Yuchtman, M.; Suzuki, A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997, 44, 867–880. [Google Scholar] [CrossRef]
- De Munck, J.C. A linear discretization of the volume conductor boundary integral equation using analytically integrated elements (electrophysiology application). IEEE Trans. Biomed. Eng. 1992, 39, 986–990. [Google Scholar] [CrossRef]
- Van Uitert, R.; Johnson, C. Can a Spherical Model Substitute for a Realistic Head Model in Forward and Inverse MEG Simulations? Biomagn. J. 2004, 25, 52–68. [Google Scholar]
- Richards, J.E.; Sanchez, C.; Phillips-Meek, M.; Xie, W. A database of age-appropriate average MRI templates. NeuroImage 2015, 124, 1254–1259. [Google Scholar] [CrossRef]
- Amjad, A.M.; Halliday, D.M.; Rosenberg, J.R.; Conway, B.A. An extended difference of coherence test for comparing and combining several independent coherence estimates: Theory and application to the study of motor units and physiological tremor. J. Neurosci. Methods 1997, 73, 69–79. [Google Scholar] [CrossRef]
- Rosenberg, J.R.; Amjad, A.M.; Breeze, P.; Brillinger, D.R.; Halliday, D.M. The Fourier approach to the identification of functional coupling between neuronal spike trains. Prog. Biophys. Mol. Biol. 1989, 53, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Jokisch, D.; Daum, I.; Suchan, B.; Troje, N.F. Structural encoding and recognition of biological motion: Evidence from event-related potentials and source analysis. Behav. Brain Res. 2005, 157, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, M.; Ding, M.; Truccolo, W.A.; Bressler, S.L. Evaluating causal relations in neural systems: Granger causality, directed transfer function and statistical assessment of significance. Biol. Cybern. 2001, 85, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Dubovik, S.; Pignat, J.-M.; Ptak, R.; Aboulafia, T.; Allet, L.; Gillabert, N.; Magnin, C.; Albert, F.; Momjian-Mayor, I.; Nahum, L.; et al. The behavioral significance of coherent resting-state oscillations after stroke. NeuroImage 2012, 61, 249–257. [Google Scholar] [CrossRef]
- Haufe, S.; Nikulin, V.V.; Müller, K.-R.; Nolte, G. A critical assessment of connectivity measures for EEG data: A simulation study. NeuroImage 2013, 64, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Cortes, C.; Vapnik, V. Support-vector networks. Mach. Learn. 1995, 20, 273–297. [Google Scholar] [CrossRef]
- Michels, L.; Muthuraman, M.; Anwar, A.R.; Kollias, S.; Leh, S.E.; Riese, F.; Unschuld, P.G.; Siniatchkin, M.; Gietl, A.F.; Hock, C. Changes of Functional and Directed Resting-State Connectivity Are Associated with Neuronal Oscillations, ApoE Genotype and Amyloid Deposition in Mild Cognitive Impairment. Front. Aging Neurosci. 2017, 9, 304. [Google Scholar] [CrossRef]
- Muthuraman, M.; Fleischer, V.; Kolber, P.; Luessi, F.; Zipp, F.; Groppa, S. Structural Brain Network Characteristics Can Differentiate CIS from Early RRMS. Front. Neurosci. 2016, 10, 14. [Google Scholar] [CrossRef]
- Foglia, V.; Siddiqui, H.; Khan, Z.; Liang, S.; Rutherford, M.D. Distinct Biological Motion Perception in Autism Spectrum Disorder: A Meta-Analysis. J. Autism. Dev. Disord. 2022, 52, 4843–4860. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Johnson, B.W. Primary motor cortex activation during action observation revealed by wavelet analysis of the EEG. Clin. Neurophysiol. 2004, 115, 1760–1766. [Google Scholar] [CrossRef]
- Arnstein, D.; Cui, F.; Keysers, C.; Maurits, N.M.; Gazzola, V. μ-suppression during action observation and execution correlates with BOLD in dorsal premotor, inferior parietal, and SI cortices. J. Neurosci. 2011, 31, 14243–14249. [Google Scholar] [CrossRef]
- O’reilly, C.; Lewis, J.D.; Elsabbagh, M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS ONE 2017, 12, e0175870. [Google Scholar] [CrossRef]
- Vogeley, K. Two social brains: Neural mechanisms of intersubjectivity. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160245. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.E.; Thomas, C.; Kravitz, D.J.; Wallace, G.L.; Baron-Cohen, S.; Martin, A.; Baker, C.I. Global motion perception deficits in autism are reflected as early as primary visual cortex. Brain 2014, 137 Pt 9, 2588–2599. [Google Scholar] [CrossRef] [PubMed]
- Lukito, S.; Norman, L.; Carlisi, C.; Radua, J.; Hart, H.; Simonoff, E.; Rubia, K. Comparative meta-analyses of brain structural and functional abnormalities during cognitive control in attention-deficit/hyperactivity disorder and autism spectrum disorder. Psychol. Med. 2020, 50, 894–919. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.; Parasuraman, R. Attention, biological motion, and action recognition. NeuroImage 2012, 59, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Brewer, J.A.; Garrison, K.A.; Whitfield-Gabrieli, S. What about the “Self” is Processed in the Posterior Cingulate Cortex? Front. Hum. Neurosci. 2013, 7, 647. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R. The brain’s default network and its adaptive role in internal mentation. Neurosci. 2012, 18, 251–270. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H. Self–other relations in social development and autism: Multiple roles for mirror neurons and other brain bases. Autism Res. 2008, 1, 73–90. [Google Scholar] [CrossRef] [PubMed]
- Ticini, L.F.; Dolk, T.; Waszak, F.; Schütz-Bosbach, S. IPL-M1 interaction shapes pre-reflective social differentiation in the human action system: New insights from TBS and TMS combined. Sci. Rep. 2018, 8, 12001. [Google Scholar] [CrossRef] [PubMed]
- Dumas, G.; Soussignan, R.; Hugueville, L.; Martinerie, J.; Nadel, J. Revisiting mu suppression in autism spectrum disorder. Brain Res. 2014, 1585, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Goodbody, S.J.; Husain, M. Maintaining internal representations: The role of the human superior parietal lobe. Nat. Neurosci. 1998, 1, 529–533. [Google Scholar] [CrossRef]
- Yan, F.; Liz, F. The involvement of mirror neuron system in visual perspective taking during action memory encoding. In Proceedings of the ACNS-2013 Australasian Cognitive Neuroscience Society Conference, Melbourne, VIC, Australia, 28 November–1 December 2013. [Google Scholar] [CrossRef]
- Mahy, C.E.; Moses, L.J.; Pfeifer, J.H. How and where: Theory-of-mind in the brain. Dev. Cogn. Neurosci. 2014, 9, 68–81. [Google Scholar] [CrossRef]
- Donnelly, N.A.; Holtzman, T.; Rich, P.D.; Nevado-Holgado, A.J.; Fernando, A.B.P.; Van Dijck, G.; Holzhammer, T.; Paul, O.; Ruther, P.; Paulsen, O.; et al. Oscillatory Activity in the Medial Prefrontal Cortex and Nucleus Accumbens Correlates with Impulsivity and Reward Outcome. PLoS ONE 2014, 9, e111300. [Google Scholar] [CrossRef]
- Rajmohan, V.; Mohandas, E. Mirror neuron system. Indian J. Psychiatry 2007, 49, 66–69. [Google Scholar] [CrossRef]
- Williams, J.; Whiten, A.; Suddendorf, T.; Perrett, D. Imitation, mirror neurons and autism. Neurosci. Biobehav. Rev. 2001, 25, 287–295. [Google Scholar] [CrossRef]
- Hadjikhani, N.; Joseph, R.M.; Snyder, J.; Tager-Flusberg, H. Anatomical Differences in the Mirror Neuron System and Social Cognition Network in Autism. Cereb. Cortex 2006, 16, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Hobson, H.M.; Bishop, D.V. Mu suppression—A good measure of the human mirror neuron system? Cortex 2016, 82, 290–310. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.-P.; Press, C.; Hobson, H.; Catmur, C.; Bird, G. Crossmodal Classification of Mu Rhythm Activity during Action Observation and Execution Suggests Specificity to Somatosensory Features of Actions. J. Neurosci. 2017, 37, 5936–5947. [Google Scholar] [CrossRef] [PubMed]
- Sowden, S.; Koehne, S.; Catmur, C.; Dziobek, I.; Bird, G. Intact Automatic Imitation and Typical Spatial Compatibility in Autism Spectrum Disorder: Challenging the Broken Mirror Theory. Autism Res. 2016, 9, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fogassi, L. The mirror mechanism: Recent findings and perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130420. [Google Scholar] [CrossRef]
- Molenberghs, P.; Brander, C.; Mattingley, J.B.; Cunnington, R. The role of the superior temporal sulcus and the mirror neuron system in imitation. Hum. Brain Mapp. 2010, 31, 1316–1326. [Google Scholar] [CrossRef]
- Gerrits, B.; Vollebregt, M.A.; Olbrich, S.; van Dijk, H.; Palmer, D.; Gordon, E.; Pascual-Marqui, R.; Kessels, R.P.; Arns, M. Probing the “Default Network Interference Hypothesisâ” with EEG: An RDoC Approach Focused on Attention. Clin. EEG Neurosci. 2019, 50, 404–412. [Google Scholar] [CrossRef]
- Salum, G.A.; Sato, J.R.; Manfro, A.G.; Pan, P.M.; Gadelha, A.; Rosário, M.C.D.; Polanczyk, G.V.; Castellanos, F.X.; Sonuga-Barke, E.; Rohde, L.A. Reaction time variability and attention-deficit/hyperactivity disorder: Is increased reaction time variability specific to attention-deficit/hyperactivity disorder? Testing predictions from the default-mode interference hypothesis. Atten. Deficit Hyperact. Disord. 2019, 11, 47–58. [Google Scholar] [CrossRef]
- Mars, R.B.; Neubert, F.-X.; Noonan, M.P.; Sallet, J.; Toni, I.; Rushworth, M.F.S. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef]
- Lancaster, K.; Venkatesan, U.M.; Lengenfelder, J.; Genova, H.M. Default Mode Network Connectivity Predicts Emotion Recognition and Social Integration after Traumatic Brain Injury. Front. Neurol. 2019, 10, 825. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, Y.; Chen, H.; Huang, J. From Self to Social Cognition: The Default Mode Network and Mirror-Neuron System. Adv. Psychol. Sci. 2015, 23, 1808. [Google Scholar] [CrossRef]
- Vaina, L.M.; Solomon, J.; Chowdhury, S.; Sinha, P.; Belliveau, J.W. Functional neuroanatomy of biological motion perception in humans. Proc. Natl. Acad. Sci. USA 2001, 98, 11656–11661. [Google Scholar] [CrossRef]
- Zikopoulos, B.; Barbas, H. Altered neural connectivity in excitatory and inhibitory cortical circuits in autism. Front. Hum. Neurosci. 2013, 7, 609. [Google Scholar] [CrossRef] [PubMed]
- Zeng, K.; Kang, J.; Ouyang, G.; Li, J.; Han, J.; Wang, Y.; Sokhadze, E.M.; Casanova, M.F.; Li, X. Disrupted Brain Network in Children with Autism Spectrum Disorder. Sci. Rep. 2017, 7, 16253. [Google Scholar] [CrossRef] [PubMed]
- Maximo, J.O.; Cadena, E.J.; Kana, R.K. The Implications of Brain Connectivity in the Neuropsychology of Autism. Neuropsychol. Rev. 2014, 24, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Van der Helm, P.A. A cognitive architecture account of the visual local advantage phenomenon in autism spectrum disorders. Vis. Res. 2016, 126, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Van Boxtel, J.J.A.; Lu, H. Impaired Global, and Compensatory Local, Biological Motion Processing in People with High Levels of Autistic Traits. Front. Psychol. 2013, 4, 209. [Google Scholar] [CrossRef] [PubMed]
- Sperduti, M.; Pieron, M.; Leboyer, M.; Zalla, T. Altered Pre-reflective Sense of Agency in Autism Spectrum Disorders as Revealed by Reduced Intentional Binding. J. Autism Dev. Disord. 2014, 44, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Parron, C.; Da Fonseca, D.; Santos, A.; Moore, D.G.; Monfardini, E.; Deruelle, C. Recognition of biological motion in children with autistic spectrum disorders. Autism 2008, 12, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Lange, J.; Georg, K.; Lappe, M. Visual perception of biological motion by form: A template-matching analysis. J. Vis. 2006, 6, 6–49. [Google Scholar] [CrossRef]
- Happé, F.; Frith, U. The Weak Coherence Account: Detail-focused Cognitive Style in Autism Spectrum Disorders. J. Autism Dev. Disord. 2006, 36, 5–25. [Google Scholar] [CrossRef]
- Barttfeld, P.; Wicker, B.; Cukier, S.; Navarta, S.; Lew, S.; Sigman, M. A big-world network in IAU ASD: Dynamical connectivity analysis reflects a deficit in long-range connections and an excess of short-range connections. Neuropsychologia 2011, 49, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.R.; Franz, E.A. Resting-state mu activity modulations are associated with aloofness. Pers. Individ. Differ. 2017, 116, 366–371. [Google Scholar] [CrossRef]
- Pavlidou, A.; Schnitzler, A.; Lange, J. Beta oscillations and their functional role in movement perception. Transl. Neurosci. 2014, 5, 286–292. [Google Scholar] [CrossRef]
- D’Cruz, A.M.; Ragozzino, M.E.; Mosconi, M.W.; Shrestha, S.; Cook, E.H.; Sweeney, J.A. Reduced behavioral flexibility in autism spectrum disorders. Neuropsychology 2013, 27, 152. [Google Scholar] [CrossRef]
- Knight, E.J.; Krakowski, A.I.; Freedman, E.G.; Butler, J.S.; Molholm, S.; Foxe, J.J. Attentional influences on neural processing of biological motion in typically developing children and those on the autism spectrum. Mol. Autism 2022, 13, 33. [Google Scholar] [CrossRef]
- Alberto, G.E.; Stapleton-Kotloski, J.R.; Klorig, D.C.; Rogers, E.R.; Constantinidis, C.; Daunais, J.B.; Godwin, D.W. MEG source imaging detects optogenetically-induced activity in cortical and subcortical networks. Nat. Commun. 2021, 12, 5259. [Google Scholar] [CrossRef]
- Seeber, M.; Cantonas, L.-M.; Hoevels, M.; Sesia, T.; Visser-Vandewalle, V.; Michel, C.M. Subcortical electrophysiological activity is detectable with high-density EEG source imaging. Nat. Commun. 2019, 10, 116. [Google Scholar] [CrossRef] [PubMed]
- Hnazaee, M.F.; Wittevrongel, B.; Khachatryan, E.; Libert, A.; Carrette, E.; Dauwe, I.; Meurs, A.; Boon, P.; Van Roost, D.; Van Hulle, M.M. Localization of deep brain activity with scalp and subdural EEG. NeuroImage 2020, 223, 117344. [Google Scholar] [CrossRef] [PubMed]


| Scramble Percept | |||
|---|---|---|---|
| Group | Coherence | ||
| TDC | Mu (putamen) | ||
| hit rate | false alarm rate | ||
| p | 0.03 | 0.04 | |
| R | 0.46 | −0.44 | |
| Beta (mPFC) | |||
| hit rate | false alarm rate | ||
| p | 0.04 | n.s. | |
| R | −0.43 | 0.37 | |
| tPDC | |||
| TDC | Mu (IFG to visual cortex) | ||
| hit rate | false alarm rate | ||
| p | 0.04 | 0.03 | |
| R | 0.43 | −0.45 | |
| Beta (PCG to HC) | |||
| hit rate | false alarm rate | ||
| p | 0.03 | 0.01 | |
| R | −0.46 | 0.53 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siemann, J.; Kroeger, A.; Bender, S.; Muthuraman, M.; Siniatchkin, M. Segregated Dynamical Networks for Biological Motion Perception in the Mu and Beta Range Underlie Social Deficits in Autism. Diagnostics 2024, 14, 408. https://doi.org/10.3390/diagnostics14040408
Siemann J, Kroeger A, Bender S, Muthuraman M, Siniatchkin M. Segregated Dynamical Networks for Biological Motion Perception in the Mu and Beta Range Underlie Social Deficits in Autism. Diagnostics. 2024; 14(4):408. https://doi.org/10.3390/diagnostics14040408
Chicago/Turabian StyleSiemann, Julia, Anne Kroeger, Stephan Bender, Muthuraman Muthuraman, and Michael Siniatchkin. 2024. "Segregated Dynamical Networks for Biological Motion Perception in the Mu and Beta Range Underlie Social Deficits in Autism" Diagnostics 14, no. 4: 408. https://doi.org/10.3390/diagnostics14040408
APA StyleSiemann, J., Kroeger, A., Bender, S., Muthuraman, M., & Siniatchkin, M. (2024). Segregated Dynamical Networks for Biological Motion Perception in the Mu and Beta Range Underlie Social Deficits in Autism. Diagnostics, 14(4), 408. https://doi.org/10.3390/diagnostics14040408







