In Vivo Effects of Joint Movement on Nerve Mechanical Properties Assessed with Shear-Wave Elastography: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Eligibility Criteria
2.3. Study Selection
2.4. Quality of the Studies
2.5. Meta-Analysis
3. Results
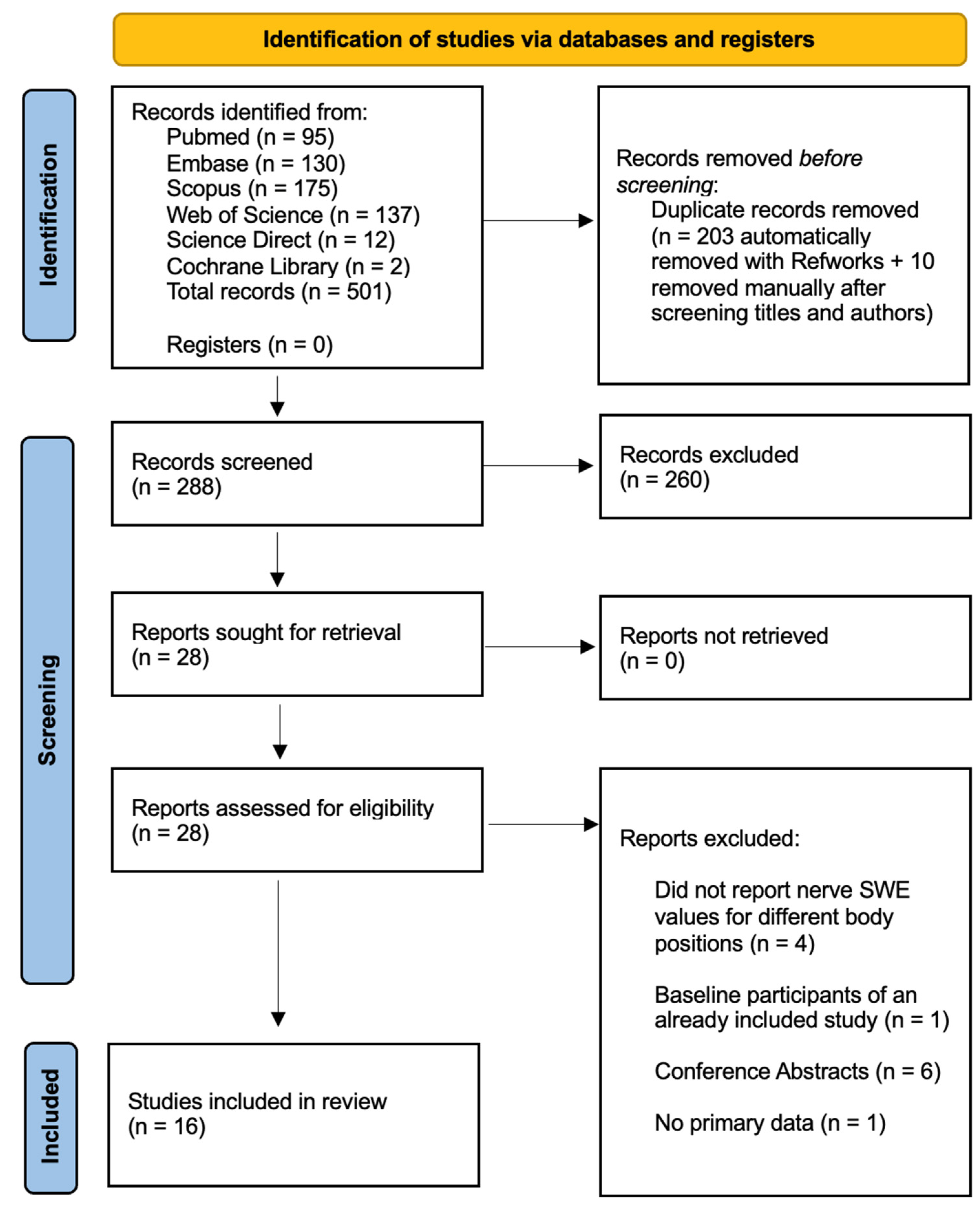
3.1. Study Selection
3.2. Quality of the Studies
3.3. Characteristics of the Studies
3.4. Median Nerve
3.4.1. Effect of Wrist Movement
3.4.2. Effect of Finger Movement
3.4.3. Effect of Elbow Movement
3.4.4. Effect of Shoulder Movement
3.4.5. Effect of Cervical Movement
3.5. Ulnar Nerve
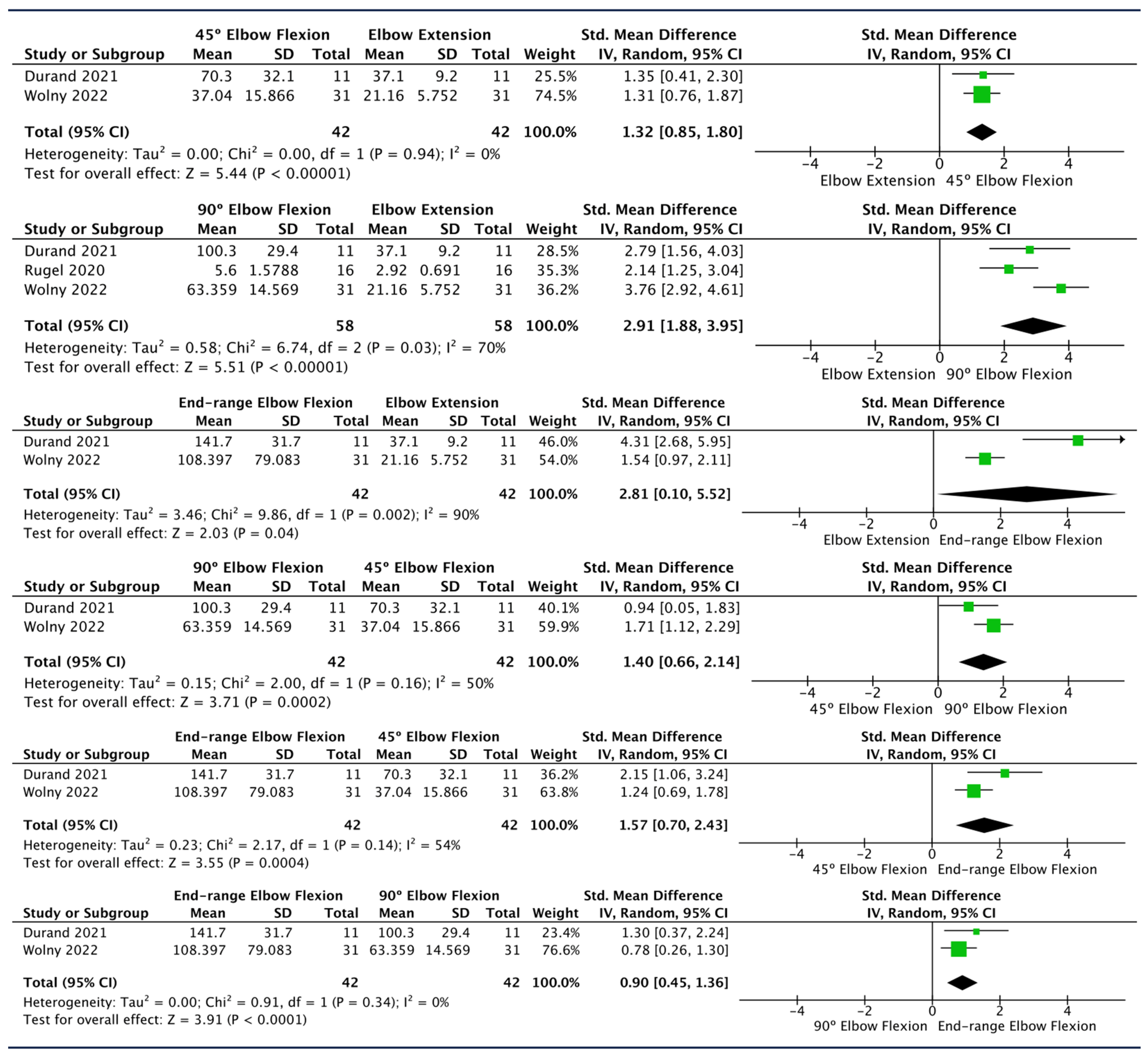
Effect of Elbow Flexion
3.6. Sciatic Nerve
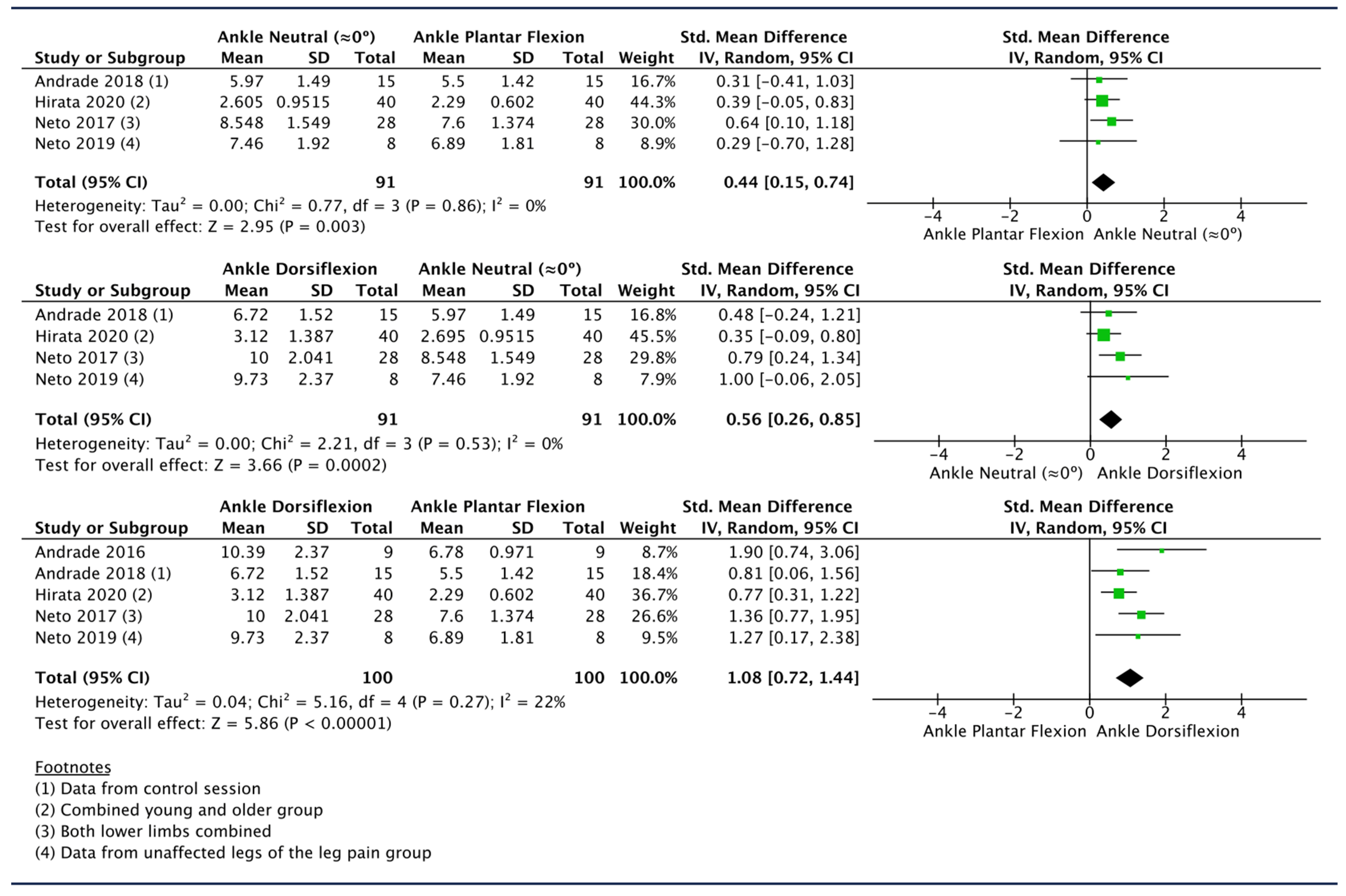
3.6.1. Effect of Ankle Movement
3.6.2. Effect of Hip Movement
3.6.3. Effect of Knee Movement
3.7. Tibial Nerve
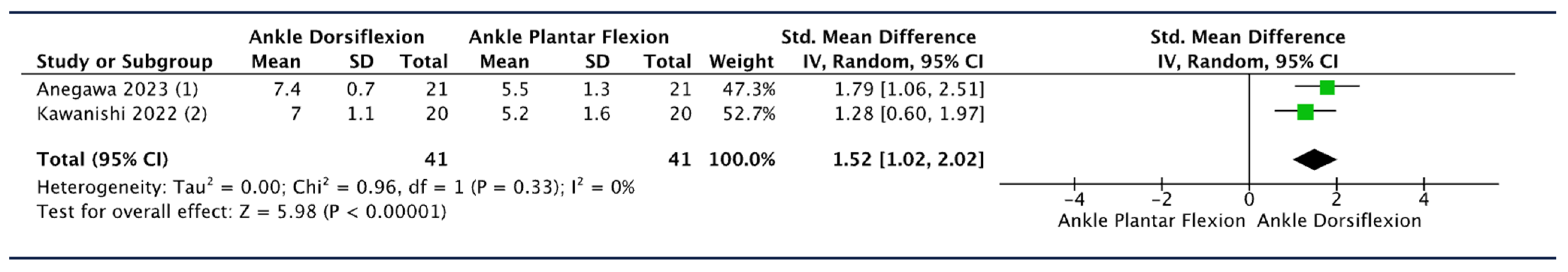
3.7.1. Effect of Ankle Movement
3.7.2. Effect of Hip Movement
3.7.3. Effect of Knee Movement
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bono, C.M.; Ghiselli, G.; Gilbert, T.J.; Kreiner, D.S.; Reitman, C.; Summers, J.T.; Baisden, J.L.; Easa, J.; Fernand, R.; Lamer, T.; et al. An Evidence-Based Clinical Guideline for the Diagnosis and Treatment of Cervical Radiculopathy from Degenerative Disorders. Spine J. 2011, 11, 64–72. [Google Scholar] [CrossRef]
- American Association of Electrodiagnostic Medicine. Guidelines in Electrodiagnostic Medicine. Muscle Nerve 1992, 15, 229–253. [Google Scholar] [CrossRef] [PubMed]
- Koulidis, K.; Veremis, Y.; Anderson, C.; Heneghan, N.R. Diagnostic Accuracy of Upper Limb Neurodynamic Tests for the Assessment of Peripheral Neuropathic Pain: A Systematic Review. Musculoskelet. Sci. Pract. 2019, 40, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Veves, A.; Backonja, M.; Malik, R.A. Painful Diabetic Neuropathy: Epidemiology, Natural History, Early Diagnosis, and Treatment Options. Pain Med. 2008, 9, 660–674. [Google Scholar] [CrossRef] [PubMed]
- Erickson, M.; Lawrence, M.; Jansen, C.W.S.; Coker, D.; Amadio, P.; Cleary, C. Hand Pain and Sensory Deficits: Carpal Tunnel Syndrome. J. Orthop. Sports Phys. Ther. 2019, 49, CPG1–CPG85. [Google Scholar] [CrossRef] [PubMed]
- Nee, R.J.; Coppieters, M.W.; Boyd, B.S. Reliability of the Straight Leg Raise Test for Suspected Lumbar Radicular Pain: A Systematic Review with Meta-Analysis. Musculoskelet. Sci. Pract. 2022, 59, 102529. [Google Scholar] [CrossRef] [PubMed]
- Haj-Mirzaian, A.; Hafezi-Nejad, N.; del Grande, F.; Endo, Y.; Nwawka, O.K.; Miller, T.T.; Carrino, J.A. Optimal Choice of Ultrasound-Based Measurements for the Diagnosis of Ulnar Neuropathy at the Elbow: A Meta-Analysis of 1961 Examinations. Am. J. Roentgenol. 2020, 215, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Torres-Costoso, A.; Martínez-Vizcaíno, V.; Álvarez-Bueno, C.; Ferri-Morales, A.; Cavero-Redondo, I. Accuracy of Ultrasonography for the Diagnosis of Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2018, 99, 758–765.e10. [Google Scholar] [CrossRef] [PubMed]
- Roomizadeh, P.; Eftekharsadat, B.; Abedini, A.; Ranjbar-Kiyakalayeh, S.; Yousefi, N.; Ebadi, S.; Babaei-Ghazani, A. Ultrasonographic Assessment of Carpal Tunnel Syndrome Severity: A Systematic Review and Meta-Analysis. Am. J. Phys. Med. Rehabil. 2019, 98, 373–381. [Google Scholar] [CrossRef]
- Fowler, J.R.; Gaughan, J.P.; Ilyas, A.M. The Sensitivity and Specificity of Ultrasound for the Diagnosis of Carpal Tunnel Syndrome: A Meta-Analysis. Clin. Orthop. Relat. Res. 2011, 469, 1089–1094. [Google Scholar] [CrossRef]
- Silva, A.; Manso, A.; Andrade, R.; Domingues, V.; Brandão, M.P.; Silva, A.G. Quantitative in Vivo Longitudinal Nerve Excursion and Strain in Response to Joint Movement: A Systematic Literature Review. Clin. Biomech. 2014, 29, 839–847. [Google Scholar] [CrossRef]
- Szikszay, T.; Hall, T.; Von Piekartz, H. In Vivo Effects of Limb Movement on Nerve Stretch, Strain, and Tension: A Systematic Review. J. Back Musculoskelet. Rehabil. 2017, 30, 1171–1186. [Google Scholar] [CrossRef]
- Taljanovic, M.S.; Gimber, L.H.; Becker, G.W.; Latt, L.D.; Klauser, A.S.; Melville, D.M.; Gao, L.; Witte, R.S. Shear-Wave Elastography: Basic Physics and Musculoskeletal Applications. Radiographics 2017, 37, 855–870. [Google Scholar] [CrossRef]
- Nightingale, K. Acoustic Radiation Force Impulse (ARFI) Imaging: A Review. Curr. Med. Imaging Rev. 2011, 7, 328–339. [Google Scholar] [CrossRef]
- Blank, J.; Blomquist, M.; Arant, L.; Cone, S.; Roth, J. Characterizing Musculoskeletal Tissue Mechanics Based on Shear Wave Propagation: A Systematic Review of Current Methods and Reported Measurements. Ann. Biomed. Eng. 2022, 50, 751–768. [Google Scholar] [CrossRef]
- Cipriano, K.J.; Wickstrom, J.; Glicksman, M.; Hirth, L.; Farrell, M.; Livinski, A.A.; Esfahani, S.A.; Maldonado, R.J.; Astrow, J.; Berrigan, W.A.; et al. A Scoping Review of Methods Used in Musculoskeletal Soft Tissue and Nerve Shear Wave Elastography Studies. Clin. Neurophysiol. 2022, 140, 181–195. [Google Scholar] [CrossRef]
- Eby, S.F.; Song, P.; Chen, S.; Chen, Q.; Greenleaf, J.F.; An, K.N. Validation of Shear Wave Elastography in Skeletal Muscle. J. Biomech. 2013, 46, 2381–2387. [Google Scholar] [CrossRef]
- Sebag, F.; Vaillant-Lombard, J.; Berbis, J.; Griset, V.; Henry, J.F.; Petit, P.; Oliver, C. Shear Wave Elastography: A New Ultrasound Imaging Mode for the Differential Diagnosis of Benign and Malignant Thyroid Nodules. J. Clin. Endocrinol. Metab. 2010, 95, 5281–5288. [Google Scholar] [CrossRef] [PubMed]
- Ashir, A.; Jerban, S.; Barrère, V.; Wu, Y.; Shah, S.B.; Andre, M.P.; Chang, E.Y. Skeletal Muscle Assessment Using Quantitative Ultrasound: A Narrative Review. Sensors 2023, 23, 4763. [Google Scholar] [CrossRef] [PubMed]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic Shear Imaging: A New Technique for Soft Tissue Elasticity Mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Youk, J.H.; Son, E.J.; Park, A.Y.; Kim, J.-A. Shear-Wave Elastography for Breast Masses: Local Shear Wave Speed (m/s) versus Young Modulus (KPa). Ultrasonography 2013, 33, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Deffieux, T.; Gennisson, J.L.; Bousquet, L.; Corouge, M.; Cosconea, S.; Amroun, D.; Tripon, S.; Terris, B.; Mallet, V.; Sogni, P.; et al. Investigating Liver Stiffness and Viscosity for Fibrosis, Steatosis and Activity Staging Using Shear Wave Elastography. J. Hepatol. 2015, 62, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Zakrzewski, J.; Zakrzewska, K.; Pluta, K.; Nowak, O.; Miłoszewska-Paluch, A. Ultrasound Elastography in the Evaluation of Peripheral Neuropathies: A Systematic Review of the Literature. Pol. J. Radiol. 2019, 84, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Lyu, G.; Yang, X.; Wang, H.; Chen, Y. Shear Wave Elastography as a Quantitative Biomarker of Diabetic Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Front. Public Health 2022, 10, 915883. [Google Scholar] [CrossRef] [PubMed]
- Wee, T.C.; Simon, N.G. Ultrasound Elastography for the Evaluation of Peripheral Nerves: A Systematic Review. Muscle Nerve 2019, 60, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.P.; Chen, I.J.; Chang, K.V.; Wu, W.T.; Özçakar, L. Utility of Ultrasound Elastography in Evaluation of Carpal Tunnel Syndrome: A Systematic Review and Meta-Analysis. Ultrasound Med. Biol. 2019, 45, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowska-Thing, A.; Zakrzewski, J.; Nowak, O.; Nitek, Ż. Ultrasound Elastography as a Potential Method to Evaluate Entrapment Neuropathies in Elite Athletes: A Mini-Review. Pol. J. Radiol. 2019, 84, e625–e629. [Google Scholar] [CrossRef]
- Rossetto, G.; Lopomo, N.F.; Shaikh, S.Z. Longitudinal Movements and Stiffness of Lower Extremity Nerves Measured by Ultrasonography and Ultrasound Elastography in Symptomatic and Asymptomatic Populations: A Systematic Review with Meta-Analysis. Ultrasound Med. Biol. 2023, 49, 1913–1929. [Google Scholar] [CrossRef]
- Schrier, V.J.M.M.; Lin, J.; Gregory, A.; Thoreson, A.R.; Alizad, A.; Amadio, P.C.; Fatemi, M. Shear Wave Elastography of the Median Nerve: A Mechanical Study. Muscle Nerve 2020, 61, 826–833. [Google Scholar] [CrossRef]
- Prado-Costa, R.; Rebelo, J.; Monteiro-Barroso, J.; Preto, A.S. Ultrasound Elastography: Compression Elastography and Shear-Wave Elastography in the Assessment of Tendon Injury. Insights Imaging 2018, 9, 791–814. [Google Scholar] [CrossRef]
- Creze, M.; Nordez, A.; Soubeyrand, M.; Rocher, L.; Maître, X.; Bellin, M.F. Shear Wave Sonoelastography of Skeletal Muscle: Basic Principles, Biomechanical Concepts, Clinical Applications, and Future Perspectives. Skelet. Radiol. 2018, 47, 457–471. [Google Scholar] [CrossRef]
- Wang, C.; Wang, H.; Zhou, Y.; Zhang, S.; Huang, M. Evaluation of the Clinical Value of Shear Wave Elastography for Early Detection and Diagnosis of Diabetic Peripheral Neuropathy: A Controlled Preliminary Prospective Clinical Study. BMC Musculoskelet. Disord. 2022, 23, 1120. [Google Scholar] [CrossRef]
- Chen, R.; Wang, X.L.; Xue, W.L.; Sun, J.W.; Dong, X.Y.; Jiang, Z.P.; Wu, H.; Ma, R.; Zhou, X.L. Application Value of Conventional Ultrasound and Real-Time Shear Wave Elastography in Patients with Type 2 Diabetic Polyneuropathy. Eur. J. Radiol. 2020, 126, 108965. [Google Scholar] [CrossRef]
- Chen, S.P.; Ye, T.T.; Hong, J.; Zhu, H. Evaluation of Sciatic Nerve Stiffness Using Shear Wave Elastography in Patients with Unilateral Diabetic Foot Ulcers. Diagnostics 2023, 13, 547. [Google Scholar] [CrossRef]
- Ruby, L.; Mutschler, T.; Martini, K.; Klingmüller, V.; Frauenfelder, T.; Rominger, M.B.; Sanabria, S.J. Which Confounders Have the Largest Impact in Shear Wave Elastography of Muscle and How Can They Be Minimized? An Elasticity Phantom, Ex Vivo Porcine Muscle and Volunteer Study Using a Commercially Available System. Ultrasound Med. Biol. 2019, 45, 2591–2611. [Google Scholar] [CrossRef] [PubMed]
- Greening, J.; Dilley, A. Posture-Induced Changes in Peripheral Nerve Stiffness Measured by Ultrasound Shear-Wave Elastography. Muscle Nerve 2017, 55, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Rugel, C.L.; Franz, C.K.; Lee, S.S.M. Influence of Limb Position on Assessment of Nerve Mechanical Properties by Using Shear Wave Ultrasound Elastography. Muscle Nerve 2020, 61, 616–622. [Google Scholar] [CrossRef]
- Ellis, R.; Blyth, R.; Arnold, N.; Miner-Williams, W. Is There a Relationship between Impaired Median Nerve Excursion and Carpal Tunnel Syndrome? A Systematic Review. J. Hand Ther. 2017, 30, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Boyd, B.S.; Dilley, A. Altered Tibial Nerve Biomechanics in Patients with Diabetes Mellitus. Muscle Nerve 2014, 50, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Hug, F.; Tucker, K.; Gennisson, J.L.; Tanter, M.; Nordez, A. Elastography for Muscle Biomechanics: Toward the Estimation of Individual Muscle Force. Exerc. Sport Sci. Rev. 2015, 43, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder Reliability and Validity of WebPlotDigitizer in Extracting Graphed Data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef]
- Downs, S.H.; Black, N. The Feasibility of Creating a Checklist for the Assessment of the Methodological Quality Both of Randomised and Non-Randomised Studies of Health Care Interventions. J. Epidemiol. Community Health 1998, 52, 377–384. [Google Scholar] [CrossRef]
- Fernando, M.; Crowther, R.; Lazzarini, P.; Sangla, K.; Cunningham, M.; Buttner, P.; Golledge, J. Biomechanical Characteristics of Peripheral Diabetic Neuropathy: A Systematic Review and Meta-Analysis of Findings from the Gait Cycle, Muscle Activity and Dynamic Barefoot Plantar Pressure. Clin. Biomech. 2013, 28, 831–845. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.3 (Updated February 2022); Wiley: Hoboken, NJ, USA, 2022. [Google Scholar]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Shi, J.; Luo, D.; Weng, H.; Zeng, X.T.; Lin, L.; Chu, H.; Tong, T. Optimally Estimating the Sample Standard Deviation from the Five-Number Summary. Res. Synth. Methods 2020, 11, 641–654. [Google Scholar] [CrossRef]
- Shi, J.; Luo, D.; Wan, X.; Liu, Y.; Liu, J.; Bian, Z.; Tong, T. Detecting the Skewness of Data from the Five-Number Summary and Its Application in Meta-Analysis. Stat. Methods Med. Res. 2023, 32, 09622802231172043. [Google Scholar] [CrossRef] [PubMed]
- Neto, T.; Freitas, S.R.; Andrade, R.J.; Vaz, J.R.; Mendes, B.; Firmino, T.; Bruno, P.M.; Nordez, A.; Oliveira, R. Noninvasive Measurement of Sciatic Nerve Stiffness in Patients with Chronic Low Back Related Leg Pain Using Shear Wave Elastography. J. Ultrasound Med. 2019, 38, 157–164. [Google Scholar] [CrossRef]
- Neto, T.; Freitas, S.R.; Andrade, R.J.; Gomes, J.; Vaz, J.; Mendes, B.; Firmino, T.; Nordez, A.; Oliveira, R. Sciatic Nerve Stiffness Is Not Changed immediately after a Slump Neurodynamics Technique. Muscles Ligaments Tendons J. 2017, 7, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for Examining and Interpreting Funnel Plot Asymmetry in Meta-Analyses of Randomised Controlled Trials. BMJ Online 2011, 343, d4002. [Google Scholar] [CrossRef] [PubMed]
- Kawanishi, K.; Nariyama, Y.; Anegawa, K.; Tsutsumi, M.; Kudo, S. Changes in Tibial Nerve Stiffness during Ankle Dorsiflexion According to In-Vivo Analysis with Shear Wave Elastography. Medicine 2022, 101, e29840. [Google Scholar] [CrossRef]
- Hirata, K.; Yamadera, R.; Akagi, R. Associations between Range of Motion and Tissue Stiffness in Young and Older People. Med. Sci. Sports Exerc. 2020, 52, 2179–2188. [Google Scholar] [CrossRef]
- Andrade, R.J.; Freitas, S.R.; Hug, F.; Coppieters, M.W.; Sierra-Silvestre, E.; Nordez, A. Spatial Variation in Mechanical Properties along the Sciatic and Tibial Nerves: An Ultrasound Shear Wave Elastography Study. J. Biomech. 2022, 136, 111075. [Google Scholar] [CrossRef]
- Zhu, B.; Yan, F.; He, Y.; Wang, L.; Xiang, X.; Tang, Y.; Yang, Y.; Qiu, L. Evaluation of the Healthy Median Nerve Elasticity: Feasibility and Reliability of Shear Wave Elastography. Medicine 2018, 97, e12956. [Google Scholar] [CrossRef]
- Anegawa, K.; Kawanishi, K.; Nakamura, M.; Izumi, M.; Tsutsumi, M.; Kudo, S. Tibial Nerve Dynamics during Ankle Dorsiflexion: The Relationship between Stiffness and Excursion of the Tibial Nerve. J. Biomech. 2023, 155, 111646. [Google Scholar] [CrossRef]
- Wolny, T.; Fernández-de-las-Peñas, C.; Granek, A.; Linek, P. Changes in Ultrasound Measurements of the Ulnar Nerve at Different Elbow Joint Positions in Patients with Cubital Tunnel Syndrome. Sensors 2022, 22, 8354. [Google Scholar] [CrossRef]
- Andrade, R.J.; Freitas, S.R.; Hug, F.; Le Sant, G.; Lacourpaille, L.; Gross, R.; McNair, P.; Nordez, A. The Potential Role of Sciatic Nerve Stiffness in the Limitation of Maximal Ankle Range of Motion. Sci. Rep. 2018, 8, 14532. [Google Scholar] [CrossRef]
- Andrade, R.J.; Nordez, A.; Hug, F.; Ates, F.; Coppieters, M.W.; Pezarat-Correia, P.; Freitas, S.R. Non-Invasive Assessment of Sciatic Nerve Stiffness during Human Ankle Motion Using Ultrasound Shear Wave Elastography. J. Biomech. 2016, 49, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Durand, S.; Raffoul, W.; Christen, T.; Pedrazzi, N. Post-Operative Assessment of Ulnar Nerve Tension Using Shear-Wave Elastography. Neurol. Int. 2021, 13, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Staber, D.; Oppold, J.; Grimm, A.; Schuhmann, M.U.; Romano, A.; Marquetand, J.; Kleiser, B. Shear-Wave-Elastography in Neurofibromatosis Type I. Diagnostics 2022, 12, 360. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Chen, Y.; Deng, W.; Liang, H.; Yu, S.; Zhang, Z.; Liu, C. Quantifying the Elasticity Properties of the Median Nerve during the Upper Limb Neurodynamic Test 1. Appl. Bionics Biomech. 2022, 2022, 3300835. [Google Scholar] [CrossRef]
- Lee, S.; Kwak, J.; Lee, S.; Cho, H.; Oh, E.; Park, J.W. Quantitative Stiffness of the Median Nerve, Flexor Tendons, and Flexor Retinaculum in the Carpal Tunnel Measured with Acoustic Radiation Force Impulse Elastography in Various Wrist and Finger Positions. Medicine 2019, 98, e17066. [Google Scholar] [CrossRef]
- Andrade, R.J.; Freitas, S.R.; Hug, X.F.; Sant, G.L.; Lacourpaille, L.; Gross, R.; Quillard, J.-B.; Mcnair, P.J.; Nordez, A. Chronic Effects of Muscle and Nerve-Directed Stretching on Tissue Mechanics. J. Appl. Physiol. 2020, 129, 1011–1023. [Google Scholar] [CrossRef]
- Dilley, A.; Lynn, B.; Greening, J.; DeLeon, N. Quantitative in Vivo Studies of Median Nerve Sliding in Response to Wrist, Elbow, Shoulder and Neck Movements. Clin. Biomech. 2003, 18, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Topp, K.S.; Boyd, B.S. Structure and Biomechanics of Peripheral Nerves: Nerve Responses to Physical Stresses and Implications for Physical Therapist Practice Perspective. Phys. Ther. 2006, 86, 92–109. [Google Scholar] [CrossRef] [PubMed]
- Alshami, A.M.; Babri, A.S.; Souvlis, T.; Coppieters, M.W. Strain in the Tibial and Plantar Nerves with Foot and Ankle Movements and the Influence of Adjacent Joint Positions. J. Appl. Biomech. 2008, 24, 368–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boyd, B.S.; Topp, K.S.; Coppieters, M.W. Impact of Movement Sequencing on Sciatic and Tibial Nerve Strain and Excursion during the Straight Leg Raise Test in Embalmed Cadavers. J. Orthop. Sports Phys. Ther. 2013, 43, 398–403. [Google Scholar] [CrossRef] [PubMed]


| Lee 2019 [63] | Lin 2022 [62] | Staber 2022 [61] | Zhu 2018 [55] | Greening 2017 [36] | Rugel 2020 [37] | Durand 2021 [60] | Wolny 2022 [57] | Andrade 2016 [59] | Andrade 2018 [58] | Andrade 2022 [54] | Hirata 2020 [53] | Neto 2017 [50] | Neto 2019 [49] | Kawanishi 2022 [52] | Anegawa 2023 [56] | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Hypothesis/aim/objective | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Main outcomes | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 3. Participants’ characteristics | 1 | 1 | 01 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 01 | 1 | 1 | 1 |
| 5. Confounders | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 6. Findings | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 7. Estimates random variability | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 10. Actual probability values | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 11. Subjects’ representative (asked) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 12. Subjects’ representative (agreed) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 15. Blinding of assessors | 01 | 01 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16. Data dredging | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 18. Appropriate statistical tests | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 20. Outcome measures valid/reliable | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 21. Internal validity (selection bias) | 01 | 01 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 22. Recruitment time period | 1 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| 25. Adjustment for confounding | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 27. Statistical power determined | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 0 |
| Total G.C. vs. Total I.A.-C. | 11 vs. 13 | 11 vs. 13 | 9 vs. 10 | 12 vs. 12 | 11 vs. 11 | 11 vs. 11 | 10 vs. 10 | 11 vs. 11 | 11 vs. 11 | 11 vs. 11 | 13 vs. 13 | 12 vs. 12 | 10 vs. 11 | 12 vs. 12 | 12 vs. 12 | 9 vs. 9 |
| Total consensus | 11 | 11 | 9 | 12 | 11 | 11 | 10 | 11 | 11 | 11 | 13 | 12 | 10 | 12 | 12 | 9 |
| Author and Year | N | Sample Characteristics | Age (Years) | Gender (M/F) | |
|---|---|---|---|---|---|
| Median nerve | |||||
| Zhu 2018 [55] | 40 | Healthy subjects | 31.20 ± 8.92 | 13/27 | |
| Greening 2017 [36] | 26 (N = 18 for position 2 of MN evaluation) | Healthy subjects | Men 37.5 (20–72); Women 38.8 (23–58). | 11/15 | |
| Lin 2022 [62] | 20 | Healthy subjects | 19.9 ± 1.4 | 7/13 | |
| Lee 2019 [63] | 26 | Healthy subjects | 24.7 ± 3.7 | 20/6 | |
| Staber 2022 [61] | 11 | Healthy controls | 30.6 ± 11.0 | - | |
| Rugel 2020 [37] | 16 | Healthy subjects | 24.9 ± 2.2 | 10/6 | |
| Ulnar nerve | |||||
| Wolny 2022 [57] | 31 | Healthy contralateral nerves from patients with unilateral ulnar tunnel syndrome | 54.2 ± 8.15 | - | |
| Durand 2021 [60] | 11 | Nerves from the contralateral healthy side, in patients with UN decompression with anterior transposition | 53.2 ± 14.9 | 6/5 | |
| Rugel 2020 [37] | 16 | Healthy subjects | 24.9 ± 2.2 | 10/6 | |
| Sciatic nerve | |||||
| Hirata 2020 [53] | 20 20 | Young males Older males | 22 ± 1 (young) 72 ± 5 (old) | 40/0 | |
| Neto 2019 [49] | 8 | Patients with chronic unilateral low back related leg pain | 30.8 ± 7.4 (leg pain) | 6/2 (leg pain) | |
| 8 | Healthy controls | 28.1 ± 8.3 (healthy controls) | 5/3 (healthy controls) | ||
| Neto 2017 [50] | 14 | Healthy subjects | 30.4 ± 10.1 | 11/3 | |
| Andrade 2016 [59] | 10 | Healthy subjects | 25.3 ± 2.5 | 10/0 | |
| Andrade 2022 [54] | 60 | Healthy subjects | 20.5 ± 2.0 | 29/31 | |
| Andrade 2018 [58] | 15 | Healthy subjects | 22 ± 3 | 13/2 | |
| Tibial nerve | |||||
| Kawanishi 2022 [52] | 20 | Healthy subjects | 23.8 ± 5.5 | 14/6 | |
| Anegawa 2023 [56] | 21 | Healthy subjects | 20.8 ± 0.5 | 10/11 | |
| Greening 2017 [36] | 26 | Healthy subjects | Men 37.5 (20–72); Women 38.8 (23–58). | 11/15 | |
| Andrade 2022 [54] | 60 | Healthy subjects | 20.5 ± 2.0 | 29/31 |
| Author and Year | Ultrasound Machine | Probe | Plane | Point of Measure | Measurement Methods | Unit | |
|---|---|---|---|---|---|---|---|
| Median nerve | |||||||
| Zhu 2018 [55] | Aixplorer | 4 to 15-MHz linear array probe | L | Mid-forearm | Circular ROI of 2 mm | m/s | |
| Greening 2017 [36] | Siemens Acuson S2000 | 4–9 MHz 38.5 mm linear array transducer | L | (1) mid-forearm; (2) immediately proximal to the elbow in the upper arm. | 4 equidistant ROIs (size = 1.5 mm × 1.5 mm) along the imaged nerve | m/s | |
| Lin 2022 [62] | Aixplorer Supersonic Imagine | 4–15 MHz and 40 mm linear transducer | L | Midpoint of the forearm | 2 mm diameters ROI | kPa | |
| Lee 2019 [63] | Siemens Acuson S2000 | 9L4 Linear Array Transducer | T | Carpal tunnel inlet | 2 × 2 mm ROI | m/s | |
| Staber 2022 [61] | Canon Aplio i800 | 14 MHz linear transducer | L | 3 cm proximal to the flexor retinaculum | 2 mm | m/s | |
| Rugel 2020 [37] | Aixplorer Supersonic Imagine | 4 to 15-MHz linear array probe | L | Lower third of the biceps and forearm. | ROI of at least 1.5 cm in length and including the entirety of the nerve visible within 0.25 cm of the SW elastography box border. | m/s | |
| Ulnar nerve | |||||||
| Durand 2021 [60] | Aixplorer Supersonic Imagine | 5–18 MHz linear array transducer (SuperLineal SL18-5) | L | Immediately proximal to the medial epicondyle | ROI of 2 mm | kPa | |
| Wolny 2022 [57] | Aixplorer 12.2.0 Supersonic Imagine | Linear transducer array 2–10 MHz; SuperLinear 10-2 | T | Ulnar tunnel | - | kPa | |
| Rugel 2020 [37] | Aixplorer Supersonic Imagine | 4 to 15-MHz linear array probe | L | Lower third of the biceps and forearm. | ROI of at least 1.5 cm in length and including the entirety of the nerve visible within 0.25 cm of the SW elastography box border. | m/s | |
| Sciatic nerve | |||||||
| Hirata 2020 [53] | Siemens Acuson S2000 | Linear transducer array 9 L4 Transducer, 4–9 MHz | L | At 60% of the thigh length from the greater trochanter to the popliteal crease. | ROI as large as possible while excluding nontarget tissues. | m/s | |
| Neto 2019 [49] | Aixplorer 10.0 Supersonic Imagine | Linear array transducer SL 10-2 MHz, Super Linear 15-4 | L | Posterior thigh, 10 cm below the gluteal fold. | The largest area within the epineurium boundaries in the elastographic window | m/s | |
| Neto 2017 [50] | Aixplorer 10.0 Supersonic Imagine | Linear array transducer SL 10-2 MHz, Super Linear | L | Posterior thigh, 10 cm below the gluteal fold. | The largest area within the epineurium boundaries in the elastographic window | m/s | |
| Andrade 2016 [59] | Aixplorer 6.1 Supersonic Imagine | L10-2 MHz, Super Linear transducer | L | 7–10 cm distal to the gluteal fold | The largest area within the epineurium boundaries in the elastographic window | m/s | |
| Andrade 2022 [54] | Aixplorer 6.1 Supersonic Imagine | L10-2 MHz, Super Linear transducer | L | Landmarks: (i) midpoint between the ischial tuberosity and the greater trochanter; (ii) SN bifurcation. Points of measure: -Sciatic PROXIMAL and Sciatic DISTAL: obtained by dividing into 2 regions between (i) and (ii). | The largest area within the epineurium boundaries in the elastographic window | m/s | |
| Andrade 2018 [58] | Aixplorer 6.1 Supersonic Imagine | L10-2 MHz, Super Linear transducer | L | Proximal third of the thigh | The largest nerve area | m/s | |
| Tibial nerve | |||||||
| Kawanishi 2022 [52] | Canon Aplio 300 | 10-MHz linear probe (PLT-1005BT) | L | 1 cm superior to the medial malleolus | 3 randomly selected ROI | m/s | |
| Anegawa 2023 [56] | Canon Aplio 300 | 10-MHz linear transducer (PLT-1005BT) | L | 1 cm superior to the medial malleolus | 3 randomly selected ROI | m/s | |
| Greening 2017 [36] | Siemens Acuson S2000 | 4–9 MHz 38.5 mm linear array transducer | L | TN: immediately proximal to the tarsal tunnel | 4 equidistant ROIs (size = 1.5 mm × 1.5 mm) along the imaged nerve | m/s | |
| Andrade 2022 [54] | Aixplorer 6.1 Supersonic Imagine | L10-2 MHz, Super Linear transducer | L | Landmarks: (ii) SN bifurcation; (iii) lateral femoral condyle; and (iv) the medial malleolus. Points of measure: -Tibial PROXIMAL: between (ii) and (iii) -Tibial INTERMEDIATE, and Tibial DISTAL: at 50% and 10% of the distance from (iv) and (iii). | The largest area within the epineurium boundaries in the elastographic window | m/s |
| Median Nerve | |||||
|---|---|---|---|---|---|
| Author and Year | N | Location | Initial Position | Movement and Involved Joints | Results |
| Zhu 2018 [55] | 40 | Forearm | Seated with the arm extended. Elbow flexed 90°, the forearm in supine position, and wrists relaxed on a flat surface with fingers semi- flexed (Posture 1) | Wrist stretched maximally (extension) while maintaining the forearm on the flat surface (Posture 2). | Significant effect of the different nerve postures was observed, and the MN in the tension condition had a higher stiffness than that in the slack condition (p < 0.001). |
| Greening 2017 [36] | 26 (n = 18 for position 2 of MN evaluation) | Forearm Proximal Elbow | Supine, with the shoulder abducted to 30°, elbow flexed to 90°, and the wrist in maximum flexion (50–60°) (Position 1) | (Position 2) shoulder abduction to 90° while maintaining 90° elbow flexion and maximum wrist flexion (not included in the article data analysis); (Position 3) the wrist was extended to end of range (60–70°), while maintaining 90° shoulder abduction and 90° elbow flexion; (Position 4) the elbow was extended maximally (135–190°) while maintaining 90° shoulder abduction and maximum wrist extension | Position 1: the mean SWV was 2.22 ± 0.07 m/s in the upper arm and 2.61 ± 0.08 m/s in the forearm, a difference that was significant. Position 2: negligible change in the MN SWV in the upper arm (mean = 2.59 ± 0.11 m/s), whereas in the forearm, there was a small but significant decrease. Position 3: increase in MN SWV in the upper arm (mean = 3.10 ± m/s) and forearm (mean = 5.87 ± 0.19 m/s). In this position, the percent increase from position 1 was significantly higher in the forearm (127 ± 7%) compared to the upper arm (40 ± 4%). Position 4: substantial increase in MN SWV in both the upper arm (mean = 6.80 ± 0.31 m/s; percent increase from position 1 = 208 ± 13%) and forearm (mean = 8.65 ± 0.19 m/s; percent increase from position 1 = 236 ± 10%). Elbow angle did not correlate with MN SWV in the forearm or upper arm. |
| Lin 2022 [62] | 20 | Forearm | On a chair with their upper arms positioned horizontally, with the shoulder abducted 90 degrees and 90 degrees externally rotated. | The MN was imaged during elbow extension in the following postures: (Position 1) with neutral posture, (Position 2) with wrist extension, (Position 3) with contralateral cervical flexion, and (Position 4) with both wrist extension and contralateral cervical flexion. | The mean shear modulus of the MN in the middle forearm was 137.71 ± 22.72 kPa at the neutral posture, only contralateral cervical flexion was 211.00 ± 30.49 kPa, and only wrist extension was 252.34 ± 40.30 kPa and 297.35 ± 64.60 kPa at contralateral cervical flexion + wrist extension. |
| Lee 2019 [63] | 26 | Wrist | Elbow at 90° | Six finger/wrist combinations: (A) wrist neutral (0°), finger neutral; (B) wrist neutral, finger grasp; (C) wrist neutral, finger extension; (D) wrist extension (30°), finger neutral; (E) wrist extension, finger grasp; and (F) wrist extension, finger extension. | Significant differences in SWV in all six motions (p < 0.001) showing an increasing trend from (A) to (F): (A) 2.3 ± 0.5 m/s; (B) 2.7 ± 0.5 m/s; (C) 2.7 ± 0.4 m/s; (D) 2.9 ± 0.5 m/s; (E) 3.0 ± 0.5 m/s; (F) 3.1 ± 0.5 m/s. |
| Staber 2022 [61] | 11 | Forearm | Seated with the back of the hand on the cushion and elbow flexed at 120°. | Three positions in the wrist joint: neutral (0°), individual maximal flexion and maximal extension. | SWV was higher in extension (5.8 m/s) than in flexion (3.2 m/s) as well as in neutral position (3.8 m/s) (extension vs. flexion (p < 0.001), extension vs. neutral (p < 0.002) and neutral vs. flexion (p = 0.071). |
| Rugel 2020 [37] | 16 | Proximal Elbow Forearm | Supine on an examination table with their shoulder at 45° abduction and wrist at neutral position. | Extension and 90° elbow flexion. | 89.3% increase in MN SWV in the proximal elbow and 64.0% increase in the forearm with elbow extension compared to flexion (p < 0.01). |
| Median Nerve—Wrist Extension vs. Neutral: SMD [95%CI]: 3.16 [1.20, 5.12], I2: 95% | |||
| Study Removed | SMD (95%CI) without the Study | I2 without the Study | % Variation of Effect Size |
| Lee 2019 [63] | 3.71 [1.25, 6.18] | 94% | 17.41% |
| Lin 2022 [62] | 3.08 [0.43, 5.73] | 96% | 2.53% |
| Staber 2022 [61] | 3.66 [1.05, 6.27] | 96% | 15.82% |
| Zhu 2018 [55] | 2.19 [1.07, 3.31] | 80% | 30.70% |
| Ulnar Nerve—Elbow Extension vs. Elbow 90° Flexion: SMD [95%CI]: 2.91 [1.88, 3.95], I2: 70% | |||
| Study Removed | SMD [95%CI] without the Study | I2 without the Study | % Variation of Effect Size |
| Durand 2021 [60] | 2.96 [1.37, 4.55] | 85% | 1.72% |
| Rugel 2020 [37] | 3.39 [2.46, 4.31] | 38% | 16.49% |
| Wolny 2022 [57] | 2.37 [1.64, 3.09] | 0% | 18.56% |
| Sciatic Nerve—Ankle Dorsiflexion vs. Plantar Flexion: SMD [95%CI]: 1.08 [0.72, 1.44], I2: 22% | |||
| Study Removed | SMD [95%CI] without the Study | I2 without the Study | % Variation of Effect Size |
| Andrade 2016 [59] | 0.98 [0.67, 1.29] | 0% | 9.26% |
| Andrade 2018 [58] | 1.17 [0.72, 1.63] | 36% | 8.33% |
| Hirata 2020 [53] | 1.26 [0.86, 1.65] | 0% | 16.67% |
| Neto 2017 [50] | 0.97 [0.56, 1.39] | 17% | 10.19% |
| Neto 2019 [49] | 1.08 [0.65, 1.51] | 40% | 0% |
| Sciatic Nerve—Ankle Dorsiflexion vs. Neutral (≈0°): SMD [95%CI]: 0.56 [0.26, 0.85], I2: 0% | |||
| Study Removed | SMD [95%CI] without the Study | I2 without the Study | % Variation of Effect Size |
| Andrade 2018 [58] | 0.58 [0.23, 0.93] | 8% | 3.57% |
| Hirata 2020 [53] | 0.73 [0.32, 1.13] | 0% | 30.36% |
| Neto 2017 [50] | 0.46 [0.10, 0.81] | 0% | 17.86% |
| Neto 2019 [49] | 0.52 [0.21, 0.83] | 0% | 7.14% |
| Sciatic Nerve—Ankle Neutral (≈0°) vs. Plantar Flexion: SMD [95%CI]: 0.44 [0.15, 0.74], I2: 0% | |||
| Study Removed | SMD [95%CI] without the Study | I2 without the Study | % Variation of Effect Size |
| Andrade 2018 [58] | 0.47 [0.15, 0.79] | 0% | 6.82% |
| Hirata 2020 [53] | 0.48 [0.09, 0.88] | 0% | 9.09% |
| Neto 2017 [50] | 0.36 [0.01, 0.71] | 0% | 18.18% |
| Neto 2019 [49] | 0.46 [0.15, 0.77] | 0% | 4.55% |
| Ulnar Nerve | |||||
|---|---|---|---|---|---|
| Author and Year | N | Location | Initial Position | Movement and Involved Joints | Results |
| Rugel 2020 [37] | 16 | Proximal Elbow Forearm | Supine on an examination table with their shoulder at 45° abduction and wrist at neutral position. | Extension and 90° elbow flexion. | 91.1% increase in UN SWV in the proximal elbow and 37.4% increase in the forearm with elbow flexion compared to extension (p < 0.01). |
| Durand 2021 [60] | 11 | Proximal Elbow | - | 0°, 45°, 90° and 120° elbow flexion. | Significant differences in the shear elastic modulus between 0° (mean 37.1 ± 9.2 kPa) and 45° of elbow flexion (mean 70.3 ± 32.1 kPa, p < 0.01), between 45° and 90° (mean 100.3 ± 29.4 kPa, p < 0.05), and between 90° and 120° (mean 141.7 ± 31.7 kPa, p < 0.005). |
| Wolny 2022 [57] | 31 | Elbow | Side lying | Full extension, 45°, 90° and maximal elbow flexion. | Share modulus increases with increasing degrees of elbow joint flexion. |
| Sciatic Nerve | |||||
|---|---|---|---|---|---|
| Author and Year | N | Location | Initial Position | Movement and Involved Joints | Results |
| Hirata 2020 [53] | 20 young males and 20 older males | Proximal thigh | Hips and knees extended | 3 different ankle positions: (1) 30° ankle plantar flexion; (2) neutral; and (3) 15° dorsal flexion. | SWV values were lower in older than in young participants at any joint angle (p ≤ 0.024, d ≥ 0.748). For both groups, SWV values became higher as the ankle dorsiflexed. SWS values at the maximal dorsiflexion angle were lower in older participants than in young participants. Young males mean SWV (m/s): (1) 2.66 ± 0.61; (2) 3.09 ± 1.12; (3) 3.77 ± 1.71. Older males mean SWV (m/s): (1) 1.92 ± 0.29; (2) 2.12 ± 0.33; (3) 2.47 ± 0.37. |
| Neto 2019 [49] | 8 patients with chronic unilateral low back-related leg pain 8 healthy controls | Proximal thigh | Prone position | 9 ankle position between 0% and 80% of maximum ankle dorsiflexion (0% = 40° ankle plantar flexion) | SN stiffness of the affected limb of patients with chronic low back-related leg pain is higher than that of the unaffected limb. No differences were observed between the unaffected limb of people with low back-related leg pain and the healthy controls. Control group left side SWV (m/s): 0% 6.93 ± 1.65; 10% 6.89 ± 1.61; 20% 6.91 ± 1.64; 30% 7.06 ± 1.42; 40% 7.26 ± 1.30; 50% 7.43 ± 1.19; 60% 7.98 ± 1.06; 70% 8.41 ± 1.16; 80% 8.67 ± 1.28. Control group right side SWV (m/s): 0% 7.02 ± 1.63; 10% 6.82 ± 1.50; 20% 6.93 ± 1.43; 30% 6.91 ± 1.51; 40% 7.38 ± 1.65; 50% 7.92 ± 1.69; 60% 8.34 ± 1.54; 70% 9.00 ± 1.74; 80% 9.11 ± 1.70. Patients group affected side SWV (m/s): 0% 7.51 ± 1.73; 10% 7.62 ± 1.96; 20% 7.81 ± 2.07; 30% 7.82 ± 2.00; 40% 8.11 ± 2.07; 50% 8.49 ± 2.32; 60% 9.22 ± 2.30; 70% 10.03 ± 2.12; 80%10.91 ± 2.92. Patients group unaffected side SWV (m/s): 0% 6.85 ± 1.81; 10% 6.89 ± 1.81; 20% 6.79 ± 1.54; 30% 7.12 ± 1.64; 40% 7.27 ± 1.88; 50% 7.46 ± 1.92; 60% 7.77 ± 2.29; 70% 8.77 ± 1.97; 80% 9.73 ± 2.37. |
| Neto 2017 [50] | 14 | Proximal thigh | Prone position | 9 ankle position between 0% and 80% of maximum ankle dorsiflexion (0% = 40° ankle plantar flexion) | Increased SWV at 50 to 80% of ankle ROM compared to the 0% of ankle ROM in both the experimental (p = 0.04) and control (p = 0.01) limbs in the pre intervention (p = 0.01). |
| Andrade 2016 [59] | 10 | Proximal thigh | 2 knee positions: Knee in full extension and knee in 90° flexion. | Ankle passively moved from 40° of plantarflexion to 80% of the maximal range of dorsiflexion (80% max. dorsiflexion = 100%). | The SWV of the SN significantly increased (p < 0.0001) during dorsiflexion when the knee was extended, but no changes were observed when the knee was flexed to 90°. For knee extension, the SWV was significantly higher at 70%, 80%, 90%, and 100% of ankle angle relative to 0% (p ≤ 0.002). SWV was significantly greater for knee 180° vs. knee 90° across all ankle angle increments (every 10% from 0% to 100%) (p values ranging from 0.001 to 0.002). |
| Andrade 2022 [54] | 60 | Proximal thigh Mid-thigh | (1) Hip neutral in supine position, knee and ankle in full-extension and neutral position | (2) Hip flexed at 90° with knee and ankle in full-extension and neutral position. | SWV increased with hip flexion (average increase +54.3%; p < 0.0001), but the increase was not different among nerve locations (p = 0.233). SWV increase of +2.4 ± 1.6 m/s for SN at the proximal thigh and +2.8 ± 1.9 m/s at the mid-thigh. Proximal thigh SWV (m/s): 4.6 ± 1.2 (hip neutral), 7.1 ± 1.2 (hip flexed). Mid-thigh SWV (m/s): 5.7 ± 1.2 (hip neutral), 8.5 ± 1.6 (hip flexed). |
| Andrade 2018 [58] | 15 | Proximal thigh | Supine with the hip in neutral position. | Progressive ankle dorsiflexion, SN imaged every 2°, from 40° of plantar flexion to the maximal ankle ROM in dorsiflexion | Exponential increase in SN stiffness during passive ankle dorsiflexion while the participants were positioned in hip-neutral position. |
| Tibial Nerve | |||||
|---|---|---|---|---|---|
| Author and Year | N | Location | Initial Position | Movement and Involved Joints | Results |
| Kawanishi 2022 [52] | 20 | Distal leg | Intermediate position of the trunk and neck, 90° hip flexion, 30° knee flexion. | 5 ankle positions: maximum dorsiflexion (100% DF), plantar flexion in the resting position (0% DF), and 3 points (25% DF, 50% DF, and 75% DF), which divided the range of motion from 0% DF to 100% DF. | SWV increased with dorsiflexion. Significant differences in SWV between 0% and 75% DF, 0% and 100% DF, and 25% and 100% DF. Significant negative correlation between the maximum ankle dorsiflexion and stiffness of the TN at 100% DF (p = 0.01) and 75% DF (p = 0.002). The SWV at 75% DF and 100% DF was higher in participants with lower maximal ankle dorsiflexion. Significant negative correlation between the total ankle range of motion and stiffness of the nerve at each joint angle. The SWV at each joint angle was high among the study participants with a reduced total ankle range. SWV (m/s): 0% DF: 4.5 ± 1.7 m/s; 25% DF: 5.2 ± 1.6 m/s; 50% DF: 6.2 ± 1.5 m/s; 75% DF: 7.0 ± 1.1 m/s; and 100% DF: 7.5 ± 0.7 m/s. |
| Anegawa 2023 [56] | 21 | Distal leg | Hip at 90° with 30° of knee flexion. | Two ankle positions: 25% and 75% of the dorsiflexion angle (25% DF and 75% DF). | The 75% DF-SWV was significantly greater than the 25% DF-SWV (7.4 ± 0.7 m/s versus 5.5 ± 1.3 m/s, p < 0.001). |
| Andrade 2022 [54] | 60 | Popliteal fossa Proximal leg Distal leg | (1) Hip neutral in supine position, knee and ankle in full-extension and neutral positions. | (2) Hip flexed at 90° with knee and ankle in full-extension and neutral positions. | Average increase in SWV by 54.3% (p < 0.0001). The increase was not different among nerve locations (p = 0.233). SWV increase of +2.4 ± 1.6 m/s for SN at the popliteal fossa 2.5 ± 1.9 m/s at the proximal leg and + 2.9 ± 1.8 m/s at the distal leg. Popliteal fossa SWV (m/s): 6.5 ± 1.5 (hip neutral); 9.4 ± 2.0 (hip flexed). Proximal leg SWV (m/s): 5.8 ± 1.1 (hip neutral); 8.2 ± 1.7 (hip flexed). Distal leg SWV (m/s): 6.2 ± 1.0 (hip neutral); 9.1 ± 1.5 (hip flexed). |
| Greening 2017 [36] | 26 | Distal leg | (1) Hip neutral, knee flexed to 90°, and foot neutral. | (2) knee maximally extended, and the ankle was dorsiflexed to end of range, while maintaining the hip in neutral; (3) the hip was positioned into maximum flexion (40–90°) while maintaining maximum knee extension and ankle dorsiflexion. | (Position 1) mean SWV: 3.25 ± 0.10 m/s. (Position 2) caused a significant increase by 60 ± 6% in SWV (mean = 5.16 ± 0.21 m/s). (Position 3) further increase (136 ± 9% from starting position) in TN SWV (mean = 7.57 ± 0.28 m/s). Hip angle did not correlate with TN SWV (rs = 0.22). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciuffreda, G.; Bueno-Gracia, E.; Albarova-Corral, I.; Montaner-Cuello, A.; Pérez-Rey, J.; Pardos-Aguilella, P.; Malo-Urriés, M.; Estébanez-de-Miguel, E. In Vivo Effects of Joint Movement on Nerve Mechanical Properties Assessed with Shear-Wave Elastography: A Systematic Review and Meta-Analysis. Diagnostics 2024, 14, 343. https://doi.org/10.3390/diagnostics14030343
Ciuffreda G, Bueno-Gracia E, Albarova-Corral I, Montaner-Cuello A, Pérez-Rey J, Pardos-Aguilella P, Malo-Urriés M, Estébanez-de-Miguel E. In Vivo Effects of Joint Movement on Nerve Mechanical Properties Assessed with Shear-Wave Elastography: A Systematic Review and Meta-Analysis. Diagnostics. 2024; 14(3):343. https://doi.org/10.3390/diagnostics14030343
Chicago/Turabian StyleCiuffreda, Gianluca, Elena Bueno-Gracia, Isabel Albarova-Corral, Alberto Montaner-Cuello, Jorge Pérez-Rey, Pilar Pardos-Aguilella, Miguel Malo-Urriés, and Elena Estébanez-de-Miguel. 2024. "In Vivo Effects of Joint Movement on Nerve Mechanical Properties Assessed with Shear-Wave Elastography: A Systematic Review and Meta-Analysis" Diagnostics 14, no. 3: 343. https://doi.org/10.3390/diagnostics14030343
APA StyleCiuffreda, G., Bueno-Gracia, E., Albarova-Corral, I., Montaner-Cuello, A., Pérez-Rey, J., Pardos-Aguilella, P., Malo-Urriés, M., & Estébanez-de-Miguel, E. (2024). In Vivo Effects of Joint Movement on Nerve Mechanical Properties Assessed with Shear-Wave Elastography: A Systematic Review and Meta-Analysis. Diagnostics, 14(3), 343. https://doi.org/10.3390/diagnostics14030343






