A Machine Learning Model for the Prediction of No-Reflow Phenomenon in Acute Myocardial Infarction Using the CALLY Index
Abstract
:1. Introduction
2. Materials and Methods
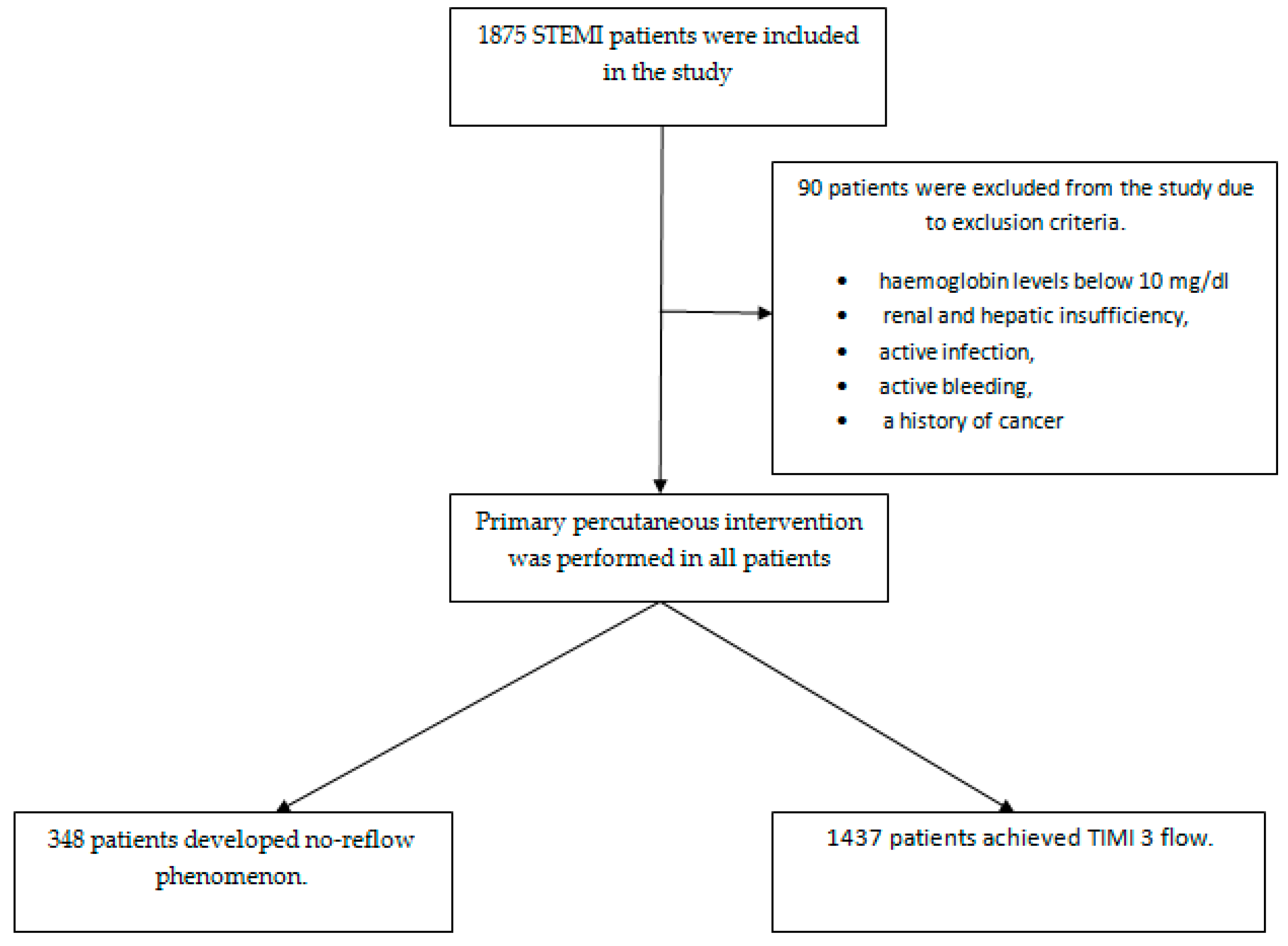
2.1. Study Design and Data
2.2. Data Collection
2.3. Machine Learning–XGBoost
2.4. Machine Learning Modeling and Performance Evaluation
3. Results
3.1. Analysis of Clinical and Demographic Data in STEMI Patients
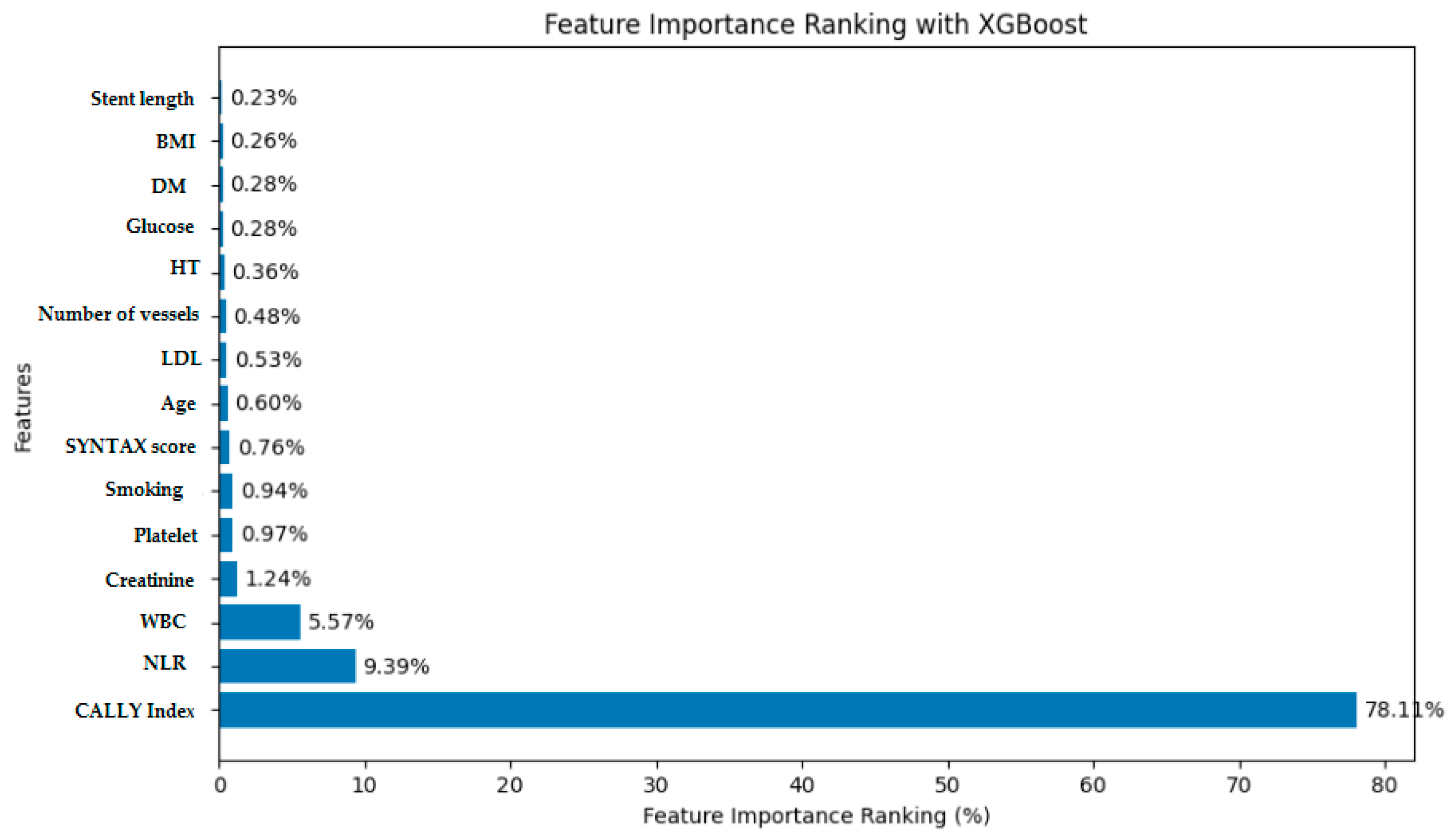
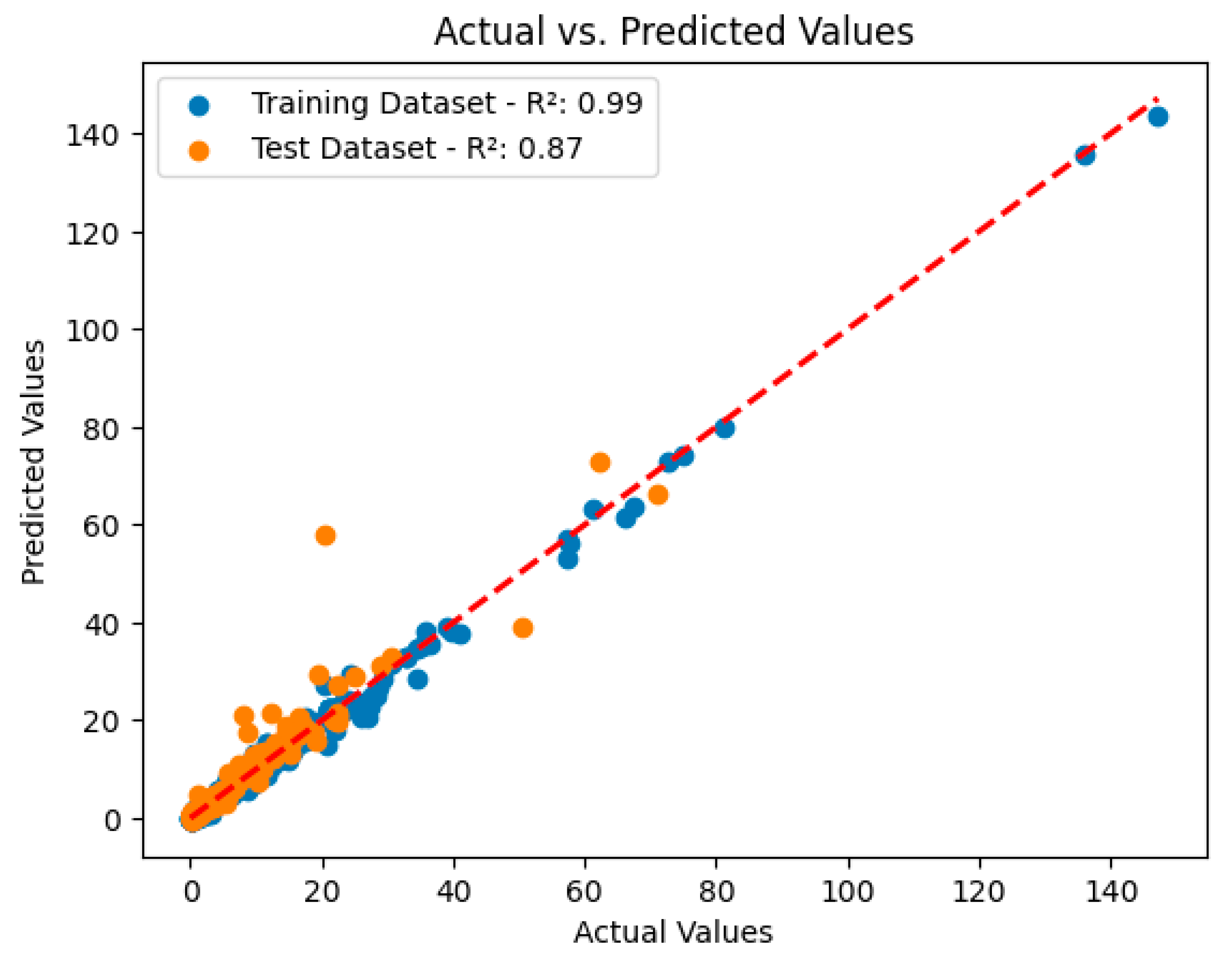
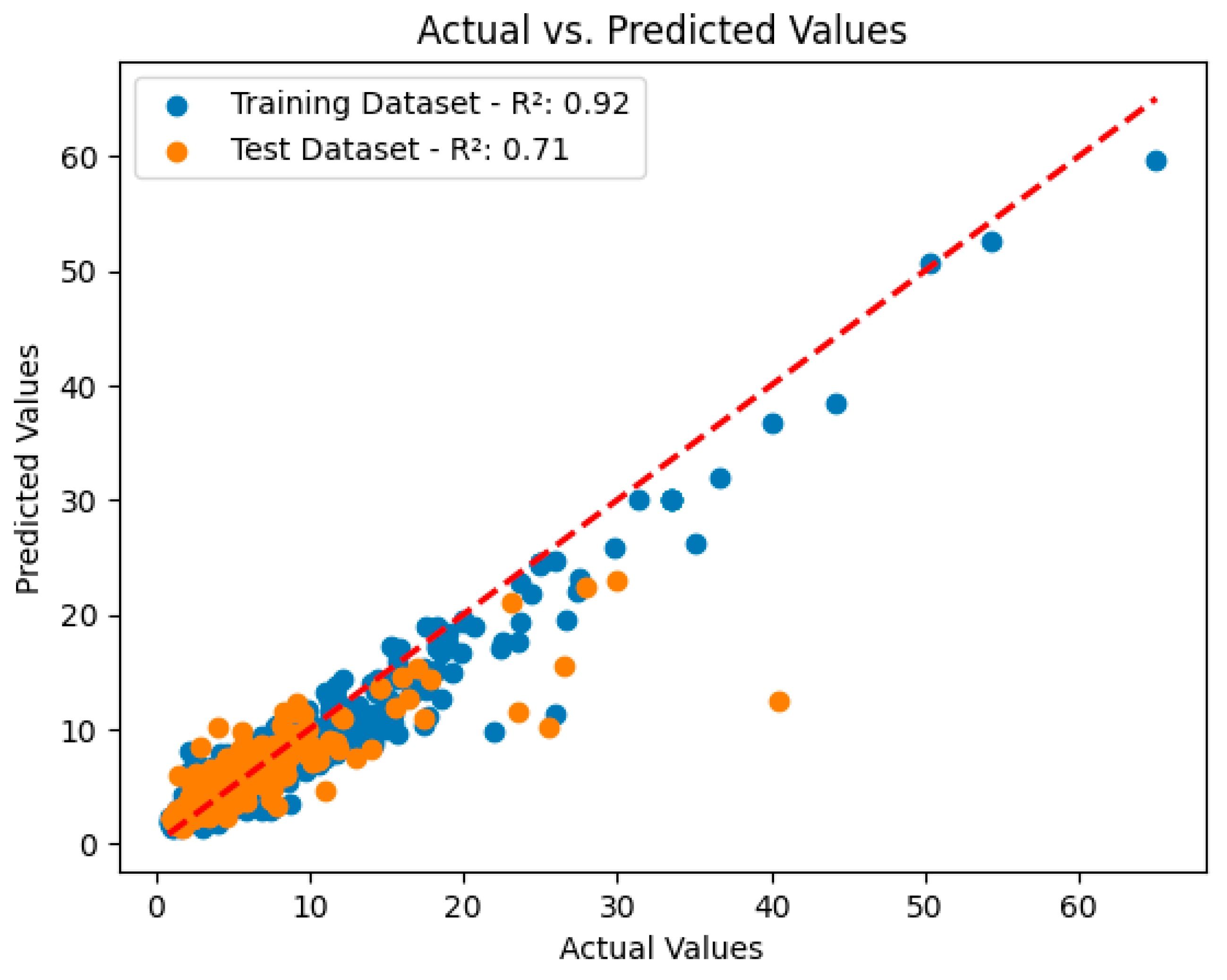
3.2. Analysis and Performance Evaluation of the XGBoost Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Kharbanda, R.K.; Crea, F.; Banning, A.P. No-reflow: Again prevention is better than treatment. Eur. Heart J. 2010, 31, 2449–2455. [Google Scholar] [CrossRef] [PubMed]
- Tasar, O.; Karabay, A.K.; Oduncu, V.; Kirma, C. Predictors and outcomes of no-reflow phenomenon in patients with acute ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Coron. Artery Dis. 2019, 30, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Niccoli, G.; Burzotta, F.; Galiuto, L.; Crea, F. Myocardial no-reflow in humans. J. Am. Coll. Cardiol. 2009, 54, 281–292. [Google Scholar] [CrossRef]
- Caiazzo, G.; Musci, R.L.; Frediani, L.; Umińska, J.; Wanha, W.; Filipiak, K.J.; Kubica, J.; Navarese, E.P. State of the Art: No-Reflow Phenomenon. Cardiol. Clin. 2020, 38, 563–573. [Google Scholar] [CrossRef]
- Kaur, G.; Baghdasaryan, P.; Natarajan, B.; Sethi, P.; Mukherjee, A.; Varadarajan, P.; Pai, R.G. Pathophysiology, Diagnosis, and Management of Coronary No-Reflow Phenomenon. Int. J. Angiol. 2021, 30, 15–21. [Google Scholar] [CrossRef]
- Dai, C.; Liu, M.; Zhou, Y.; Lu, D.; Li, C.; Chang, S.; Chen, Z.; Qian, J.; Ge, J. A score system to predict no-reflow in primary percutaneous coronary intervention: The PIANO Score. Eur. J. Clin. Investig. 2022, 52, e13686. [Google Scholar] [CrossRef]
- Toprak, K.; Toprak, İ.H.; Acar, O.; Ermiş, M.F. The predictive value of the HALP score for no-reflow phenomenon and short-term mortality in patients with ST-elevation myocardial infarction. Postgrad. Med. 2024, 136, 169–179. [Google Scholar] [CrossRef]
- Wang, J.W.; Zhou, Z.Q.; Chen, Y.D.; Wang, C.H.; Zhu, X.L. A risk score for no reflow in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Clin. Cardiol. 2015, 38, 208–215. [Google Scholar] [CrossRef]
- Celık, T.; Balta, S.; Demır, M.; Yıldırım, A.O.; Kaya, M.G.; Ozturk, C.; Demırkol, S.; Unlu, M.; Kılıc, S.; Aydın, İ.; et al. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol. J. 2016, 23, 84–92. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, M.; Ma, S.; Niu, T. New R2-CHA2DS2-VASc score predicts no-reflow phenomenon and long-term prognosis in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Front. Cardiovasc. Med. 2022, 13, 899739. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Lin, S.Q.; Liu, X.Y.; Tang, M.; Hu, C.L.; Wang, Z.W.; Zhang, Q.; Zhang, X.; Song, M.M.; Ruan, G.T.; et al. Association between C-reactive protein-albumin-lymphocyte (CALLY) index and overall survival in patients with colorectal cancer: From the investigation on nutrition status and clinical outcome of common cancers study. Front. Immunol. 2023, 14, 1131496. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, J.; Xie, H.; Liu, X.; Ruan, G.; Lin, S.; Ge, Y.; Liu, C.; Chen, Y.; Zheng, X.; et al. Superiority of CRP-albumin-lymphocyte index as a prognostic biomarker for patients with gastric cancer. Nutrition 2023, 116, 112191. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Zhang, X.; Zhang, Q.; Ruan, G.T.; Liu, T.; Xie, H.L.; Ge, Y.Z.; Song, M.M.; Deng, L.; Shi, H.P. The value of CRP-albumin-lymphocyte index (CALLY index) as a prognostic biomarker in patients with non-small cell lung cancer. Support Care Cancer 2023, 31, 533. [Google Scholar] [CrossRef] [PubMed]
- Iida, H.; Tani, M.; Komeda, K.; Nomi, T.; Matsushima, H.; Tanaka, S.; Ueno, M.; Nakai, T.; Maehira, H.; Mori, H.; et al. Superiority of CRP-albumin-lymphocyte index (CALLY index) as a non-invasive prognostic biomarker after hepatectomy for hepatocellular carcinoma. HPB 2022, 24, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Polikar, R. Ensemble Learning. Ensemble Machine Learning; Springer: Boston, MA, USA, 2012; pp. 1–34. [Google Scholar]
- Nassif, A.B.; Talib, M.A.; Nasir, Q.; Afadar, Y.; Elgendy, O. Breast cancer detection using artificial intelligence techniques: A systematic literature review. Artif. Intell. Med. 2022, 127, 102276. [Google Scholar] [CrossRef]
- Guan, C.; Gong, A.; Zhao, Y.; Yin, C.; Geng, L.; Liu, L.; Yang, X.; Lu, J.; Xiao, B. Interpretable machine learning model for new-onset atrial fibrillation prediction in critically ill patients: A multi-center study. Crit. Care 2024, 28, 349. [Google Scholar] [CrossRef]
- Liu, Z.; Luo, C.; Chen, X.; Feng, Y.; Feng, J.; Zhang, R.; Ouyang, F.; Li, X.; Tan, Z.; Deng, L.; et al. Noninvasive prediction of perineural invasion in intrahepatic cholangiocarcinoma by clinicoradiological features and computed tomography radiomics based on interpretable machine learning: A multicenter cohort study. Int. J. Surg. 2024, 110, 1039–1051. [Google Scholar]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826, Erratum in Eur. Heart J. 2024, 45, 1145. [Google Scholar] [CrossRef]
- TIMI Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial. Phase I findings. N. Engl. J. Med. 1985, 312, 932–936. [Google Scholar] [CrossRef]
- Müller, L.; Hahn, F.; Mähringer-Kunz, A.; Stoehr, F.; Gairing, S.J.; Michel, M.; Foerster, F.; Weinmann, A.; Galle, P.R.; Mittler, J.; et al. Immunonutritive Scoring for Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization: Evaluation of the CALLY Index. Cancers 2021, 13, 5018. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, P.; Ran, R.; Che, Y.; Zhou, Y. A short-term photovoltaic power prediction model based on the gradient boost decision tree. Appl. Sci. 2018, 8, 689. [Google Scholar] [CrossRef]
- Salam Patrous, Z. Evaluating XGBoost for User Classification by Using Behavioral Features Extracted from Smartphone Sensors. Master’s Thesis, KTH Royal Institute of Technology, Stockholm, Sweden, 2018. [Google Scholar]
- Mohite, K.; Kulkarni, C.S.; Bidwe, R.V.; Kamble, A.; Mane, D.; Magar, A.; Sangve, S. Amalgam based cardiovascular disease prediction using Xception with XGBoost model. Front. Health Inform. 2024, 13, 43–55. [Google Scholar]
- Mao, Q.; Jiang, W.; Sun, G.; Zeng, D. XGBoost-Enhanced Prediction and Interpretation of Heart Disease Using SHAP Values. In Proceedings of the 2023 4th International Conference on Computer, Big Data and Artificial Intelligence (ICCBD + AI), Guiyang, China, 15–17 December 2023; pp. 738–742. [Google Scholar]
- Bergamaschi, L.; Arangalage, D.; Maurizi, N.; Pizzi, C.; Valgimigli, M.; Iglesias, J.F.; Landi, A.; Leo, L.A.; Eeckhout, E.; Schwitter, J.; et al. Hepatic T1 Mapping as a Novel Cardio-Hepatic Axis Imaging Biomarker Early after STEMI. Eur. Heart J. Cardiovasc. Imaging 2024. [Google Scholar] [CrossRef]
- Gupta, S.; Gupta, M.M. No reflow phenomenon in percutaneous coronary interventions in ST-segment elevation myocardial infarction. Indian Heart J. 2016, 68, 539–551. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Kloner, R.A. Coronary No-reflow Phenomenon. Curr Treat Options Cardiovasc. Med. 2005, 7, 75–80. [Google Scholar] [CrossRef]
- Kanal, Y.; Şeyda Kanal, H.E.; Yakut, İ.; Özen, Y.; Özbay, M.B.; Gülcihan, B.K.; Yayla, C. CRP Albumin Ratio May Predict No Reflow in Patients Undergoing Percutaneous Coronary Intervention for Saphenous Vein Graft Stenosis. Angiology 2023, 74, 55–61. [Google Scholar] [CrossRef]
- Somuncu, M.U.; Akgun, T.; Cakır, M.O.; Akgul, F.; Serbest, N.G.; Karakurt, H.; Can, M.; Demir, A.R. The Elevated Soluble ST2 Predicts No-Reflow Phenomenon in ST-Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. J. Atheroscler. Thromb. 2019, 26, 970–978. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Stankowski, R.V.; Hanna, J.; Kloner, R.A. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 215–223. [Google Scholar] [CrossRef]
- Allencherril, J.; Jneid, H.; Atar, D.; Alam, M.; Levine, G.; Kloner, R.A.; Birnbaum, Y. Pathophysiology, Diagnosis, and Management of the No-Reflow Phenomenon. Cardiovasc. Drugs Ther. 2019, 33, 589–597. [Google Scholar] [CrossRef]
- Kumar, R.; Khan, K.A.; Shah, J.A.; Ammar, A.; Kumar, D.; Khowaja, S.; Sial, J.A.; Kazmi, S.; Murtaza, M.; Karim, M. Quantification Of Thrombus Burden As An Independent Predictor Of Intra-Procedural No-Reflow In Patients With St-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Revascularization. J. Ayub Med. Coll. Abbottabad 2022, 34, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Badran, H.M.; Fatah, A.A.; Soltan, G. Platelet/lymphocyte ratio for prediction of no-reflow phenomenon in ST-elevation myocardial infarction managed with primary percutaneous coronary intervention. J. Clin. Transl. Res. 2020, 6, 20–26. [Google Scholar] [PubMed]
- Namazi, M.; Mahmoudi, E.; Safi, M.; Jenab, Y.; Vakili, H.; Saadat, H.; Alipour Parsa, S.; Khaheshi, I.; Talasaz, A.H.; Hosseini, S.H.; et al. The No-reflow Phenomenon: Is it Predictable by Demographic factors and Routine Laboratory Data? Acta Biomed. 2021, 92, e2021297. [Google Scholar]
- Gao, G.; Xu, H.; Zhang, D.; Song, C.; Guan, C.; Xu, B.; Yin, D.; Dou, K. The Predictive Value of Baseline Target Lesion SYNTAX Score for No-Reflow during Urgent Percutaneous Coronary Intervention in Acute Myocardial Infarction. J. Interv. Cardiol. 2021, 2021, 9987265. [Google Scholar] [CrossRef]
- Adlung, L.; Cohen, Y.; Mor, U.; Elinav, E. Machine learning in clinical decision making. Med 2021, 2, 642–665. [Google Scholar] [CrossRef]
- Rahman, A.; Karmakar, M.; Debnath, P. Predictive Analytics for Healthcare: Improving Patient Outcomes in the US through Machine Learning. Rev. Intel. Artif. Med. 2023, 14, 595–624. [Google Scholar]
| One-Sample Statistics | ||||
|---|---|---|---|---|
| N | Mean | Standart Deviation | Standart Error Mean | |
| Age | 1785 | 58.527 | 12.1213 | 0.2869 |
| BMI | 1785 | 26.503 | 2.0020 | 0.0474 |
| Diabetes mellitus (%) | 1785 (35.1) | |||
| Hypertension (%) | 1785 (66.5) | |||
| Smoking (%) | 1785 (62.3) | |||
| Glucose | 1785 | 127.841 | 54.6558 | 1.2937 |
| Creatinine | 1785 | 0.872 | 0.2756 | 0.0065 |
| LDL | 1782 | 108.899 | 36.6572 | 0.8684 |
| CRP | 1785 | 1.332 | 1.5668 | 0.0371 |
| WBC | 1785 | 10.649 | 3.2771 | 0.0776 |
| Hct | 1785 | 42.906 | 5.6940 | 0.1348 |
| Number of vessels | 1785 | 1.918 | 0.7987 | 0.0189 |
| Stent length | 1785 | 30.299 | 6.5334 | 0.1546 |
| SYNTAX score | 1785 | 14.670 | 5.3117 | 0.1257 |
| CALLY index | 1785 | 3.8224 | 8.82368 | 0.20885 |
| NLR | 1785 | 5.3271 | 5.10681 | 0.12091 |
| One-Sample Test | ||||||
|---|---|---|---|---|---|---|
| Test Value = 0 | ||||||
| T | df | Sig. (Two-Tailed) | Mean Difference | 95% Confidence Interval of Difference | ||
| Lower | Upper | |||||
| Age | 203.996 | 1785 | 0.001 | 58.5266 | 57.964 | 59.089 |
| BMI | 559.298 | 1785 | 0.001 | 26.5025 | 26.410 | 26.595 |
| Glucose | 98.822 | 1785 | 0.001 | 127.8409 | 125.304 | 130.378 |
| Creatinine | 133.735 | 1785 | 0.001 | 0.8723 | 0.860 | 0.885 |
| LDL | 125.406 | 1785 | 0.001 | 108.8986 | 107.195 | 110.602 |
| CRP | 35.905 | 1785 | 0.001 | 1.3316 | 1.259 | 1.404 |
| White blood cell | 137.294 | 1785 | 0.001 | 10.6493 | 10.497 | 10.801 |
| Hematocrit | 318.362 | 1785 | 0.001 | 42.9059 | 42.642 | 43.170 |
| Number of vessels | 101.468 | 1785 | 0.001 | 1.9182 | 1.881 | 1.955 |
| Stent length | 195.935 | 1785 | 0.001 | 30.2992 | 29.996 | 30.602 |
| SYNTAX score | 116.685 | 1785 | 0.001 | 14.6700 | 14.423 | 14.917 |
| CALLY index | 18.302 | 1785 | 0.001 | 3.82238 | 3.4128 | 4.2320 |
| NLR | 44.059 | 1785 | 0.001 | 5.32709 | 5.0900 | 5.5642 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fedai, H.; Sariisik, G.; Toprak, K.; Taşcanov, M.B.; Efe, M.M.; Arğa, Y.; Doğanoğulları, S.; Gez, S.; Demirbağ, R. A Machine Learning Model for the Prediction of No-Reflow Phenomenon in Acute Myocardial Infarction Using the CALLY Index. Diagnostics 2024, 14, 2813. https://doi.org/10.3390/diagnostics14242813
Fedai H, Sariisik G, Toprak K, Taşcanov MB, Efe MM, Arğa Y, Doğanoğulları S, Gez S, Demirbağ R. A Machine Learning Model for the Prediction of No-Reflow Phenomenon in Acute Myocardial Infarction Using the CALLY Index. Diagnostics. 2024; 14(24):2813. https://doi.org/10.3390/diagnostics14242813
Chicago/Turabian StyleFedai, Halil, Gencay Sariisik, Kenan Toprak, Mustafa Beğenç Taşcanov, Muhammet Mucip Efe, Yakup Arğa, Salih Doğanoğulları, Sedat Gez, and Recep Demirbağ. 2024. "A Machine Learning Model for the Prediction of No-Reflow Phenomenon in Acute Myocardial Infarction Using the CALLY Index" Diagnostics 14, no. 24: 2813. https://doi.org/10.3390/diagnostics14242813
APA StyleFedai, H., Sariisik, G., Toprak, K., Taşcanov, M. B., Efe, M. M., Arğa, Y., Doğanoğulları, S., Gez, S., & Demirbağ, R. (2024). A Machine Learning Model for the Prediction of No-Reflow Phenomenon in Acute Myocardial Infarction Using the CALLY Index. Diagnostics, 14(24), 2813. https://doi.org/10.3390/diagnostics14242813







