Mammographic Vascular Microcalcifications as a Surrogate Parameter for Coronary Heart Disease: Correlation to Cardiac Computer Tomography and Proposal of a Classification Score
Abstract
- The classification of breast calcifications into (1) visible or sporadically scattered microcalcifications, (2) suspicious microcalcifications indistinguishable from breast arterial calcification, (3) minor breast arterial calcification, and (4) major breast arterial calcification is feasible with substantial inter-rater agreement and may improve patient management.
- Breast arterial calcification on mammography correlates with coronary artery calcification (p < 0.001). Therefore, evaluation of potential coronary heart disease in patients with breast arterial calcification in mammography may be feasible and worthwile in large-scale mammography screening programs.
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. CT-Imaging
2.3. Mammography Protocol
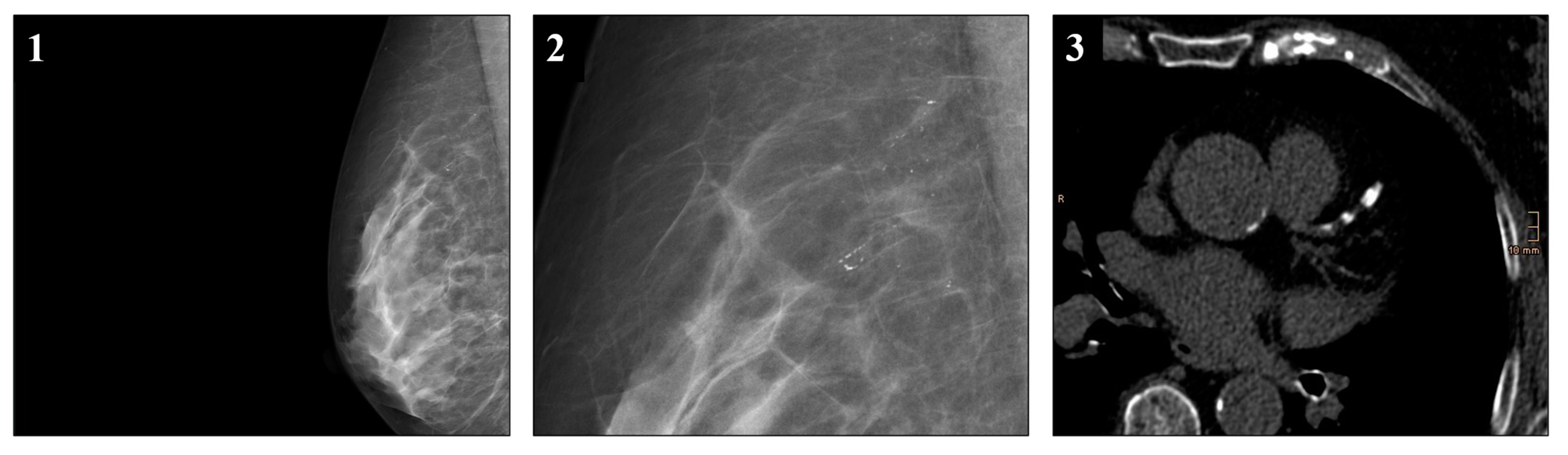
2.4. Definitions and Score Generation
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Cardiac CT Results
3.3. Breast Densities and BI-RADS Classifications in Mammographies
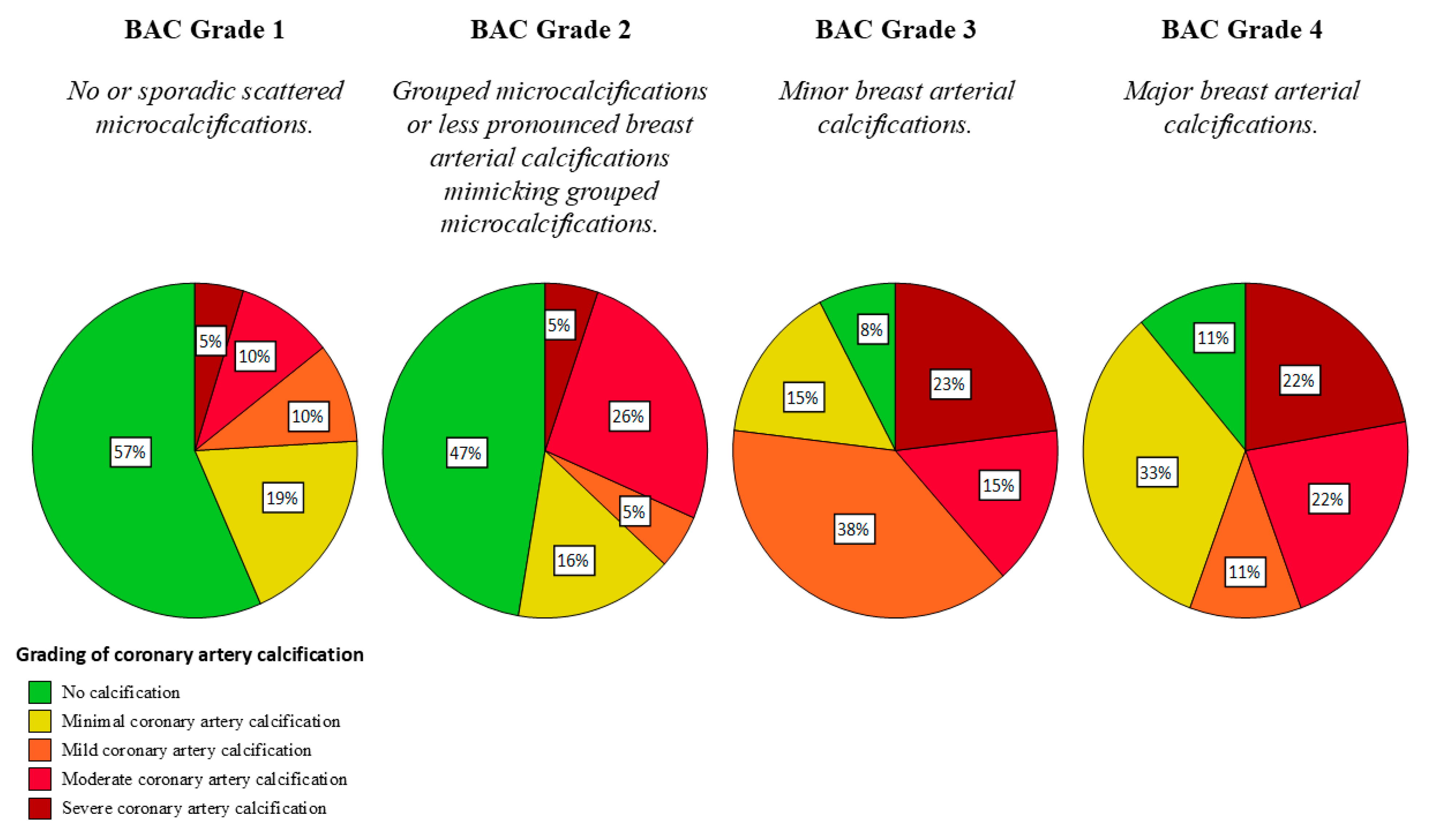
3.4. Vascular Calcifications in Mammographies
3.5. Correlation Between Mammography and CT Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAC | Breast arterial calcification |
| BI-RADS | Breast Imaging Reporting and Data System |
| CAC | Coronary artery calcification |
| CHD | Coronary heart disease |
| CT | Computed tomography |
| DCIS | Ductal carcinoma in situ |
| MC | Microcalcifications |
References
- BHF. Global Heart & Circulatory Diseases Factsheet; British Heart Foundation: London, UK, 2023; pp. 1–12. Available online: https://www.bhf.org.uk/-/media/files/for-professionals/research/heart-statistics/bhf-cvd-statistics-global-factsheet.pdf?rev=169125b6b7e2474985f8a0c419ba9676&hash=79BA5336A597F7180A0B8898F5C2ADEB (accessed on 10 December 2024).
- Mehta, L.S.; Beckie, T.M.; DeVon, H.A.; Grines, C.L.; Krumholz, H.M.; Johnson, M.N.; Lindley, K.J.; Vaccarino, V.; Wang, T.Y.; Watson, K.E.; et al. Acute Myocardial Infarction in Women. Circulation 2016, 133, 916–947. [Google Scholar] [CrossRef]
- Heron, M. Deaths: Leading Causes for 2017. Natl. Vital Stat. Rep. 2019, 68, 1–77. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32501203 (accessed on 3 July 2024). [PubMed]
- Bleyer, A.; Welch, H.G. Effect of Three Decades of Screening Mammography on Breast-Cancer Incidence. N. Engl. J. Med. 2012, 367, 1998–2005. [Google Scholar] [CrossRef]
- Duffy, S.W. Reduction in Breast Cancer Mortality from Organized Service Screening with Mammography: 1. Further Confirmation with Extended Data. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 45–51. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Heart Disease Facts. Natl. Cent. Chronic Dis. Prev. Heal Promot. Div. Hear. Dis. Stroke Prev. 2012, 22–25. Available online: https://www.cdc.gov/heart-disease/data-research/facts-stats/index.html (accessed on 4 July 2024).
- Marcon, M.; Dedes, K.; Varga, Z.; Frauenfelder, T.; Boss, A. Influence of breast cancer opportunistic screening on aesthetic surgical outcome: A single-center retrospective study in Switzerland. Breast J. 2018, 24, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Duffy, S.W.; Vulkan, D.; Cuckle, H.; Parmar, D.; Sheikh, S.; Smith, R.A.; Evans, A.; Blyuss, O.; Johns, L.; Ellis, I.O.; et al. Effect of mammographic screening from age 40 years on breast cancer mortality (UK Age trial): Final results of a randomised, controlled trial. Lancet Oncol. 2020, 21, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Naseem, M.; Murray, J.; Hilton, J.F.; Karamchandani, J.; Muradali, D.; Faragalla, H.; Polenz, C.; Han, D.; Bell, D.C.; Brezden-Masley, C. Mammographic microcalcifications and breast cancer tumorigenesis: A radiologic-pathologic analysis. BMC Cancer 2015, 15, 307. [Google Scholar] [CrossRef] [PubMed]
- Strax, P.; Venet, L.; Shapiro, S.; Gross, S. Mammography and clinical examination in mass screening for cancer of the breast. Cancer 1967, 20, 2184–2188. [Google Scholar] [CrossRef] [PubMed]
- Nelson, H.D.; Fu, R.; Cantor, A.; Pappas, M.; Daeges, M.; Humphrey, L. Effectiveness of Breast Cancer Screening: Systematic Review and Meta-analysis to Update the 2009 U.S. Preventive Services Task Force Recommendation. Ann. Intern. Med. 2016, 164, 244. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.; Thomas, V.; Sharma, N. Microcalcification on mammography: Approaches to interpretation and biopsy. Br. J. Radiol. 2017, 90, 20160594. [Google Scholar] [CrossRef] [PubMed]
- Kenkel, D.; Varga, Z.; Heuer, H.; Dedes, K.J.; Berger, N.; Filli, L.; Boss, A. A Micro CT Study in Patients with Breast Microcalcifications Using a Mathematical Algorithm to Assess 3D Structure. PLoS ONE 2017, 12, e0169349. [Google Scholar] [CrossRef]
- Spak, D.A.; Plaxco, J.S.; Santiago, L.; Dryden, M.J.; Dogan, B.E. BI-RADS® fifth edition: A summary of changes. Diagn. Interv. Imaging 2017, 98, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Greenberg, J.S.; Javitt, M.C. Breast Calcifications due to Mönckeberg Medial Calcific Sclerosis. RadioGraphics 1999, 19, 1401–1403. [Google Scholar] [CrossRef][Green Version]
- Oliveira, E.L.C.; Freitas-Junior, R.; Afiune-Neto, A.; Murta, E.F.C.; Ferro, J.E.; Melo, A.F.B. Vascular Calcifications Seen on Mammography: An Independent Factor Indicating Coronary Artery Disease. Clinics 2009, 64, 763–767. [Google Scholar] [CrossRef]
- Zuin, M.; Rigatelli, G.; Scaranello, F.; Ribecco, S.G.; Picariello, C.; Zuliani, G.; Faggian, G.; Zonzin, P.; Roncon, L. Breast arterial calcifications on mammography and coronary artery disease: A new screening tool for cardiovascular disease? Int. J. Cardiol. 2016, 220, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, E.J.E.; de Jong, P.A.; van der Graaf, Y.; Mali, W.P.T.M.; van der Schouw, Y.T.; Beulens, J.W.J. Breast arterial calcifications: A systematic review and meta-analysis of their determinants and their association with cardiovascular events. Atherosclerosis 2015, 239, 11–20. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Yu, C.M.; Ji, Q.W.; Cai, M.; Zhao, Y.X.; Zhou, Y.J. Current understanding of coronary artery calcification. J. Geriatr. Cardiol. 2015, 12, 668–675. [Google Scholar] [CrossRef]
- Reddy, J.; Son, H.; Smith, S.J.; Paultre, F.; Mosca, L. Prevalence of Breast Arterial Calcifications in an Ethnically Diverse Population of Women. Ann. Epidemiol. 2005, 15, 344–350. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, C.J.; Sickler, E.A.; Mendelson, E.B.; Morris, E.S.; Creech, W.E.; Butler, P.F.; Wiegmann, P.G.; Chatfield, M.B.; Meyer, L.W.; Wilcox, P.A. ACR BI-RADS®-Atlas der Mammadiagnostik; Springer: Berlin/Heidelberg, Germany, 2016; ISBN 978-3-662-48817-1. [Google Scholar]
- Rumberger, J.A. Coronary Artery Calcium Scanning Using Computed Tomography: Clinical Recommendations for Cardiac Risk Assessment and Treatment. Semin. Ultrasound CT MRI 2008, 29, 223–229. [Google Scholar] [CrossRef]
- Houn, F.; Brown, M.L. Current practice of screening mammography in the United States: Data from the National Survey of Mammography Facilities. Radiology 1994, 190, 209–215. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. Available online: http://www.ncbi.nlm.nih.gov/pubmed/843571 (accessed on 21 August 2024). [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Janzen, K.; Janzen, J. Arterial Microcalcifications in the Breast Mimicking Malignancy. Case Rep. Radiol. 2012, 2012, 946317. [Google Scholar] [CrossRef] [PubMed][Green Version]
- George, J.T.; Green, L. Calciphylaxis of the breast, mimicking advanced breast cancer with skin involvement. Radiol. Case Rep. 2021, 16, 1211–1215. [Google Scholar] [CrossRef]
- Maxwell, A.J.; Ridley, N.T.; Rubin, G.; Wallis, M.G.; Gilbert, F.J.; Michell, M.J. The Royal College of Radiologists Breast Group breast imaging classification. Clin. Radiol. 2009, 64, 624–627. [Google Scholar] [CrossRef]
- Maas, A.H.E.M.; van der Schouw, Y.T.; Atsma, F.; Beijerinck, D.; Deurenberg, J.J.M.; Mali, W.P.T.M.; van der Graaf, Y. Breast arterial calcifications are correlated with subsequent development of coronary artery calcifications, but their aetiology is predominantly different. Eur. J. Radiol. 2007, 63, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Phillips, M.; Bellinge, J.; Stone, J.; Wylie, E.; Schultz, C. Is breast arterial calcification associated with coronary artery disease?—A systematic review and meta-analysis. PLoS ONE 2020, 15, e0236598. [Google Scholar] [CrossRef]
- Matsumura, M.E.; Maksimik, C.; Martinez, M.W.; Weiss, M.; Newcomb, J.; Harris, K.; Rossi, M.A. Breast artery calcium noted on screening mammography is predictive of high risk coronary calcium in asymptomatic women: A case control study. Vasa 2013, 42, 429–433. [Google Scholar] [CrossRef]
- Iribarren, C.; Go, A.S.; Tolstykh, I.; Sidney, S.; Johnston, S.C.; Spring, D.B. Breast Vascular Calcification and Risk of Coronary Heart Disease, Stroke, and Heart Failure. J. Women’s Health 2004, 13, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kemmeren, J.M.; Beijerinck, D.; van Noord, P.A.; Banga, J.D.; Deurenberg, J.J.; Pameijer, F.A.; van der Graaf, Y. Breast arterial calcifications: Association with diabetes mellitus and cardiovascular mortality. Work in progress. Radiology 1996, 201, 75–78. [Google Scholar] [CrossRef]
- Schnatz, P.F.; Marakovits, K.A.; O’Sullivan, D.M. The Association of Breast Arterial Calcification and Coronary Heart Disease. Obstet. Gynecol. 2011, 117, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Kemmeren, J.M.; van Noord, P.A.H.; Beijerinck, D.; Fracheboud, J.; Banga, J.-D.; van der Graaf, Y. Arterial Calcification Found on Breast Cancer Screening Mammograms and Cardiovascular Mortality in Women: The DOM Project. Am. J. Epidemiol. 1998, 147, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Margolies, L.; Salvatore, M.; Hecht, H.S.; Kotkin, S.; Yip, R.; Baber, U.; Bishay, V.; Narula, J.; Yankelevitz, D.; Henschke, C. Digital Mammography and Screening for Coronary Artery Disease. JACC Cardiovasc. Imaging 2016, 9, 350–360. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. MQSA National Statistics. MQSA Insights 2022, 10–12. Available online: http://www.fda.gov/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/FacilityScorecard/ucm113858.htm (accessed on 2 July 2024).
- Gendarme, S.; Goussault, H.; Assié, J.-B.; Taleb, C.; Chouaïd, C.; Landre, T. Impact on All-Cause and Cardiovascular Mortality Rates of Coronary Artery Calcifications Detected during Organized, Low-Dose, Computed-Tomography Screening for Lung Cancer: Systematic Literature Review and Meta-Analysis. Cancers 2021, 13, 1553. [Google Scholar] [CrossRef]
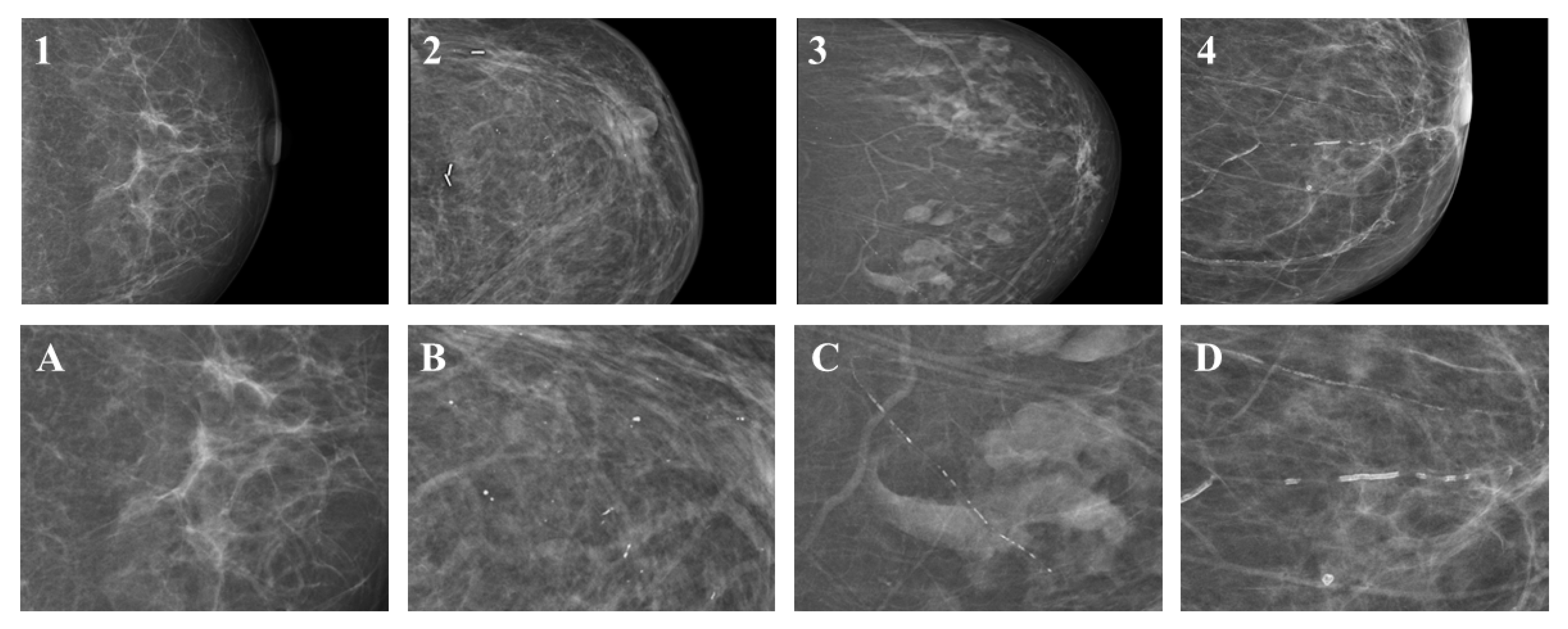
| Characteristic | Overall, N = 124 | No BAC, N = 102 | BAC, N = 22 | p-Value 1 |
|---|---|---|---|---|
| Age at mammography, median (IQR) | 57 (15) | 52 (14) | 62 (12) | |
| Time between mammography and cardiac CT in years (IQR) | 2.94 (17.0) | 3.03 (15.8) | 2.94 (17.0) | |
| Maximum diameter of breast vessels (mm) | ||||
| Left breast only | 2.06 | 2.06 | 2.02 | 0.07 |
| Right breast only | 1.95 | 1.95 | 2.03 | 0.66 |
| BAC laterality | <0.01 | |||
| Unilateral | 25 (20) | 17 (17) | 8 (36) | |
| Bilateral | 18 (15) | 4 (4) | 14 (64) | |
| BAC Side | 0.89 2 | |||
| Left breast only | 12 (10) | 8 (8) | 4 (18) | |
| Right breast only | 13 (10) | 9 (9) | 4 (18) | |
| ACR | 0.25 | |||
| a | 19 (15) | 13 (13) | 6 (27) | |
| b | 48 (39) | 41 (40) | 7 (32) | |
| c | 42 (34) | 34 (33) | 8 (36) | |
| d | 15 (12) | 14 (14) | 1 (5) | |
| BIRADs, n (%) | 0.24 | |||
| 0 | 0 (0) | 0 (0) | 0 (0) | |
| 1 | 5 (4) | 5 (5) | 0 (0) | |
| 2 | 105 (85) | 86 (84) | 19 (86) | |
| 3 | 10 (8) | 9 (9) | 1 (0) | |
| 4 | 3 (2) | 2 (2) | 1 (5) | |
| 5 | 1 (1) | 0 (0) | 1 (5) |
| Characteristics | Overall, N = 124 | No BAC, N = 102 | BAC, N = 22 | p-Value 1 |
|---|---|---|---|---|
| Maximum diameter of proximal coronary vessel (mm) | ||||
| LM | 5.44 | 5.30 | 6.05 | 0.08 |
| LAD | 4.45 | 4.41 | 4.61 | 0.13 |
| CX | 4.25 | 4.16 | 4.64 | 0.68 |
| RCA | 4.57 | 4.50 | 4.89 | 0.02 |
| Coronary Artery Calcification, n (%) | 66 (53) | 46 (45) | 20 (91) | 0.01 |
| Number of affected coronary vessels, n (%) | <0.01 | |||
| 0 | 58 (47) | 56 (55) | 2 (9) | |
| 1 | 29 (23) | 22 (22) | 7 (32) | |
| 2 | 18 (15) | 11 (11) | 7 (32) | |
| 3 | 12 (10) | 8 (8) | 4 (18) | |
| 4 | 7 (6) | 5 (5) | 2 (9) | |
| Affected vessels | ||||
| LM | 16 (13) | 13 (13) | 3 (13) | 1 |
| LAD | 59 (48) | 40 (39) | 19 (86) | <0.01 |
| CX | 25 (20) | 16 (16) | 9 (41) | 0.02 |
| RCA | 29 (23) | 19 (19) | 10 (41) | 0.01 |
| Agatston score, n (%) | <0.01 | |||
| No CAC: CS = 0 | 58 (47) | 56 (55) | 2 (9) | |
| Minimal CAC: CS = 1–10 | 24 (19) | 19 (19) | 5 (22) | |
| Mild CAC: CS = 11–100 | 15 (12) | 9 (9) | 6 (27) | |
| Moderate CAC: CS = 100–400 | 17 (14) | 13 (12) | 4 (18) | |
| Severe CAC: CS > 400 | 10 (8) | 5 (5) | 5 (23) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saenger, J.A.; Uenal, E.; Mann, E.; Winnik, S.; Eriksson, U.; Boss, A. Mammographic Vascular Microcalcifications as a Surrogate Parameter for Coronary Heart Disease: Correlation to Cardiac Computer Tomography and Proposal of a Classification Score. Diagnostics 2024, 14, 2803. https://doi.org/10.3390/diagnostics14242803
Saenger JA, Uenal E, Mann E, Winnik S, Eriksson U, Boss A. Mammographic Vascular Microcalcifications as a Surrogate Parameter for Coronary Heart Disease: Correlation to Cardiac Computer Tomography and Proposal of a Classification Score. Diagnostics. 2024; 14(24):2803. https://doi.org/10.3390/diagnostics14242803
Chicago/Turabian StyleSaenger, Jonathan Andreas, Ela Uenal, Eugen Mann, Stephan Winnik, Urs Eriksson, and Andreas Boss. 2024. "Mammographic Vascular Microcalcifications as a Surrogate Parameter for Coronary Heart Disease: Correlation to Cardiac Computer Tomography and Proposal of a Classification Score" Diagnostics 14, no. 24: 2803. https://doi.org/10.3390/diagnostics14242803
APA StyleSaenger, J. A., Uenal, E., Mann, E., Winnik, S., Eriksson, U., & Boss, A. (2024). Mammographic Vascular Microcalcifications as a Surrogate Parameter for Coronary Heart Disease: Correlation to Cardiac Computer Tomography and Proposal of a Classification Score. Diagnostics, 14(24), 2803. https://doi.org/10.3390/diagnostics14242803






