Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. CCM in Chemotherapy Induced Polyneuropathy
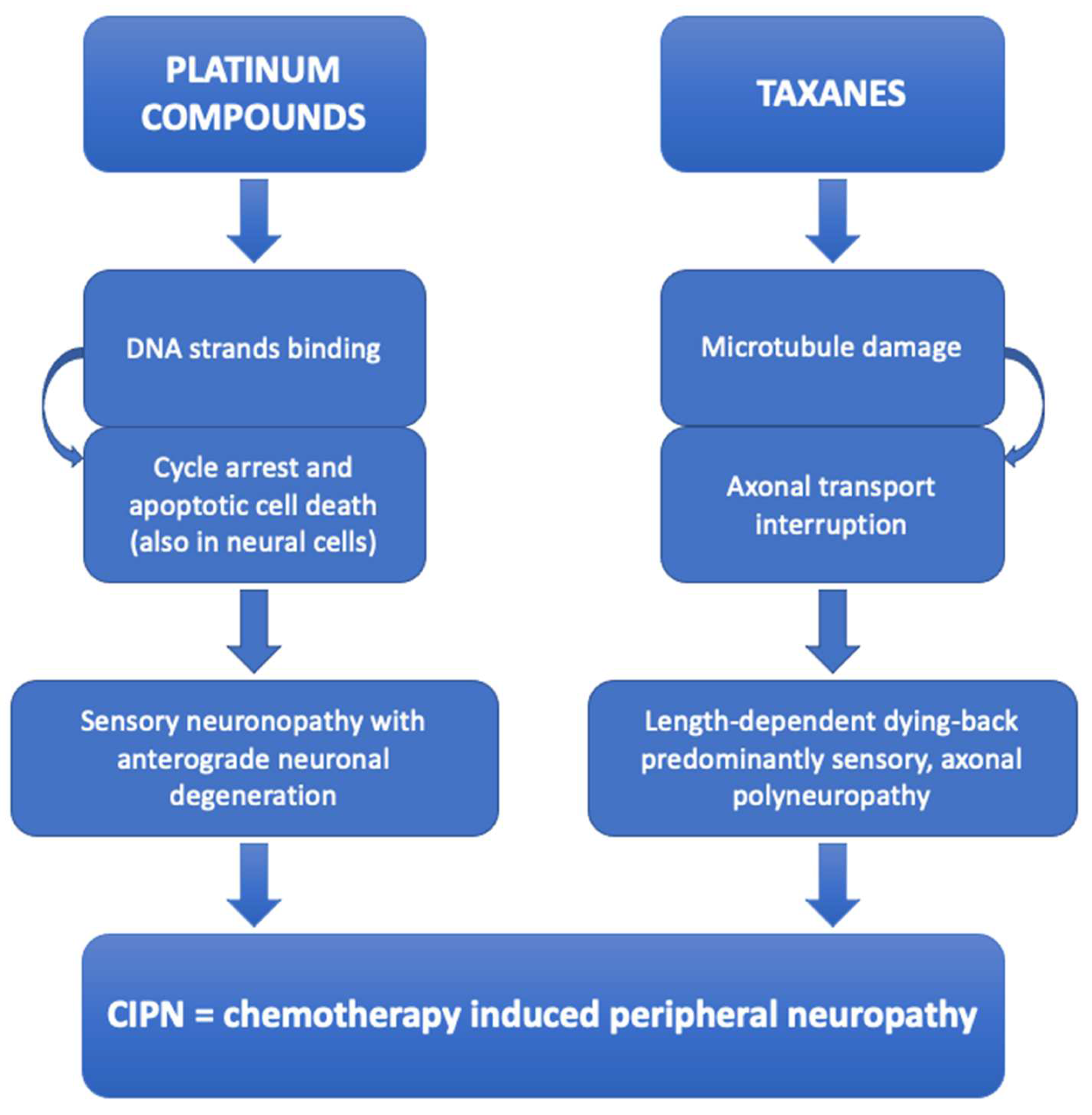
3.1.1. Platinum Compounds and Taxanes
3.1.2. Other Chemotherapy Drugs
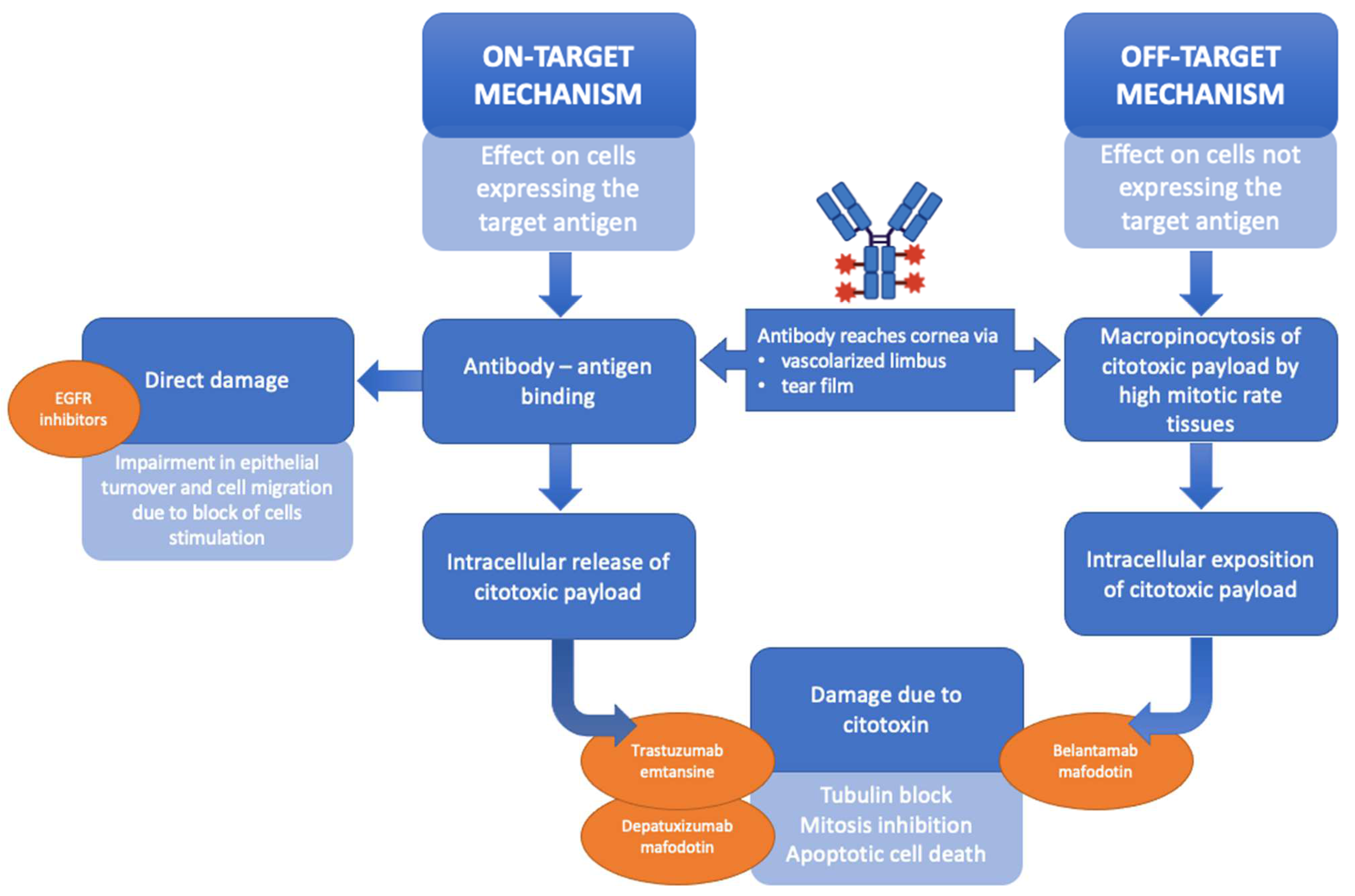
3.2. CCM in Other Chemotherapy Induced Damage on Corneal Structure
3.3. Limits and Future Directions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, T.; Niu, X.; He, Q.; Liu, M.; Qiao, S.; Qi, R.Q. Development, Efficacy and Side Effects of Antibody–Drug Conjugates for Cancer Therapy (Review). Mol. Clin. Oncol. 2023, 18, 47. [Google Scholar] [CrossRef]
- Schirrmacher, V. From Chemotherapy to Biological Therapy: A Review of Novel Concepts to Reduce the Side Effects of Systemic Cancer Treatment. Int. J. Oncol. 2019, 54, 407–419. [Google Scholar] [CrossRef]
- Kunkler, A.L.; Binkley, E.M.; Mantopoulos, D.; Hendershot, A.J.; Ohr, M.P.; Kendra, K.L.; Davidorf, F.H.; Cebulla, C.M. Known and Novel Ocular Toxicities of Biologics, Targeted Agents, and Traditional Chemotherapeutics. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1771–1781. [Google Scholar] [CrossRef]
- DelMonte, D.W.; Kim, T. Anatomy and Physiology of the Cornea. J. Cataract Refract. Surg. 2011, 37, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-Soto, L.; Efron, N. Morphology of Corneal Nerves Using Confocal Microscopy. Cornea 2001, 20, 374–384. [Google Scholar] [CrossRef]
- Cosmo, E.; Midena, G.; Frizziero, L.; Bruno, M.; Cecere, M.; Midena, E. Corneal Confocal Microscopy as a Quantitative Imaging Biomarker of Diabetic Peripheral Neuropathy: A Review. J. Clin. Med. 2022, 11, 5130. [Google Scholar] [CrossRef] [PubMed]
- Midena, E.; Gambato, C.; Miotto, S.; Cortese, M.; Salvi, R.; Ghirlando, A. Long-Term Effects on Corneal Keratocytes of Mitomycin C during Photorefractive Keratectomy: A Randomized Contralateral Eye Confocal Microscopy Study. J. Refract. Surg. 2007, 23, S1011–S1014. [Google Scholar] [CrossRef]
- Parrozzani, R.; Lazzarini, D.; Alemany-Rubio, E.; Urban, F.; Midena, E. Topical 1% 5-Fluorouracil in Ocular Surface Squamous Neoplasia: A Long-Term Safety Study. Br. J. Ophthalmol. 2011, 95, 355–359. [Google Scholar] [CrossRef]
- Leonardi, A.; Lazzarini, D.; Bortolotti, M.; Piliego, F.; Midena, E.; Fregona, I. Corneal Confocal Microscopy in Patients with Vernal Keratoconjunctivitis. Ophthalmology 2012, 119, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Sturniolo, G.C.; Lazzarini, D.; Bartolo, O.; Berton, M.; Leonardi, A.; Fregona, I.A.; Parrozzani, R.; Midena, E. Small Fiber Peripheral Neuropathy in Wilson Disease: An in Vivo Documentation by Corneal Confocal Microscopy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1390–1395. [Google Scholar] [CrossRef]
- Midena, E.; Cosmo, E.; Cattelan, A.M.; Briani, C.; Leoni, D.; Capizzi, A.; Tabacchi, V.; Parrozzani, R.; Midena, G.; Frizziero, L. Small Fibre Peripheral Alterations Following COVID-19 Detected by Corneal Confocal Microscopy. J. Pers. Med. 2022, 12, 563. [Google Scholar] [CrossRef]
- Stone, J.B.; DeAngelis, L.M. Cancer-Treatment-Induced Neurotoxicity--Focus on Newer Treatments. Nat. Rev. Clin. Oncol. 2016, 13, 92–105. [Google Scholar] [CrossRef]
- Park, S.B.; Goldstein, D.; Krishnan, A.V.; Lin, C.S.-Y.; Friedlander, M.L.; Cassidy, J.; Koltzenburg, M.; Kiernan, M.C. Chemotherapy-Induced Peripheral Neurotoxicity: A Critical Analysis. CA Cancer J. Clin. 2013, 63, 419–437. [Google Scholar] [CrossRef]
- Chiang, J.C.B.; Goldstein, D.; Park, S.B.; Krishnan, A.V.; Markoulli, M. Corneal Nerve Changes Following Treatment with Neurotoxic Anticancer Drugs. Ocul. Surf. 2021, 21, 221–237. [Google Scholar] [CrossRef]
- Argyriou, A.A.; Park, S.B.; Islam, B.; Tamburin, S.; Velasco, R.; Alberti, P.; Bruna, J.; Psimaras, D.; Cavaletti, G.; Cornblath, D.R. Neurophysiological, Nerve Imaging and Other Techniques to Assess Chemotherapy-Induced Peripheral Neurotoxicity in the Clinical and Research Settings. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1361–1369. [Google Scholar] [CrossRef]
- Shah, A.; Hoffman, E.M.; Mauermann, M.L.; Loprinzi, C.L.; Windebank, A.J.; Klein, C.J.; Staff, N.P. Incidence and Disease Burden of Chemotherapy-Induced Peripheral Neuropathy in a Population-Based Cohort. J. Neurol. Neurosurg. Psychiatry 2018, 89, 636–641. [Google Scholar] [CrossRef]
- Zajaczkowską, R.; Kocot-Kępska, M.; Leppert, W.; Wrzosek, A.; Mika, J.; Wordliczek, J. Mechanisms of Chemotherapy-Induced Peripheral Neuropathy. Int. J. Mol. Sci. 2019, 20, 1451. [Google Scholar] [CrossRef]
- Bonomo, R.; Cavaletti, G. Clinical and Biochemical Markers in CIPN: A Reappraisal. Rev. Neurol. (Paris) 2021, 177, 890–907. [Google Scholar] [CrossRef]
- Campagnolo, M.; Lazzarini, D.; Fregona, I.; Cacciavillani, M.; Bergamo, F.; Parrozzani, R.; Midena, E.; Briani, C. Corneal Confocal Microscopy in Patients with Oxaliplatin-Induced Peripheral Neuropathy. J. Peripher. Nerv. Syst. 2013, 18, 269–271. [Google Scholar] [CrossRef]
- Ferdousi, M.; Azmi, S.; Petropoulos, I.N.; Fadavi, H.; Ponirakis, G.; Marshall, A.; Tavakoli, M.; Malik, I.; Mansoor, W.; Malik, R.A. Corneal Confocal Microscopy Detects Small Fibre Neuropathy in Patients with Upper Gastrointestinal Cancer and Nerve Regeneration in Chemotherapy Induced Peripheral Neuropathy. PLoS ONE 2015, 10, e0139394. [Google Scholar] [CrossRef]
- Bennedsgaard, K.; Ventzel, L.; Andersen, N.T.; Themistocleous, A.C.; Bennett, D.L.; Jensen, T.S.; Tankisi, H.; Finnerup, N.B. Oxaliplatin- and Docetaxel-Induced Polyneuropathy: Clinical and Neurophysiological Characteristics. J. Peripher. Nerv. Syst. 2020, 25, 377–387. [Google Scholar] [CrossRef]
- Chiang, J.C.B.; Goldstein, D.; Trinh, T.; Au, K.; Mizrahi, D.; Muhlmann, M.; Crowe, P.; O’neill, S.; Edwards, K.; Park, S.B.; et al. A Cross-Sectional Study of Sub-Basal Corneal Nerve Reduction Following Neurotoxic Chemotherapy. Transl. Vis. Sci. Technol. 2021, 10, 24. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Trinh, T.; Au, K.; Park, S.B.; Krishnan, A.V.; Markoulli, M. A Cross-Sectional Study of Ocular Surface Discomfort and Corneal Nerve Dysfunction after Paclitaxel Treatment for Cancer. Sci. Rep. 2021, 11, 1786. [Google Scholar] [CrossRef] [PubMed]
- Tyler, E.F.; McGhee, C.N.J.; Lawrence, B.; Braatvedt, G.D.; Mankowski, J.L.; Oakley, J.D.; Sethi, S.; Misra, S.L. Corneal Nerve Changes Observed by In Vivo Confocal Microscopy in Patients Receiving Oxaliplatin for Colorectal Cancer: The COCO Study. J. Clin. Med. 2022, 11, 4770. [Google Scholar] [CrossRef] [PubMed]
- Riva, N.; Bonelli, F.; Lasagni Vitar, R.M.; Barbariga, M.; Fonteyne, P.; Lopez, I.D.; Domi, T.; Scarpa, F.; Ruggeri, A.; Reni, M.; et al. Corneal and Epidermal Nerve Quantification in Chemotherapy Induced Peripheral Neuropathy. Front. Med. 2022, 9, 832344. [Google Scholar] [CrossRef]
- Ferrari, G.; Gemignani, F.; Macaluso, C. Chemotherapy-Associated Peripheral Sensory Neuropathy Assessed Using in Vivo Corneal Confocal Microscopy. Arch. Neurol. 2010, 67, 364–365. [Google Scholar] [CrossRef]
- Cocito, F.; Ricciardelli, G.; Mangiacavalli, S.; Pompa, A.; Pochintesta, L.; Ferretti, V.; Ceccuzzi, R.; Cazzola, M.; Bianchi, P.E.; Corso, A. Corneal Sub-Basal Neural Damage Pattern in Multiple Myeloma Patients Treated with Bortezomib: An in Vivo Confocal Study. Leuk. Lymphoma 2015, 56, 3440–3441. [Google Scholar] [CrossRef]
- Parrozzani, R.; Lombardi, G.; Midena, E.; Londei, D.; Padovan, M.; Marchione, G.; Caccese, M.; Midena, G.; Zagonel, V.; Frizziero, L. Ocular Side Effects of EGFR-Inhibitor ABT-414 in Recurrent Glioblastoma: A Long-Term Safety Study. Front. Oncol. 2020, 10, 593461. [Google Scholar] [CrossRef]
- Park, S.B.; Lin, C.S.Y.; Krishnan, A.V.; Friedlander, M.L.; Lewis, C.R.; Kiernan, M.C. Early, Progressive, and Sustained Dysfunction of Sensory Axons Underlies Paclitaxel-Induced Neuropathy. Muscle Nerve 2011, 43, 367–374. [Google Scholar] [CrossRef]
- Briani, C.; Argyriou, A.A.; Izquierdo, C.; Velasco, R.; Campagnolo, M.; Alberti, P.; Frigeni, B.; Cacciavillani, M.; Bergamo, F.; Cortinovis, D.; et al. Long-Term Course of Oxaliplatin-Induced Polyneuropathy: A Prospective 2-Year Follow-up Study. J. Peripher. Nerv. Syst. 2014, 19, 299–306. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.A.; Wilson, L. Microtubules as a Target for Anticancer Drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Tavakoli, A.; Trinh, T.; Klisser, J.; Lewis, C.R.; Friedlander, M.; Naduvilath, T.J.; Au, K.; Park, S.B.; et al. Corneal Dendritic Cells and the Subbasal Nerve Plexus Following Neurotoxic Treatment with Oxaliplatin or Paclitaxel. Sci. Rep. 2021, 11, 22884. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.C.B.; Goldstein, D.; Trinh, T.; Au, K.; Park, S.B.; Krishnan, A.V.; Markoulli, M. Tear Film Substance P in Patients Treated with Neurotoxic Chemotherapy. Exp. Eye Res. 2022, 224, 109253. [Google Scholar] [CrossRef]
- Sterenczak, K.A.; Stache, N.; Bohn, S.; Allgeier, S.; Köhler, B.; Bartschat, A.; George, C.; Guthoff, R.F.; Stachs, O.; Stachs, A. Burst of Corneal Dendritic Cells during Trastuzumab and Paclitaxel Treatment. Diagnostics 2021, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Bohn, S.; Stache, N.; Sperlich, K.; Allgeier, S.; Köhler, B.; Bartschat, A.; Do, H.V.; George, C.; Guthoff, R.F.; Stachs, A.; et al. In Vivo Monitoring of Corneal Dendritic Cells in the Subbasal Nerve Plexus during Trastuzumab and Paclitaxel Breast Cancer Therapy-A One-Year Follow-Up. Diagnostics 2022, 12, 1180. [Google Scholar] [CrossRef]
- Saif, M.W.; Wood, T.E.; McGee, P.J.; Diasio, R.B. Peripheral Neuropathy Associated with Capecitabine. Anticancer Drugs 2004, 15, 767–771. [Google Scholar] [CrossRef]
- Saint-Jean, A.; Reguart, N.; Eixarch, A.; Adán, A.; Castellà, C.; Sánchez-Dalmau, B.; Sainz-de-la-Maza, M. Ocular Surface Adverse Events of Systemic Epidermal Growth Factor Receptor Inhibitors (EGFRi): A Prospective Trial. J. Fr. Ophtalmol. 2018, 41, 955–962. [Google Scholar] [CrossRef]
- Parrozzani, R.; Lombardi, G.; Midena, E.; Leonardi, F.; Londei, D.; Padovan, M.; Caccese, M.; Marchione, G.; Bini, S.; Zagonel, V.; et al. Corneal Side Effects Induced by EGFR-Inhibitor Antibody–Drug Conjugate ABT-414 in Patients with Recurrent Glioblastoma: A Prospective Clinical and Confocal Microscopy Study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920907543. [Google Scholar] [CrossRef]
- Tarafdar, S.; Lim, L.T.; Collins, C.E.; Ramaesh, K. Tamoxifen Keratopathy as Seen with In-Vivo Confocal Microscopy. Semin. Ophthalmol. 2012, 27, 27–28. [Google Scholar] [CrossRef]
- Kreps, E.O.; Derveaux, T.; Denys, H. Corneal Changes in Trastuzumab Emtansine Treatment. Clin. Breast Cancer 2018, 18, e427–e429. [Google Scholar] [CrossRef] [PubMed]
- Deklerck, E.; Denys, H.; Kreps, E.O. Corneal Features in Trastuzumab Emtansine Treatment: Not a Rare Occurrence. Breast Cancer Res. Treat. 2019, 175, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Arriola-Villalobos, P.; Benito-Pascual, B.; Díaz-Valle, D.; Benítez-Del-Castillo, J.M. Confocal Microscopy Observation of Cornea Verticillata After Vandetanib Therapy for Medullary Thyroid Carcinoma. Cornea 2018, 37, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Farooq, A.V.; Degli, S.; Rakesh, E.; Praneetha, P.; Sagar, T. Corneal Epithelial Findings in Patients with Multiple Myeloma Treated with Antibody–Drug Conjugate Belantamab Mafodotin in the Pivotal, Randomized, DREAMM-2 Study. Ophthalmol. Ther. 2020, 9, 889–911. [Google Scholar] [CrossRef]
- Marquant, K.; Quinquenel, A.; Arndt, C.; Denoyer, A. Corneal in Vivo Confocal Microscopy to Detect Belantamab Mafodotin-Induced Ocular Toxicity Early and Adjust the Dose Accordingly: A Case Report. J. Hematol. Oncol. 2021, 14, 159. [Google Scholar] [CrossRef]
- Mencucci, R.; Cennamo, M.; Alonzo, L.; Senni, C.; Vagge, A.; Desideri, L.F.; Scorcia, V.; Giannaccare, G. Corneal Findings Associated to Belantamab-Mafodotin (Belamaf) Use in a Series of Patients Examined Longitudinally by Means of Advanced Corneal Imaging. J. Clin. Med. 2022, 11, 2884. [Google Scholar] [CrossRef]
- Di Staso, F.; Gattazzo, I.; Salimbeni, B.T.; Lambiase, A.; Scuderi, G.; Di Staso, S.; Ciancaglini, M. Treatment of Capecitabine Corneal Side Effects with Autologous Blood-Derived Serum Eye Drops. In Vivo 2021, 35, 1881–1884. [Google Scholar] [CrossRef]
- Özcan, G.; Uçakhan, Ö.Ö. Cytarabine-Induced Corneal Toxicity: Clinical Features and Relief of Symptoms with Loteprednol Etabonate 0.5% in Two Patients. Turk. J. Ophthalmol. 2021, 51, 114–117. [Google Scholar] [CrossRef] [PubMed]
- Therssen, R.; Jansen, E.; Leys, A.; Rutten, J.; Meyskens, J. Screening for Tamoxifen Ocular Toxicity: A Prospective Study. Eur. J. Ophthalmol. 1995, 5, 230–234. [Google Scholar] [CrossRef]
- Eaton, J.S.; Miller, P.E.; Mannis, M.J.; Murphy, C.J. Ocular Adverse Events Associated with Antibody-Drug Conjugates in Human Clinical Trials. J. Ocul. Pharmacol. Ther. 2015, 31, 589–604. [Google Scholar] [CrossRef]
- Liu, Z.; Carvajal, M.; Carothers Carraway, C.A.; Carraway, K.; Pflugfelder, S.C. Expression of the Receptor Tyrosine Kinases, Epidermal Growth Factor Receptor, ErbB2, and ErbB3, in Human Ocular Surface Epithelia. Cornea 2001, 20, 81–85. [Google Scholar] [CrossRef]
- Nakamura, Y.; Sotozono, C.; Kinoshita, S. The Epidermal Growth Factor Receptor (EGFR): Role in Corneal Wound Healing and Homeostasis. Exp. Eye Res. 2001, 72, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Lonial, S.; Lee, H.C.; Badros, A.; Trudel, S.; Nooka, A.K.; Chari, A.; Abdallah, A.O.; Callander, N.; Lendvai, N.; Sborov, D.; et al. Belantamab Mafodotin for Relapsed or Refractory Multiple Myeloma (DREAMM-2): A Two-Arm, Randomised, Open-Label, Phase 2 Study. Lancet Oncol. 2020, 21, 207–221. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. Quantitative Analysis of Invivo Confocal Microscopy Images: A Review. Surv. Ophthalmol. 2013, 58, 466–475. [Google Scholar] [CrossRef]
- Chen, X.; Graham, J.; Dabbah, M.A.; Petropoulos, I.N.; Tavakoli, M.; Malik, R.A. An Automatic Tool for Quantification of Nerve Fibres in Corneal Confocal Microscopy Images. IEEE Trans. Biomed. Eng. 2017, 64, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.M.; Borroni, D.; Liu, R.; Zhao, Y.; Zhang, J.; Lim, J.; Ma, B.; Romano, V.; Qi, H.; Ferdousi, M.; et al. An Artificial Intelligence-Based Deep Learning Algorithm for the Diagnosis of Diabetic Neuropathy Using Corneal Confocal Microscopy: A Development and Validation Study. Diabetologia 2020, 63, 419–430. [Google Scholar] [CrossRef] [PubMed]
- Mehrgardt, P.; Zandavi, S.M.; Poon, S.K.; Kim, J.; Markoulli, M.; Khushi, M. U-Net Segmented Adjacent Angle Detection (USAAD) for Automatic Analysis of Corneal Nerve Structures. Data 2020, 5, 37. [Google Scholar] [CrossRef]
- Wei, S.; Shi, F.; Wang, Y.; Chou, Y.; Li, X. A Deep Learning Model for Automated Sub-Basal Corneal Nerve Segmentation and Evaluation Using In Vivo Confocal Microscopy. Transl. Vis. Sci. Technol. 2020, 9, 32. [Google Scholar] [CrossRef]
- Scarpa, F.; Colonna, A.; Ruggeri, A. Multiple-Image Deep Learning Analysis for Neuropathy Detection in Corneal Nerve Images. Cornea 2020, 39, 342–347. [Google Scholar] [CrossRef]
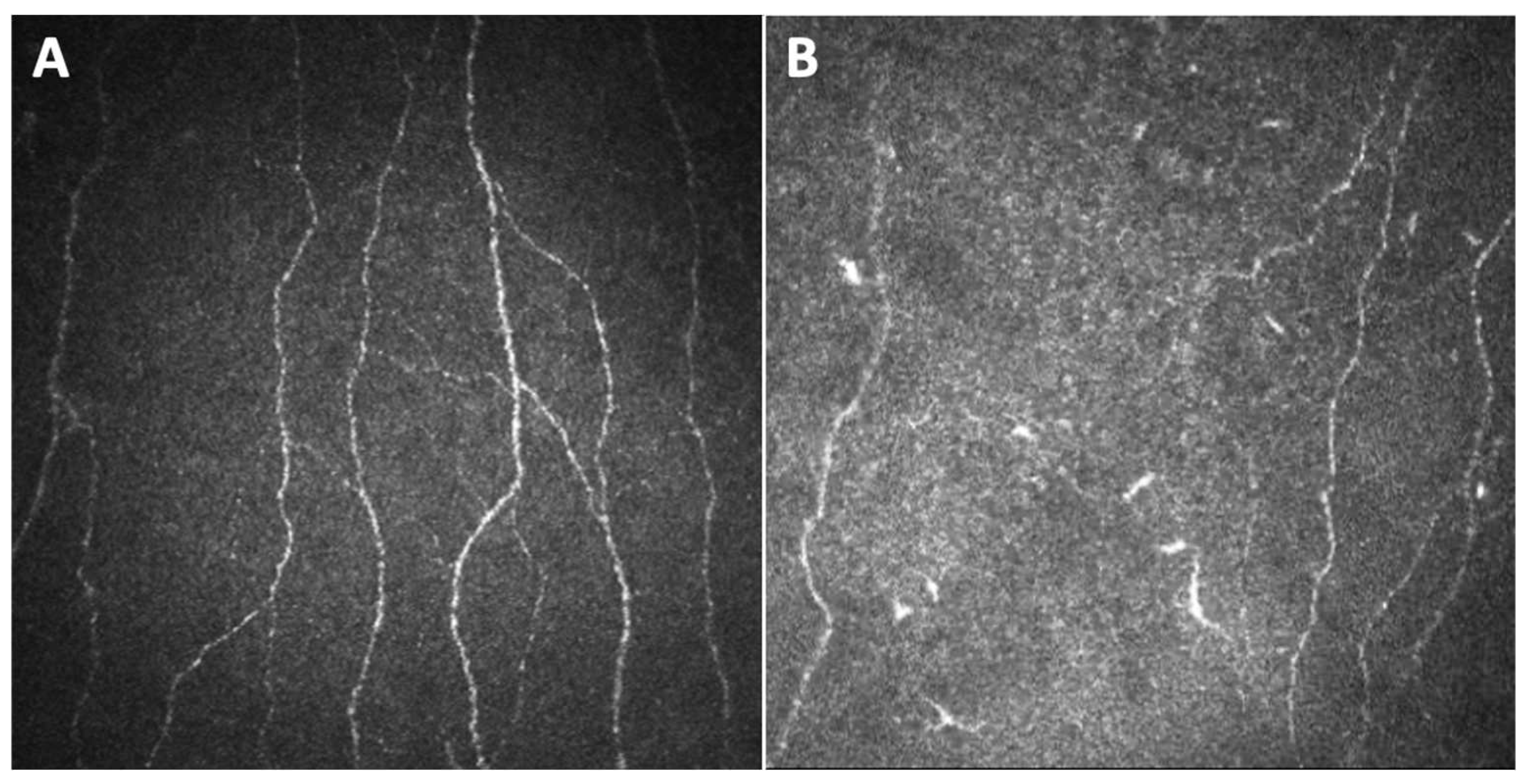

| Study | Chemotherapy Agent | Design, no. of Patients | Nerve Parameters | Significant Results | Methods | Clinical Relevance |
|---|---|---|---|---|---|---|
| Campagnolo et al., 2013 [19] | Oxaliplatin | Longitudinal, 15 | Length density | → reduction | n.s. | Prediction of coasting effect |
| Number of fibers | → reduction | |||||
| Number of beading | / | |||||
| Tortuosity | / | |||||
| Ferdousi et al., 2015 [20] | Oxaliplatin, cisplatin | Cross-sectional, 21 Longitudinal, 13 | CNFD | → reduction vs. controls | Analysis of 6 images (3 from each eye) with CCMetrics * | Aid in diagnosis of CIPN |
| CNBD | → reduction vs. controls | |||||
| CNFL | → reduction vs. controls, increase in follow-up | |||||
| Bennedsgaard et al., 2020 [21] | Oxaliplatin, docetaxel | Cross-sectional, 63 | CNFL | / | Fiber counted automatically | / |
| CNFD | / | |||||
| CNBD | / | |||||
| Chiang et al., 2021 [22] | Oxaliplatin, paclitaxel | Cross-sectional, 70 | CNFL | → reduction | Analysis of 8 images from the central cornea and 3 to 5 images from the inferior whorl region with ACCMetrics # | Aid in the monitoring of nerve function in patients undergoing chemotherapy |
| IWL | → reduction | |||||
| ANFL | → reduction | |||||
| CNFD | → reduction | |||||
| CNFA | → reduction | |||||
| CNBD | / | |||||
| Chiang et al., 2021 [23] | Paclitaxel | Cross-sectional, 29 | CNFL | → reduction | Analysis of 8 images from the central cornea and 5 images from the inferior whorl region with ACCMetrics # | / |
| IWL | → reduction | |||||
| CNFD | → reduction | |||||
| CNBD | / | |||||
| Tyler et al., 2022 [24] | Oxaliplatin | Cross-sectional, 23 | Corneal nerve density | → correlation with clinical peripheral neuropathy | Semi-automated analysis with software ImageJ—NeuronJ §; Automated analysis with customized deep learning-based approach deepNerve | Aid in diagnosis of CIPN |
| Corneal nerve lenght | / | |||||
| Riva et al., 2022 [25] | Platinum compounds, paclitaxel, bortezomib-thalidomide-dexamethasone, cyclophosphamide-combined treatments | Longitudinal, 73 | CNFL | / | Deep learning technique Convolutional Neural Network | Aid in diagnosis of CIPN |
| Tortuosity | / | |||||
| CNFL/tortuosity | → reduction | |||||
| Ferrari et al., 2010 [26] | Capecitabine | Case report, 1 | Beading, tortuosity and sprouting | → increase vs. age-matched control | n.s. | Aid in diagnosis and follow-up of CIPN |
| Cocito et al., 2015 [27] | Bortezomib | Cross-sectional, 26 | Nerve fiber length | → reduction | n.s | Prediction for clinically significant peripheral neuropathy |
| Nerve fiber number | → reduction | |||||
| Beadings number | → reduction | |||||
| Nerve fiber tortuosity | → increase | |||||
| Parrozzani et al., 2020 [28] | EGFRi (depatuxizumab mafodotin) | Longitudinal, 15 | Fragmentation/disappearance of fibers | → present in all patients | n.s. | Ocular side effects due to ABT-414 can be manageable |
| Chemotherapy Agent | Study | CCM Findings | Corneal Layer Affected | Clinical Relevance |
|---|---|---|---|---|
| Tamoxifen | Tarafdar et al., 2012 [40] | Multiple tiny crystalline deposits | Stroma | Aid in monitoring tamoxifen crystalline keratopathy |
| Trastuzumab emtansine | Kreps et al., 2018 [41] | Multiple hyperreflective lesions associated with pleiomorphic cells | Basal epithelium | / |
| Deklerck et al., 2019 [42] | Coarse cystoid lesions | Deep epithelium | Indication for adjusting systemic treatment | |
| EGFRi | ||||
| Vandetanib | Arriola-Villalobos et al., 2018 [43] | Hyperreflective deposits | Epithelium and subepithelial nerve plexus | / |
| Bright microdots | Stroma | |||
| Depatuxizumab mafodotin | Parrozzani et al., 2020 [28] | Multiple and diffuse hyperreflective white round spots and round cystic structures | Basal epithelium | Indication for adjusting systemic treatment |
| Belantamab mafodotin | Farooq et al., 2020 (DREAMM-2 Study) [44] | Hyperreflective opacities | Epithelium (mainly basal) | Indication for adjusting systemic treatment |
| Marquant et al., 2021 [45] | Clusters of hyperreflective material and small degenerative intraepithelial microcysts, mainly consisting of a hyper-reflective wall | Basal epithelium and sub-basal nerve plexus | ||
| Mencucci et al., 2022 [46] | Hyperreflective opacities | Epithelium | ||
| Capecitabine and lapatinib | Di Staso et al., 2021 [47] | Irregular cellular population and a mosaic pattern consisting mostly of hypo-reflective cells and cystic changes | Epithelium | Indication on adequate therapy for ocular side effects of capecitabine |
| Cytarabine | Özcan et al., 2021 [48] | Highly reflective disseminated granular and irregular opacities | Basal epithelium | Indication on adequate therapy for ocular side effects of cytarabine |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosmo, E.; Midena, G.; Parrozzani, R.; Midena, E. Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review. Diagnostics 2024, 14, 2399. https://doi.org/10.3390/diagnostics14212399
Cosmo E, Midena G, Parrozzani R, Midena E. Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review. Diagnostics. 2024; 14(21):2399. https://doi.org/10.3390/diagnostics14212399
Chicago/Turabian StyleCosmo, Eleonora, Giulia Midena, Raffaele Parrozzani, and Edoardo Midena. 2024. "Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review" Diagnostics 14, no. 21: 2399. https://doi.org/10.3390/diagnostics14212399
APA StyleCosmo, E., Midena, G., Parrozzani, R., & Midena, E. (2024). Chemotherapy Induced Corneal Changes Assessed by Corneal Confocal Microscopy: A Review. Diagnostics, 14(21), 2399. https://doi.org/10.3390/diagnostics14212399









