The Impact of Molecular and Genetic Analysis on the Treatment of Patients with Atypical Meningiomas
Abstract
1. Introduction
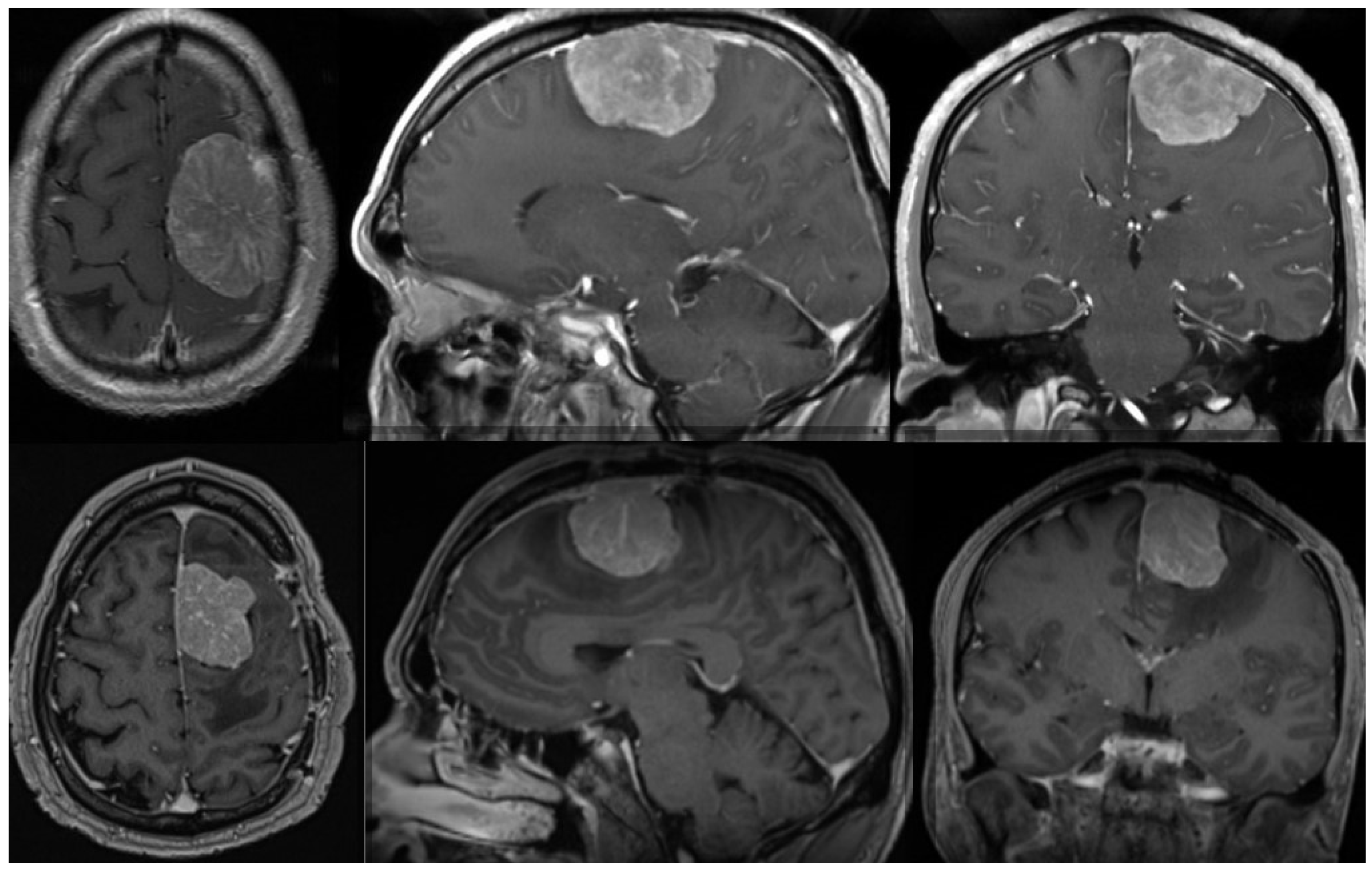
2. Histopathological Features
3. Cytogenetic Features
3.1. Copy Number Alterations
3.2. Molecular Models for Risk Assessment
3.3. Cytogenetic Features in Post-Radiation Meningiomas
4. Molecular Genetics and Classification of Meningiomas
4.1. The Role of NF2 Gene Mutations in Meningiomas
4.2. Epigenetic Modifications
4.3. Genomic Analysis-Based Meningioma Division
4.4. Integrated Molecular–Morphological Grading System of Meningiomas
5. Management
6. Clinical Application of Cytogenetic Features of Atypical Meningiomas
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Price, M.; Neff, C.; Cioffi, G.; Waite, K.A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2015–2019. Neuro-Oncol. 2022, 24 (Suppl. S5), v1–v95. [Google Scholar]
- Zouaoui, S.; Darlix, A.; Rigau, V.; Mathieu-Daudé, H.; Bauchet, F.; Bessaoud, F.; Fabbro-Peray, P.; Trétarre, B.; Figarella-Branger, D.; Taillandier, L.; et al. Descriptive epidemiology of 13,038 newly diagnosed and histologically confirmed meningiomas in France: 2006–2010. Neurochirurgie 2018, 64, 15–21. [Google Scholar] [CrossRef]
- James, Z.; Makwana, M.; Hayhurst, C. De Novo Skull Base Atypical Meningioma: Incidence and Outcome. J. Neurol. Surg. Part B Skull Base. 2023, 84, 113–118. [Google Scholar] [CrossRef]
- Nobee, A.; Xu, M.; Seth, A.; Rong, Y. Atypical Intraparenchymal Meningioma with YAP1-MAML2 Fusion in a Young Adult Male: A Case Report and Mini Literature Review. Int. J. Mol. Sci. 2023, 24, 12814. [Google Scholar] [CrossRef]
- Shibuya, M. Pathology and Molecular Genetics of Meningioma: Recent Advances. Neurol. Med. Chir. 2015, 55, 14–27. [Google Scholar] [CrossRef]
- Yamashima, T.; Sakuda, K.; Tohma, Y.; Yamashita, J.; Oda, H.; Irikura, D.; Eguchi, N.; Beuckmann, C.T.; Kanaoka, Y.; Urade, Y.; et al. Prostaglandin D Synthase (β-Trace) in Human Arachnoid and Meningioma Cells: Roles as a Cell Marker or in Cerebrospinal Fluid Absorption, Tumorigenesis, and Calcification Process. J. Neurosci. 1997, 17, 2376–2382. [Google Scholar] [CrossRef]
- Yasuda, K.; Cline, C.; Vogel, P.; Onciu, M.; Fatima, S.; Sorrentino, B.P.; Thirumaran, R.K.; Ekins, S.; Urade, Y.; Fujimori, K.; et al. Drug Transporters on Arachnoid Barrier Cells Contribute to the Blood–Cerebrospinal Fluid Barrier. Drug Metab. Dispos. 2013, 41, 923–931. [Google Scholar] [CrossRef]
- Marastoni, E.; Barresi, V. Atypical meningioma: Histopathological, genetic, and epigenetic features to predict recurrence risk. Histol. Histopathol. 2024, 39, 293–302. [Google Scholar]
- Marastoni, E.; Barresi, V. Meningioma Grading beyond Histopathology: Relevance of Epigenetic and Genetic Features to Predict Clinical Outcome. Cancers 2023, 15, 2945. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D. The recurrence of intracranial meningiomas after surgical treatment. J. Neurol. Neurosurg. Psychiatry 1957, 20, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Louis, D.N.; Perry, A.; Reifenberger, G.; Von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed]
- Van Den Bent, M.J. Interobserver variation of the histopathological diagnosis in clinical trials on glioma: A clinician’s perspective. Acta Neuropathol. 2010, 120, 297–304. [Google Scholar] [CrossRef] [PubMed]
- Korte, B.; Mathios, D. Innovation in Non-Invasive Diagnosis and Disease Monitoring for Meningiomas. Int. J. Mol. Sci. 2024, 25, 4195. [Google Scholar] [CrossRef] [PubMed]
- Fioravanzo, A.; Caffo, M.; Di Bonaventura, R.; Gardiman, M.P.; Ghimenton, C.; Ius, T.; Maffeis, V.; Marti-ni, M.; Nicolato, A.; Pallini, R.; et al. A Risk Score Based on 5 Clinico-Pathological Variables Predicts Recurrence of Atypical Meningiomas. J. Neuropathol. Exp. Neurol. 2020, 79, 500–507. [Google Scholar] [CrossRef]
- Goldbrunner, R.; Stavrinou, P.; Jenkinson, M.D.; Sahm, F.; Mawrin, C.; Weber, D.C.; Preusser, M.; Minniti, G.; Lund-Johansen, M.; Lefranc, F.; et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-Oncology 2021, 23, 1821–1834. [Google Scholar] [CrossRef]
- Corona, A.M.; Di, L.; Shah, A.H.; Crespo, R.; Eichberg, D.G.; Lu, V.M.; Luther, E.M.; Komotar, R.J.; Ivan, M.E. Current experimental therapies for atypical and malignant meningiomas. J. Neuro-Oncol. 2021, 153, 203–210. [Google Scholar] [CrossRef]
- Marosi, C.; Hassler, M.; Roessler, K.; Reni, M.; Sant, M.; Mazza, E.; Vecht, C. Meningioma. Crit. Rev. Oncol. Hematol. 2008, 67, 153–171. [Google Scholar] [CrossRef]
- Zhu, H.; Bi, W.L.; Aizer, A.; Hua, L.; Tian, M.; Den, J.; Tang, H.; Chen, H.; Wang, Y.; Mao, Y.; et al. Efficacy of adjuvant radiotherapy for atypical and anaplastic meningioma. Cancer Med. 2019, 8, 13–20. [Google Scholar] [CrossRef]
- Durand, A.; Labrousse, F.; Jouvet, A.; Bauchet, L.; Kalamaridès, M.; Menei, P.; Deruty, R.; Moreau, J.J.; Fèvre-Montange, M.; Guyotat, J. WHO grade II and III meningiomas: A study of prognostic factors. J. Neuro-Oncol. 2009, 95, 367–375. [Google Scholar] [CrossRef]
- Apra, C.; Peyre, M.; Kalamarides, M. Current treatment options for meningioma. Expert. Rev. Neurother. 2018, 18, 241–249. [Google Scholar] [CrossRef]
- Magill, S.T.; Young, J.S.; Chae, R.; Aghi, M.K.; Theodosopoulos, P.V.; McDermott, M.W. Relationship between tumor location, size, and WHO grade in meningioma. Neurosurg. Focus 2018, 44, E4. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Ning, B.; Hua, X.; Liang, Z.; Ye, J.; Yu, F.; Xu, Z.; Chen, J. Atypical meningioma: A retrospective analysis of six cases and literature review. Transl. Cancer Res. 2021, 10, 1509–1518. [Google Scholar] [CrossRef] [PubMed]
- Surov, A.; Gottschling, S.; Mawrin, C.; Prell, J.; Spielmann, R.P.; Wienke, A.; Fiedler, E. Diffusion-Weighted Imaging in Meningioma: Prediction of Tumor Grade and Association with Histopathological Parameters. Transl. Oncol. 2015, 8, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Nagar, V.A.; Ye, J.R.; Ng, W.H.; Chan, Y.H.; Hui, F.; Lee, C.K.; Lim, C.C.T. Diffusion-Weighted MR Imaging: Diagnosing Atypical or Malignant Meningiomas and Detecting Tumor Dedifferentiation. Am. J. Neuroradiol. 2008, 29, 1147–1152. [Google Scholar] [CrossRef] [PubMed]
- Yarabarla, V.; Mylarapu, A.; Han, T.J.; McGovern, S.L.; Raza, S.M.; Beckham, T.H. Intracranial meningiomas: An update of the 2021 World Health Organization classifications and review of management with a focus on radiation therapy. Front. Oncol. 2023, 13, 1137849. [Google Scholar] [CrossRef] [PubMed]
- Paldor, I.; Awad, M.; Sufaro, Y.Z. Review of controversies in management of non-benign meningioma. J. Clin. Neurosci. 2016, 31, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, F.; Mariniello, G.; Guadagno, E.; Barbato, M.; Corvino, S.; Del Basso De Caro, M. WHO grade, proliferation index, and progesterone receptor expression are different according to the location of meningioma. Acta Neurochir. 2019, 161, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Meling, T.R.; Da Broi, M.; Scheie, D.; Helseth, E. Meningiomas: Skull base versus non-skull base. Neurosurg. Rev. 2019, 42, 163–173. [Google Scholar] [CrossRef]
- Wang, Y.C.; Chuang, C.C.; Wei, K.C.; Hsu, Y.H.; Hsu, P.W.; Lee, S.T.; Wu, C.-T.; Tseng, C.-K.; Wang, C.-C.; Chen, Y.-L.; et al. Skull base atypical meningioma: Long term surgical outcome and prognostic factors. Clin. Neurol. Neurosurg. 2015, 128, 112–116. [Google Scholar] [CrossRef]
- Buttrick, S.; Shah, A.H.; Komotar, R.J.; Ivan, M.E. Management of Atypical and Anaplastic Meningiomas. Neurosurg. Clin. N. Am. 2016, 27, 239–247. [Google Scholar] [CrossRef]
- Aizer, A.A.; Bi, W.L.; Kandola, M.S.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; Beroukhim, R.; Reardon, D.A.; Wen, P.Y.; et al. Extent of resection and overall survival for patients with atypical and malignant meningioma. Cancer 2015, 121, 4376–4381. [Google Scholar] [CrossRef] [PubMed]
- Aizer, A.A.; Arvold, N.D.; Catalano, P.; Claus, E.B.; Golby, A.J.; Johnson, M.D.; Al-Mefty, O.; Wen, P.Y.; Reardon, D.A.; Lee, E.Q.; et al. Adjuvant radiation therapy, local recurrence, and the need for salvage therapy in atypical meningioma. Neuro-Oncology 2014, 16, 1547–1553. [Google Scholar] [CrossRef] [PubMed]
- Pisćević, I.; Villa, A.; Milićević, M.; Ilić, R.; Nikitović, M.; Cavallo, L.M.; Grujičić, D. The Influence of Adjuvant Radiotherapy in Atypical and Anaplastic Meningiomas: A Series of 88 Patients in a Single Institution. World Neurosurg. 2015, 83, 987–995. [Google Scholar] [CrossRef]
- Pizem, J.; Velnar, T.; Prestor, B.; Mlakar, J.; Popovic, M. Brain invasion assessability in meningiomas is related to meningioma size and grade, and can be improved by extensive sampling of the surgically removed meningioma specimen. Clin. Neuropathol. 2014, 33, 354–363. [Google Scholar]
- Baumgarten, P.; Gessler, F.; Schittenhelm, J.; Skardelly, M.; Tews, D.S.; Senft, C.; Dunst, M.; Imoehl, L.; Plate, K.H.; Wagner, M.; et al. Brain invasion in otherwise benign meningiomas does not predict tumor recurrence. Acta Neuropathol. 2016, 132, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Biczok, A.; Jungk, C.; Egensperger, R.; Von Deimling, A.; Suchorska, B.; Tonn, J.C.; Herold-Mende, C.; Schichor, C. Microscopic brain invasion in meningiomas previously classified as WHO grade I is not associated with patient outcome. J. Neuro-Oncol. 2019, 145, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Chun, S.W.; Dho, Y.S.; Seo, Y.; Lee, J.H.; Won, J.K.; Kim, J.W.; Park, C.-K.; Park, S.-H.; Kim, Y.H. Histopathological predictors of progression-free survival in atypical meningioma: A single-center retrospective cohort and meta-analysis. Brain Tumor Pathol. 2022, 39, 99–110. [Google Scholar] [CrossRef]
- Kwon, S.M.; Kim, J.H.; Kim, Y.H.; Hong, S.H.; Cho, Y.H.; Kim, C.J.; Nam, S.J. Clinical Implications of the Mitotic Index as a Predictive Factor for Malignant Transformation of Atypical Meningiomas. J. Korean Neurosurg. Soc. 2022, 65, 297–306. [Google Scholar] [CrossRef]
- Devaprasath, A.; Chacko, G. Diagnostic validity of the Ki-67 labeling index using the MIB-1 monoclonal antibody in the grading of meningiomas. Neurol. India 2003, 51, 336–340. [Google Scholar]
- Barresi, V.; Ammendola, S.; Simbolo, M.; Pedron, S.; Caffo, M.; Scarpa, A. Atypical meningiomas with an immunohistochemical profile consistent with hypermetabolic or proliferative molecular groups show high mitotic index, chromosomal instability, and higher recurrence risk. Virchows Arch. 2023, 483, 97–104. [Google Scholar] [CrossRef]
- Bertero, L.; Dea, G.D.; Osella-Abate, S.; Botta, C.; Castellano, I.; Morra, I.; Pollo, B.; Calatozzolo, C.; Patri-arca, S.; Mantovani, C.; et al. Prognostic Characterization of Higher-Grade Meningiomas: A Histopathological Score to Predict Progression and Outcome. J. Neuropathol. Exp. Neurol. 2019, 78, 248–256. [Google Scholar] [PubMed]
- Rogers, L.; Barani, I.; Chamberlain, M.; Kaley, T.J.; McDermott, M.; Raizer, J.; Schiff, D.; Weber, D.C.; Wen, P.Y.; Vogelbaum, M.A. Meningiomas: Knowledge base, treatment outcomes, and uncertainties. A RANO review. J. Neurosurg. 2015, 122, 4–23. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Jung, S.; Moon, K.S.; Pei, J.; Lee, K.H.; Jin, S.G.; Li, S.Y.; Ryu, H.H. Immunohistochemical profile of the dural tail in intracranial meningiomas. Acta Neurochir. 2014, 156, 2263–2273. [Google Scholar] [CrossRef] [PubMed]
- Tollefsen, S.; Jarmund, A.; Ytterhus, B.; Salvesen, Ø.; Mjønes, P.; Torp, S. Somatostatin Receptors in Human Meningiomas—Clinicopathological Aspects. Cancers 2021, 13, 5704. [Google Scholar] [CrossRef]
- Durand, A.; Champier, J.; Jouvet, A.; Labrousse, F.; Honnorat, J.; Guyotat, J.; Fèvre-Montange, M. Expression of c-Myc, neurofibromatosis Type 2, somatostatin receptor 2 and erb-B2 in human meningiomas: Relation to grades or histotypes. Clin. Neuropathol. 2008, 27, 334–345. [Google Scholar] [CrossRef] [PubMed]
- Fodi, C.; Skardelly, M.; Hempel, J.M.; Hoffmann, E.; Castaneda, S.; Tabatabai, G.; Honegger, J.; Tatagiba, M.; Schittenhelm, J.; Behling, F. The immunohistochemical expression of SSTR2A is an independent prognostic factor in meningioma. Neurosurg. Rev. 2022, 45, 2671–2679. [Google Scholar] [CrossRef]
- Mnango, L.; Mwakimonga, A.; Ngaiza, A.I.; Yahaya, J.J.; Vuhahula, E.; Mwakigonja, A.R. Expression of Progesterone Receptor and Its Association with Clinicopathological Characteristics in Meningiomas: A Cross-Sectional Study. World Neurosurg. X. 2021, 12, 100111. [Google Scholar] [CrossRef]
- Marcos, D.S.; Paiva Neto, M.A.; Góes, P.; Oshima, C.T.F.; Silva, M.S.; Stávale, J.N. Grade I meningiomas with atypical characteristics: A worse prognosis. Arq. Neuropsiquiatr. 2018, 76, 756–759. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.G.; Boström, J.; Wolter, M.; Baudis, M.; Collins, V.P.; Reifenberger, G.; Lichter, P. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: Toward a genetic model of meningioma progression. Proc. Natl. Acad. Sci. USA 1997, 94, 14719–14724. [Google Scholar] [CrossRef]
- Zang, K.D. Meningioma: A cytogenetic model of a complex benign human tumor, including data on 394 karyotyped cases. Cytogenet. Cell Genet. 2001, 93, 207–220. [Google Scholar] [CrossRef]
- Williams, E.A.; Santagata, S.; Wakimoto, H.; Shankar, G.M.; Barker, F.G.; Sharaf, R.; Reddy, A.; Spear, P.; Alexander, B.M.; Ross, J.S.; et al. Distinct genomic subclasses of high-grade/progressive meningiomas: NF2-associated, NF2-exclusive, and NF2-agnostic. Acta Neuropathol. Commun. 2020, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Yuzawa, S.; Nishihara, H.; Tanaka, S. Genetic landscape of meningioma. Brain Tumor Pathol. 2016, 33, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Clark, V.E.; Erson-Omay, E.Z.; Serin, A.; Yin, J.; Cotney, J.; Özduman, K.; Avşar, T.; Li, J.; Murray, P.B.; Henegariu, O.; et al. Genomic Analysis of Non-NF2 Meningiomas Reveals Mutations in TRAF7, KLF4, AKT1, and SMO. Science 2013, 339, 1077–1080. [Google Scholar] [CrossRef] [PubMed]
- Choy, W.; Kim, W.; Nagasawa, D.; Stramotas, S.; Yew, A.; Gopen, Q.; Parsa, A.T.; Yang, I. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg. Focus 2011, 30, E6. [Google Scholar] [CrossRef] [PubMed]
- Hansson, C.M.; Buckley, P.G.; Grigelioniene, G.; Piotrowski, A.; Hellström, A.R.; Mantripragada, K.; Jarbo, C.; Mathiesen, T.; Dumanski, J.P. Comprehensive genetic and epigenetic analysis of sporadic meningioma for macro-mutations on 22q and micro-mutations within the NF2 locus. BMC Genom. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Greenwald, N.F.; Abedalthagafi, M.; Wala, J.; Gibson, W.J.; Agarwalla, P.K.; Horowitz, P.; Schu-macher, S.E.; Esaulova, E.; Mei, Y.; et al. Genomic landscape of high-grade meningiomas. Npj Genom. Med. 2017, 2, 15. [Google Scholar] [CrossRef]
- Mawrin, C.; Perry, A. Pathological classification and molecular genetics of meningiomas. J. Neuro-Oncol. 2010, 99, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.X.; Banerjee, R.; Scheithauer, B.W.; Lohse, C.M.; Kleinschmidt-Demasters, B.K.; Perry, A. Chromosome 1p and 14q FISH Analysis in Clinicopathologic Subsets of Meningioma: Diagnostic and prognostic Implications. J. Neuropathol. Exp. Neurol. 2001, 60, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Lamszus, K. Meningioma pathology, genetics, and biology. J. Neuropathol. Exp. Neurol. 2004, 63, 275–286. [Google Scholar] [CrossRef]
- Al-Mefty, O.; Kadri, P.A.; Pravdenkova, S.; Sawyer, J.R.; Stangeby, C.; Husain, M. Malignant progression in meningioma: Documentation of a series and analysis of cytogenetic findings. J. Neurosurg. 2004, 101, 210–218. [Google Scholar] [CrossRef]
- Aizer, A.A.; Abedalthagafi, M.; Linda Bi, W.; Horvath, M.C.; Arvold, N.D.; Al-Mefty, O.; Lee, E.Q.; Nayak, L.; Rinne, M.L.; Norden, A.D.; et al. A prognostic cytogenetic scoring system to guide the adjuvant management of patients with atypical meningioma. Neuro-Oncology 2016, 18, 269–274. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Simbolo, M.; Fioravanzo, A.; Piredda, M.; Caffo, M.; Ghimenton, C.; Pinna, G.; Longhi, M.; Nicolato, A.; Scarpa, A. Molecular Profiling of 22 Primary Atypical Meningiomas Shows the Prognostic Significance of 18q Heterozygous Loss and CDKN2A/B Homozygous Deletion on Recurrence-Free Survival. Cancers 2021, 13, 903. [Google Scholar] [CrossRef] [PubMed]
- Maas, S.L.N.; Stichel, D.; Hielscher, T.; Sievers, P.; Berghoff, A.S.; Schrimpf, D.; Sill, M.; Euskirchen, P.; Blume, C.; Patel, A.; et al. Integrated Molecular-Morphologic Meningioma Classification: A Multicenter Retrospective Analysis, Retrospectively and Prospectively Validated. J. Clin. Oncol. 2021, 39, 3839–3852. [Google Scholar] [CrossRef] [PubMed]
- Pfisterer, W.K.; Hank, N.C.; Preul, M.C.; Hendricks, W.P.; Pueschel, J.; Coons, S.W.; Scheck, A.C. Diagnostic and prognostic significance of genetic regional heterogeneityin meningiomas. Neuro-Oncology 2004, 6, 290–299. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Pham, M.H.; Pease, M.; Zada, G.; Giannotta, S.L.; Wang, K.; Mack, W.J. A review of epigenetic and gene expression alterations associated with intracranial meningiomas. Neurosurg. Focus 2013, 35, E5. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, H.; Chen, R.; Yang, H.; Zou, Y.; Ke, C.; Chen, J.; Yu, J. Integration of molecular pathology with histopathology to accurately evaluate the biological behaviour of WHO grade 2 meningiomas and patient prognosis. J. Neuro-Oncol. 2022, 160, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Vaubel, R.A.; Kumar, R.; Weiskittel, T.M.; Jenkins, S.; Dasari, S.; Uhm, J.H.; Lachance, D.H.; Brown, P.D.; Van Gompel, J.J.; Jenkins, R.B.; et al. Genomic markers of recurrence risk in atypical meningioma following gross total resection. Neuro-Oncol. Adv. 2023, 5, vdad004. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.B.; English, C.W.; Chen, W.C.; Athukuri, P.; Bayley, J.C.; Brandt, V.L.; Shetty, A.; Hadley, C.C.; Choudhury, A.; Lu, H.-C.; et al. Even heterozygous loss of CDKN2A/B greatly accelerates recurrence in aggressive meningioma. Acta Neuropathol. 2023, 145, 501–503. [Google Scholar] [CrossRef] [PubMed]
- Neglia, J.P.; Robison, L.L.; Stovall, M.; Liu, Y.; Packer, R.J.; Hammond, S.; Yasui, Y.; Kasper, C.E.; Mertens, A.C.; Donaldson, S.S.; et al. New Primary Neoplasms of the Central Nervous System in Survivors of Childhood Cancer: A Report From the Childhood Cancer Survivor Study. JNCI J. Natl. Cancer Inst. 2006, 98, 1528–1537. [Google Scholar] [CrossRef]
- Broniscer, A.; Ke, W.; Fuller, C.E.; Wu, J.; Gajjar, A.; Kun, L.E. Second neoplasms in pediatric patients with primary central nervous system tumors: The St. Jude Children’s Research Hospital experience. Cancer 2004, 100, 2246–2252. [Google Scholar] [CrossRef]
- Shoshan, Y.; Chernova, O.; Jeun, S.S.; Somerville, R.P.; Israel, Z.; Barnett, G.H.; Cowell, J.K. Radiation-Induced Meningioma: A Distinct Molecular Genetic Pattern? J. Neuropathol. Exp. Neurol. 2000, 59, 614–620. [Google Scholar] [CrossRef]
- Yamanaka, R.; Hayano, A.; Kanayama, T. Radiation-Induced Meningiomas: An Exhaustive Review of the Literature. World Neurosurg. 2017, 97, 635–644.e8. [Google Scholar] [CrossRef]
- Flint-Richter, P.; Sadetzki, S. Genetic predisposition for the development of radiation-associated meningioma: An epidemiological study. Lancet Oncol. 2007, 8, 403–410. [Google Scholar] [CrossRef]
- Sahm, F.; Schrimpf, D.; Olar, A.; Koelsche, C.; Reuss, D.; Bissel, J.; Kratz, A.; Capper, D.; Schefzyk, S.; Hielscher, T.; et al. TERT Promoter Mutations and Risk of Recurrence in Meningioma. J. Natl. Cancer Inst. 2016, 108, djv377. [Google Scholar] [CrossRef] [PubMed]
- Sievers, P.; Hielscher, T.; Schrimpf, D.; Stichel, D.; Reuss, D.E.; Berghoff, A.S.; Neidert, M.C.; Wirsching, H.-G.; Mawrin, C.; Ketter, R.; et al. CDKN2A/B homozygous deletion is associated with early recurrence in meningiomas. Acta Neuropathol. 2020, 140, 409–413. [Google Scholar] [CrossRef]
- Krimpenfort, P.; Snoek, M.; Lambooij, J.P.; Song, J.Y.; Van Der Weide, R.; Bhaskaran, R.; Teunissen, H.; Adams, D.J.; De Wit, E.; Berns, A. A natural WNT signaling variant potently synergizes with Cdkn2ab loss in skin carcinogenesis. Nat. Commun. 2019, 10, 1425. [Google Scholar] [CrossRef]
- Guyot, A.; Duchesne, M.; Robert, S.; Lia, A.S.; Derouault, P.; Scaon, E.; Lemnos, L.; Salle, H.; Durand, K.; Labrousse, F. Analysis of CDKN2A gene alterations in recurrent and non-recurrent meningioma. J. Neuro-Oncol. 2019, 145, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Peyre, M.; Stemmer-Rachamimov, A.; Clermont-Taranchon, E.; Quentin, S.; El-Taraya, N.; Walczak, C.; Volk, A.; Niwa-Kawakita, M.; Karboul, N.; Giovannini, M.; et al. Meningioma progression in mice triggered by Nf2 and Cdkn2ab inactivation. Oncogene 2013, 32, 4264–4272. [Google Scholar] [CrossRef] [PubMed]
- Harmancı, A.S.; Youngblood, M.W.; Clark, V.E.; Coşkun, S.; Henegariu, O.; Duran, D.; Erson-Omay, E.Z.; Kaulen, L.D.; Lee, T.I.; Abraham, B.J.; et al. Integrated genomic analyses of de novo pathways underlying atypical meningiomas. Nat. Commun. 2017, 8, 14433. [Google Scholar] [CrossRef]
- Patel, A.J.; Wan, Y.W.; Al-Ouran, R.; Revelli, J.P.; Cardenas, M.F.; Oneissi, M. Molecular profiling predicts meningioma recurrence and reveals loss of DREAM complex repression in aggressive tumors. Proc. Natl. Acad. Sci. USA 2019, 116, 21715–21726. [Google Scholar] [CrossRef] [PubMed]
- Berghoff, A.S.; Hielscher, T.; Ricken, G.; Furtner, J.; Schrimpf, D.; Widhalm, G.; Rajky, U.; Marosi, C.; Hainfellner, J.A.; Von Deimling, A.; et al. Prognostic impact of genetic alterations and methylation classes in meningioma. Brain Pathol. 2022, 32, e12970. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Gómez, A.; Molnar, C.; Holguín, H.; Mayor, F.; De Celis, J.F. The cell biology of Smo signalling and its relationships with GPCRs. Biochim. Biophys. Acta BBA Biomembr. 2007, 1768, 901–912. [Google Scholar] [CrossRef] [PubMed]
- Boetto, J.; Bielle, F.; Sanson, M.; Peyre, M.; Kalamarides, M. SMO mutation status defines a distinct and frequent molecular subgroup in olfactory groove meningiomas. Neuro-Oncology 2017, 19, 345–351. [Google Scholar]
- Brastianos, P.K.; Horowitz, P.M.; Santagata, S.; Jones, R.T.; McKenna, A.; Getz, G.; Ligon, K.L.; Palescan-dolo, E.; Van Hummelen, P.; Ducar, M.D.; et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nat. Genet. 2013, 45, 285–289. [Google Scholar] [CrossRef]
- Karakas, B.; Bachman, K.E.; Park, B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer 2006, 94, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Abedalthagafi, M.; Bi, W.L.; Aizer, A.A.; Merrill, P.H.; Brewster, R.; Agarwalla, P.K.; Listewnik, M.L.; Di-as-Santagata, D.; Thorner, A.R.; Van Hummelen, P.; et al. Oncogenic PI3K mutations are as common as AKT1 and SMO mutations in meningioma. Neuro-Oncology 2016, 18, 649–655. [Google Scholar] [CrossRef]
- Laurendeau, I.; Ferrer, M.; Garrido, D.; D’Haene, N.; Ciavarelli, P.; Basso, A.; Vidaud, M.; Bieche, I.; Salmon, I.; Szijan, I. Gene Expression Profiling of the Hedgehog Signaling Pathway in Human Meningiomas. Mol. Med. 2010, 16, 262–270. [Google Scholar] [CrossRef]
- Goutagny, S.; Bah, A.B.; Henin, D.; Parfait, B.; Grayeli, A.B.; Sterkers, O.; Kalamarides, M. Long-term follow-up of 287 meningiomas in neurofibromatosis type 2 patients: Clinical, radiological, and molecular features. Neuro-Oncology 2012, 14, 1090–1096. [Google Scholar] [CrossRef]
- Bachir, S.; Shah, S.; Shapiro, S.; Koehler, A.; Mahammedi, A.; Samy, R.N.; Zuccarello, M.; Schorry, E.; Sengupta, S. Neurofibromatosis Type 2 (NF2) and the Implications for Vestibular Schwannoma and Meningioma Pathogenesis. Int. J. Mol. Sci. 2021, 22, 690. [Google Scholar] [CrossRef]
- Plotkin, S.R.; Messiaen, L.; Legius, E.; Pancza, P.; Avery, R.A.; Blakeley, J.O.; Babovic-Vuksanovic, D.; Ferner, R.; Fisher, M.J.; Friedman, J.M.; et al. Updated diagnostic criteria and nomenclature for neurofibromatosis type 2 and schwannomatosis: An international consensus recommendation. Genet. Med. 2022, 24, 1967–1977. [Google Scholar] [CrossRef]
- Halabi, R.; Dakroub, F.; Haider, M.Z.; Patel, S.; Amhaz, N.A.; Reslan, M.A.; Eid, A.H.; Mechref, Y.; Dar-wiche, N.; Kobeissy, F.; et al. Unveiling a Biomarker Signature of Meningioma: The Need for a Panel of Genomic, Epigenetic, Proteomic, and RNA Biomarkers to Advance Diagnosis and Prognosis. Cancers 2023, 15, 5339. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Lee, Y.S. Molecular characteristics of meningiomas. J. Pathol. Transl. Med. 2020, 54, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Sievers, P.; Chiang, J.; Schrimpf, D.; Stichel, D.; Paramasivam, N.; Sill, M.; Gayden, T.; Casalini, B.; Reuss, D.E.; Dalton, J.; et al. YAP1-fusions in pediatric NF2-wildtype meningioma. Acta Neuropathol. 2020, 139, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Guo, S.; Liu, D.; Chu, J.; Li, Y.; Wang, X.; Zhang, X.; Song, C.; Huang, Q. Pediatric meningioma with a Novel MAML2-YAP1 fusion variant: A case report and literature review. BMC Pediatr. 2022, 22, 694. [Google Scholar] [CrossRef]
- Esposito, S.; Marucci, G.; Antonelli, M.; Miele, E.; Modena, P.; Giagnacovo, M.; Massimino, M.; Biassoni, V.; Taddei, M.; Schiariti, M.; et al. Interhemispheric Pediatric Meningioma, YAP1 Fusion-Positive. Diagnostics 2022, 12, 2367. [Google Scholar] [CrossRef]
- Yagi, R.; Chen, L.F.; Shigesada, K.; Murakami, Y.; Ito, Y. A WW domain-containing Yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 1999, 18, 2551–2562. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Dev. Biol. 2021, 475, 205–221. [Google Scholar] [CrossRef]
- Huang, J.; Wu, S.; Barrera, J.; Matthews, K.; Pan, D. The Hippo Signaling Pathway Coordinately Regulates Cell Proliferation and Apoptosis by Inactivating Yorkie, the Drosophila Homolog of YAP. Cell 2005, 122, 421–434. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Arora, S.; Arakaki, A.K.S.; Sievers, P.; Almiron Bonnin, D.A.; Paddison, P.J.; Sahm, F.; Cimino, P.J.; Gujral, T.S.; Holland, E.C. Both YAP1-MAML2 and constitutively active YAP1 drive the formation of tumors that resemble NF2 mutant meningiomas in mice. Genes Dev. 2022, 36, 857–870. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Molecular insights into NF2/Merlin tumor suppressor function. FEBS Lett. 2014, 588, 2743–2752. [Google Scholar] [CrossRef]
- Loewenstern, J.; Rutland, J.; Gill, C.; Arib, H.; Pain, M.; Umphlett, M.; Kinoshita, Y.; McBride, R.; Do-novan, M.; Sebra, R.; et al. Comparative genomic analysis of driver mutations in matched primary and recurrent meningiomas. Oncotarget. 2019, 10, 3506–3517. [Google Scholar] [CrossRef]
- Pemov, A.; Dewan, R.; Hansen, N.F.; Chandrasekharappa, S.C.; Ray-Chaudhury, A.; Jones, K.; Luo, W.; Heiss, J.D.; Mullikin, J.C.; Chittiboina, P.; et al. Comparative clinical and genomic analysis of neurofibromatosis type 2-associated cranial and spinal meningiomas. Sci. Rep. 2020, 10, 12563. [Google Scholar] [CrossRef] [PubMed]
- Laraba, L.; Hillson, L.; De Guibert, J.G.; Hewitt, A.; Jaques, M.R.; Tang, T.T.; Post, L.; Ercolano, E.; Rai, G.; Yang, S.-M.; et al. Inhibition of YAP/TAZ-driven TEAD activity prevents growth of NF2-null schwannoma and meningioma. Brain 2023, 146, 1697–1713. [Google Scholar] [CrossRef]
- Szulzewsky, F.; Arora, S.; Hoellerbauer, P.; King, C.; Nathan, E.; Chan, M.; Cimino, P.J.; Ozawa, T.; Ka-wauchi, D.; Pajtler, K.W.; et al. Comparison of tumor-associated YAP1 fusions identifies a recurrent set of functions critical for oncogenesis. Genes Dev. 2020, 34, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Behling, F.; Fodi, C.; Gepfner-Tuma, I.; Kaltenbach, K.; Renovanz, M.; Paulsen, F.; Skardelly, M.; Honeg-ger, J.; Tatagiba, M.; International Consortium on Meningiomas; et al. H3K27me3 loss indicates an increased risk of recurrence in the Tübingen meningioma cohort. Neuro-Oncology 2021, 23, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Katz, L.M.; Hielscher, T.; Liechty, B.; Silverman, J.; Zagzag, D.; Sen, R.; Wu, P.; Golfinos, J.G.; Reuss, D.; Neidert, M.C.; et al. Loss of histone H3K27me3 identifies a subset of meningiomas with increased risk of recurrence. Acta Neuropathol. 2018, 135, 955–963. [Google Scholar] [CrossRef]
- Ammendola, S.; Rizzo, P.C.; Longhi, M.; Zivelonghi, E.; Pedron, S.; Pinna, G.; Sala, F.; Nicolato, A.; Scarpa, A.; Barresi, V. The Immunohistochemical Loss of H3K27me3 in Intracranial Meningiomas Predicts Shorter Progression-Free Survival after Stereotactic Radiosurgery. Cancers 2022, 14, 1718. [Google Scholar] [CrossRef]
- Hua, L.; Ren, L.; Wu, Q.; Deng, J.; Chen, J.; Cheng, H.; Wang, D.; Chen, H.; Xie, Q.; Wakimoto, H.; et al. Loss of H3K27me3 expression enriches in recurrent grade 1&2 meningiomas and maintains as a biomarker stratifying progression risk. J. Neuro-Oncol. 2023, 161, 267–275. [Google Scholar]
- Vasudevan, K.; Saad, H.; Oyesiku, M.N. The Role of Three-Dimensional Endoscopy in Pituitary Adenoma Surgery. Neurosurg. Clin. N. Am. 2019, 30, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Sahm, F.; Schrimpf, D.; Stichel, D.; Jones, D.T.W.; Hielscher, T.; Schefzyk, S.; Okonechnikov, K.; Koelsche, C.; Reuss, D.E.; Capper, D.; et al. DNA methylation-based classification and grading system for meningioma: A multicentre, retrospective analysis. Lancet Oncol. 2017, 18, 682–694. [Google Scholar] [CrossRef]
- Nassiri, F.; Liu, J.; Patil, V.; Mamatjan, Y.; Wang, J.Z.; Hugh-White, R.; Macklin, A.M.; Khan, S.; Singh, O.; Karimi, S.; et al. A clinically applicable integrative molecular classification of meningiomas. Nature 2021, 597, 119–125. [Google Scholar] [CrossRef]
- Olar, A.; Wani, K.M.; Wilson, C.D.; Zadeh, G.; DeMonte, F.; Jones, D.T.W.; Pfister, S.M.; Sulman, E.P.; Al-dape, K.D. Global epigenetic profiling identifies methylation subgroups associated with recurrence-free survival in meningioma. Acta Neuropathol. 2017, 133, 431–444. [Google Scholar] [CrossRef]
- Hielscher, T.; Sill, M.; Sievers, P.; Stichel, D.; Brandner, S.; Jones, D.T.W.; Von Deimling, A.; Sahm, F.; Maas, S.L.N. Clinical implementation of integrated molecular-morphologic risk prediction for meningioma. Brain Pathol. 2023, 33, e13132. [Google Scholar] [CrossRef]
- Driver, J.; Hoffman, S.E.; Tavakol, S.; Woodward, E.; Maury, E.A.; Bhave, V.; Greenwald, N.F.; Nassiri, F.; Aldape, K.; Zadeh, G.; et al. A molecularly integrated grade for meningioma. Neuro-Oncology 2022, 24, 796–808. [Google Scholar] [CrossRef]
- Choudhury, A.; Magill, S.T.; Eaton, C.D.; Prager, B.C.; Chen, W.C.; Cady, M.A.; Seo, K.; Lucas, C.-H.G.; Casey-Clyde, T.J.; Vasudevan, H.N.; et al. Meningioma DNA methylation groups identify biological drivers and therapeutic vulnerabilities. Nat. Genet. 2022, 54, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Bi, W.L.; Nayak, L.; Meredith, D.M.; Driver, J.; Du, Z.; Hoffman, S.; Li, Y.; Lee, E.Q.; Beroukhim, R.; Rinne, M.; et al. Activity of PD-1 blockade with nivolumab among patients with recurrent atypical/anaplastic meningioma: Phase II trial results. Neuro-Oncology 2022, 24, 101–113. [Google Scholar] [CrossRef]
- Islim, A.I.; Kolamunnage-Dona, R.; Mohan, M.; Moon, R.D.C.; Crofton, A.; Haylock, B.J.; Rathi, N.; Brod-belt, A.R.; Mills, S.J.; Jenkinson, M.D. A prognostic model to personalize monitoring regimes for patients with incidental asymptomatic meningiomas. Neuro-Oncology 2020, 22, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Ferrarotto, R.; Curcio, A.; Metro, L.; Pasqualetti, F.; Gaviani, P.; Barresi, V.; Angileri, F.F.; Caffo, M. Novel Advances in Treatment of Meningiomas: Prognostic and Therapeutic Implications. Cancers 2023, 15, 4521. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Gilbert, M.; Vogelbaum, M.A. Intracranial meningiomas of atypical (WHO grade II) histology. J. Neuro-Oncol. 2010, 99, 393–405. [Google Scholar] [CrossRef]
- Palma, L.; Cantore, G. Long-term prognosis for atypical and malignant meningiomas: A study of 71 surgical cases. Neurosurg. Focus 1997, 2, e3. [Google Scholar] [CrossRef]
- Morales-Valero, S.F.; Van Gompel, J.J.; Loumiotis, I.; Lanzino, G. Craniotomy for anterior cranial fossa meningiomas: Historical overview. Neurosurg. Focus 2014, 36, E14. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.A.; Huang, L.; Ramanathan, D.; Lopez-Gonzalez, M.; Pillai, P.; De Los Reyes, K.; Kumal, M.; Boling, W. Review of Atypical and Anaplastic Meningiomas: Classification, Molecular Biology, and Management. Front. Oncol. 2020, 10, 565582. [Google Scholar] [CrossRef] [PubMed]
- Perry, A. Unmasking the secrets of meningioma: A slow but rewarding journey. Surg. Neurol. 2004, 61, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Buerki, R.A.; Horbinski, C.M.; Kruser, T.; Horowitz, P.M.; James, C.D.; Lukas, R.V. An Overview of Meningiomas. Future Oncol. 2018, 14, 2161–2177. [Google Scholar] [CrossRef] [PubMed]
- Graffeo, C.S.; Leeper, H.E.; Perry, A.; Uhm, J.H.; Lachance, D.J.; Brown, P.D.; Ma, D.J.; Gompel, J.J.V.; Giannini, C.; Johnson, D.R.; et al. Revisiting Adjuvant Radiotherapy After Gross Total Resection of World Health Organization Grade II Meningioma. World Neurosurg. 2017, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Demchuk, A.M.; Menon, B.K.; Eesa, M.; Rempel, J.L.; Thornton, J.; Roy, D.; Jovin, T.G.; Willin-sky, R.A.; Sapkota, B.L.; et al. Randomized Assessment of Rapid Endovascular Treatment of Ischemic Stroke. N. Engl. J. Med. 2015, 372, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Englot, D.J.; Magill, S.T.; Han, S.J.; Chang, E.F.; Berger, M.S.; McDermott, M.W. Seizures in supratentorial meningioma: A systematic review and meta-analysis. J. Neurosurg. 2016, 124, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Magill, S.T.; Englot, D.J.; Baal, J.D.; Wagle, S.; Rick, J.W.; McDermott, M.W. Factors Associated with Pre- and Postoperative Seizures in 1033 Patients Undergoing Supratentorial Meningioma Resection. Neurosurgery 2017, 81, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.; Young, M.; Albert, T.; Shah, A.H.; Okoye, C.; Bregy, A.; Lo, S.S.; Ishkanian, F.; Komotar, R.J. The Role of Adjuvant Radiotherapy After Gross Total Resection of Atypical Meningiomas. World Neurosurg. 2015, 83, 808–815. [Google Scholar] [CrossRef]
- Attia, A.; Chan, M.D.; Mott, R.T.; Russell, G.B.; Seif, D.; Daniel Bourland, J.; Deguzman, A.F.; Ellis, T.L.; McMullen, K.P.; Munley, M.T.; et al. Patterns of failure after treatment of atypical meningioma with gamma knife radiosurgery. J. Neuro-Oncol. 2012, 108, 179–185. [Google Scholar] [CrossRef]
- Jimenez, R.B.; Alexander, B.M.; Mahadevan, A.; Niemierko, A.; Rajakesari, S.; Arvold, N.D.; Floyd, S.R.; Oh, K.S.; Loeffler, J.S.; Shih, H.A. The impact of different stereotactic radiation therapy regimens for brain metastases on local control and toxicity. Adv. Radiat. Oncol. 2017, 2, 391–397. [Google Scholar] [CrossRef]
- Cohen-Inbar, O.; Lee C chia Sheehan, J.P. The Contemporary Role of Stereotactic Radiosurgery in the Treatment of Meningiomas. Neurosurg. Clin. N. Am. 2016, 27, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.K.; Niranjan, A.; McInerney, J.; Kondziolka, D.; Flickinger, J.C.; Lunsford, L.D. Stereotactic radiosurgery providing long-term tumor control of cavernous sinus meningiomas. J. Neurosurg. 2002, 97, 65–72. [Google Scholar] [CrossRef]
- Kano, H.; Takahashi, J.A.; Katsuki, T.; Araki, N.; Oya, N.; Hiraoka, M.; Hashimoto, N. Stereotactic radiosurgery for atypical and anaplastic meningiomas. J. Neuro-Oncol. 2007, 84, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.E.; Lee, J.Y.K.; Omalu, B.; Flickinger, J.C.; Kondziolka, D.; Lunsford, L.D. The effect of radiosurgeryduring management of aggressive meningiomas. Surg. Neurol. 2003, 60, 298–305. [Google Scholar] [CrossRef]
- Rogers, C.L.; Won, M.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Fogh, S.E.; Youssef, E.; et al. High-risk Meningioma: Initial Outcomes from NRG Oncology/RTOG 0539. Int. J. Radiat. Oncol. 2020, 106, 790–799. [Google Scholar] [CrossRef]
- Madani, I.; Lomax, A.J.; Albertini, F.; Trnková, P.; Weber, D.C. Dose-painting intensity-modulated proton therapy for intermediate- and high-risk meningioma. Radiat. Oncol. 2015, 10, 72. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hammouche, S.; Clark, S.; Wong, A.H.L.; Eldridge, P.; Farah, J.O. Long-term survival analysis of atypical meningiomas: Survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir. 2014, 156, 1475–1481. [Google Scholar] [CrossRef]
- Komotar, R.J.; Iorgulescu, J.B.; Raper, D.M.S.; Holland, E.C.; Beal, K.; Bilsky, M.H.; Brennan, C.W.; Tabar, V.; Sherman, J.H.; Yamada, Y.; et al. The role of radiotherapy following gross-total resection of atypical meningiomas: Clinical article. J. Neurosurg. 2012, 117, 679–686. [Google Scholar] [CrossRef]
- Pasquier, D.; Bijmolt, S.; Veninga, T.; Rezvoy, N.; Villa, S.; Krengli, M.; Weber, D.C.; Baumert, B.G.; Canyilmaz, E.; Yalman, D.; et al. Atypical and Malignant Meningioma: Outcome and Prognostic Factors in 119 Irradiated Patients. A Multicenter, Retrospective Study of the Rare Cancer Network. Int. J. Radiat. Oncol. 2008, 71, 1388–1393. [Google Scholar] [CrossRef]
- Jääskeläinen, J.; Haltia, M.; Laasonen, E.; Wahlström, T.; Valtonen, S. The growth rate of intracranial meningiomas and its relation to histology. An analysis of 43 patients. Surg. Neurol. 1985, 24, 165–172. [Google Scholar] [CrossRef]
- Jenkinson, M.D.; Javadpour, M.; Haylock, B.J.; Young, B.; Gillard, H.; Vinten, J.; Bulbeck, H.; Das, K.; Far-rell, M.; Looby, S.; et al. The ROAM/EORTC-1308 trial: Radiation versus Observation following surgical resection of Atypical Meningioma: Study protocol for a randomised controlled trial. Trials 2015, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.C.; Ares, C.; Villa, S.; Peerdeman, S.M.; Renard, L.; Baumert, B.G.; Lucas, A.; Veninga, T.; Pica, A.; Jefferies, S.; et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: A phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother. Oncol. 2018, 128, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Rogers, L.; Zhang, P.; Vogelbaum, M.A.; Perry, A.; Ashby, L.S.; Modi, J.M.; Alleman, A.M.; Galvin, J.; Brachman, D.; Jenrette, J.M.; et al. Intermediate-risk meningioma: Initial outcomes from NRG Oncology RTOG 0539. J. Neurosurg. 2018, 129, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Sioka, C.; Kyritsis, A.P. Chemotherapy, hormonal therapy, and immunotherapy for recurrent meningiomas. J. Neuro-Oncol. 2009, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Raizer, J.J.; Abrey, L.E.; Lamborn, K.R.; Lassman, A.B.; Chang, S.M.; Yung, W.K.A.; Gilbert, M.R.; Fine, H.A.; Mehta, M.; et al. Phase II trials of erlotinib or gefitinib in patients with recurrent meningioma. J. Neuro-Oncol. 2010, 96, 211–217. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Tsao-Wei, D.D.; Groshen, S. Salvage chemotherapy with CPT-11 for recurrent meningioma. J. Neuro-Oncol. 2006, 78, 271–276. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.H.; Kim, Y.Z. The Clinical Outcome of Hydroxyurea Chemotherapy after Incomplete Resection of Atypical Meningiomas. Brain Tumor Res. Treat. 2017, 5, 77–86. [Google Scholar] [CrossRef]
- Abdel Karim, K.; El Shehaby, A.; Emad, R.; Reda, W.; El Mahdy, M.; Ghali, R.; Nabeel, A. Role of hydroxyurea as an adjuvant treatment after Gamma knife radiosurgery for atypical (WHO grade II) meningiomas. J. Egypt Natl. Cancer Inst. 2018, 30, 69–72. [Google Scholar] [CrossRef]
- Chamberlain, M.C.; Glantz, M.J. Interferon-α for recurrent World Health Organization grade 1 intracranial meningiomas. Cancer 2008, 113, 2146–2151. [Google Scholar] [CrossRef]
- Kaley, T.J.; Wen, P.; Schiff, D.; Ligon, K.; Haidar, S.; Karimi, S.; Lassman, A.B.; Nolan, C.P.; DeAngelis, L.M.; Gavrilovic, I.; et al. Phase II trial of sunitinib for recurrent and progressive atypical and anaplastic meningioma. Neuro-Oncology 2015, 17, 116–121. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Abrey, L.E.; Lassman, A.B.; Chang, S.M.; Lamborn, K.R.; Kuhn, J.G.; Yung, W.K.A.; Gilbert, M.R.; Aldape, K.D.; Wen, P.Y.; et al. A phase I trial of erlotinib in patients with nonprogressive glioblastoma multiforme postradiation therapy, and recurrent malignant gliomas and meningiomas. Neuro-Oncology 2010, 12, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Raizer, J.J.; Grimm, S.A.; Rademaker, A.; Chandler, J.P.; Muro, K.; Helenowski, I.; Rice, L.; McCarthy, K.; Johnston, S.K.; Mrugala, M.M.; et al. A phase II trial of PTK787/ZK 222584 in recurrent or progressive radiation and surgery refractory meningiomas. J. Neuro-Oncol. 2014, 117, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Caffo, M.; Pino, M.A.; Caruso, G.; Tomasello, F. Antisense Molecular Therapy in Cerebral Gliomas. J. Anal. Oncol. 2012, 1, 135–144. Available online: https://neoplasiaresearch.com/pms/index.php/jao/article/view/92 (accessed on 15 July 2024).
- Sakuma, T.; Nakagawa, T.; Ido, K.; Takeuchi, H.; Sato, K.; Kubota, T. Expression of vascular endothelial growth factor-A and mRNA stability factor HuR in human meningiomas. J. Neuro-Oncol. 2008, 88, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Barresi, V.; Tuccari, G. Increased ratio of vascular endothelial growth factor to semaphorin3A is a negative prognostic factor in human meningiomas. Neuropathology 2010, 30, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Baxter, D.S.; Orrego, A.; Rosenfeld, J.V.; Mathiesen, T. An audit of immunohistochemical marker patterns in meningioma. J. Clin. Neurosci. 2014, 21, 421–426. [Google Scholar] [CrossRef]
- Winter, R.C.; Antunes, A.C.M.; Oliveira, F.H.D. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. Surg. Neurol. Int. 2020, 11, 328. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Kshettry, V.R.; Selman, W.R.; Bambakidis, N.C. Peritumoral brain edema in intracranial meningiomas: The emergence of vascular endothelial growth factor–directed therapy. Neurosurg. Focus 2013, 35, E2. [Google Scholar] [CrossRef]
- Salokorpi, N.; Yrjänä, S.; Tuominen, H.; Karttunen, A.; Heljasvaara, R.; Pihlajaniemi, T.; Heikkinen, E.; Koivukangas, J. Expression of VEGF and collagen XVIII in meningiomas: Correlations with histopathological and MRI characteristics. Acta Neurochir. 2013, 155, 989–996. [Google Scholar] [CrossRef]
- Shih, K.C.; Chowdhary, S.; Rosenblatt, P.; Weir, A.B.; Shepard, G.C.; Williams, J.T.; Shastry, M.; Burris, H.A.; Hainsworth, J.D. A phase II trial of bevacizumab and everolimus as treatment for patients with refractory, progressive intracranial meningioma. J. Neuro-Oncol. 2016, 129, 281–288. [Google Scholar] [CrossRef] [PubMed]
- Pachow, D.; Andrae, N.; Kliese, N.; Angenstein, F.; Stork, O.; Wilisch-Neumann, A.; Kirches, E.; Mawrin, C. mTORC1 Inhibitors Suppress Meningioma Growth in Mouse Models. Clin. Cancer Res. 2013, 19, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Graillon, T.; Sanson, M.; Campello, C.; Idbaih, A.; Peyre, M.; Peyrière, H.; Basset, N.; Autran, D.; Roche, C.; Kalamarides, M.; et al. Everolimus and Octreotide for Patients with Recurrent Meningioma: Results from the Phase II CEVOREM Trial. Clin. Cancer Res. 2020, 26, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Wolfsberger, S.; Doostkam, S.; Boecher-Schwarz, H.G.; Roessler, K.; Van Trotsenburg, M.; Hainfellner, J.A.; Knosp, E. Progesterone-receptor index in meningiomas: Correlation with clinico-pathological parameters and review of the literature. Neurosurg. Rev. 2004, 27, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Norden, A.D.; Ligon, K.L.; Hammond, S.N.; Muzikansky, A.; Reardon, D.A.; Kaley, T.J.; Batchelor, T.T.; Plotkin, S.R.; Raizer, J.J.; Wong, E.T.; et al. Phase II study of monthly pasireotide LAR (SOM230C) for recurrent or progressive meningioma. Neurology 2015, 84, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Zhi, F.; Zhou, G.; Wang, S.; Shi, Y.; Peng, Y.; Shao, N.; Guan, W.; Qu, H.; Zhang, Y.; Wang, Q.; et al. A microRNA expression signature predicts meningioma recurrence. Int. J. Cancer. 2013, 132, 128–136. [Google Scholar] [CrossRef]
- Deng, J.; Hua, L.; Bian, L.; Chen, H.; Chen, L.; Cheng, H.; Dou, C.; Geng, D.; Hong, T.; Ji, H.; et al. Molecular diagnosis and treatment of meningiomas: An expert consensus (2022). Chin. Med. J. 2022, 135, 1894–1912. (In English) [Google Scholar] [CrossRef]
| Major Criteria |
| 4–19 mitotic figures/10 high-power fields |
| Brain invasion |
| Minor Criteria |
| Increased cellularity |
| Small cells with high N/C ratio |
| Large and prominent nucleoli |
| Pattern-less or sheet-like growth (loss of lobular architecture) |
| Foci of spontaneous or geographic necrosis |
| Factor | |
|---|---|
| Clinical | Incomplete removal (Simpson grade 2–4) |
| Fast growth | |
| Histopathological | High mitotic index/Ki-67 |
| Major diagnostic criteria | |
| Chromosomal | Loss of 1p, 14q, 18q, 10q, 6q, 7p Gain of 20q, 12q, 15q, 1q, 9q, 17q |
| Genetic | NF2 mutation |
| AKT1, SMARCB1, SMO, PIK3CA. SUFU, PTEN, ARID1A gene mutations | |
| Epigenetic | H3K27me2 loss |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ravnik, J.; Rowbottom, H. The Impact of Molecular and Genetic Analysis on the Treatment of Patients with Atypical Meningiomas. Diagnostics 2024, 14, 1782. https://doi.org/10.3390/diagnostics14161782
Ravnik J, Rowbottom H. The Impact of Molecular and Genetic Analysis on the Treatment of Patients with Atypical Meningiomas. Diagnostics. 2024; 14(16):1782. https://doi.org/10.3390/diagnostics14161782
Chicago/Turabian StyleRavnik, Janez, and Hojka Rowbottom. 2024. "The Impact of Molecular and Genetic Analysis on the Treatment of Patients with Atypical Meningiomas" Diagnostics 14, no. 16: 1782. https://doi.org/10.3390/diagnostics14161782
APA StyleRavnik, J., & Rowbottom, H. (2024). The Impact of Molecular and Genetic Analysis on the Treatment of Patients with Atypical Meningiomas. Diagnostics, 14(16), 1782. https://doi.org/10.3390/diagnostics14161782





