Assessment of the Diagnostic Efficacy of Low-Field Magnetic Resonance Imaging: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
- Level 1: technical quality of the test;
- Level 2: diagnostic accuracy, sensitivity and specificity of the test, interpretation;
- Level 3: whether the test result leads to a change in the diagnostic thinking of the physician;
- Level 4: impact of the test on the patient’s treatment plan;
- Level 5: impact of the test on patient outcomes;
- Level 6: societal costs and benefits of the diagnostic test.
2.1. Sources and Search Strategies
2.2. Inclusion and Exclusion Criteria
2.2.1. Inclusion Criteria
- These are clinical trials, i.e., randomised controlled trials, cohort studies or case-control studies;
- Were published in a peer-reviewed journal;
- LF MRI technology was compared with one or more currently available 1.5 T MRI technologies for one (or more) of the areas listed in the key questions;
- Technologies were compared according to their diagnostic efficacy (see Fryback and Thornbury’s model above) [9].
2.2.2. Exclusion Criteria
- Overview articles, reviews, casuistries, letters to the editor, commentaries, case studies;
- Studies with less than 5 patients;
- Animal, in vitro or cadaver studies;
- Abstracts from conferences that did not result in a peer-reviewed publication.
2.3. Screening and Assessment of Studies
2.4. Data Extraction
2.5. Critical Assessment
2.6. Data Analysis
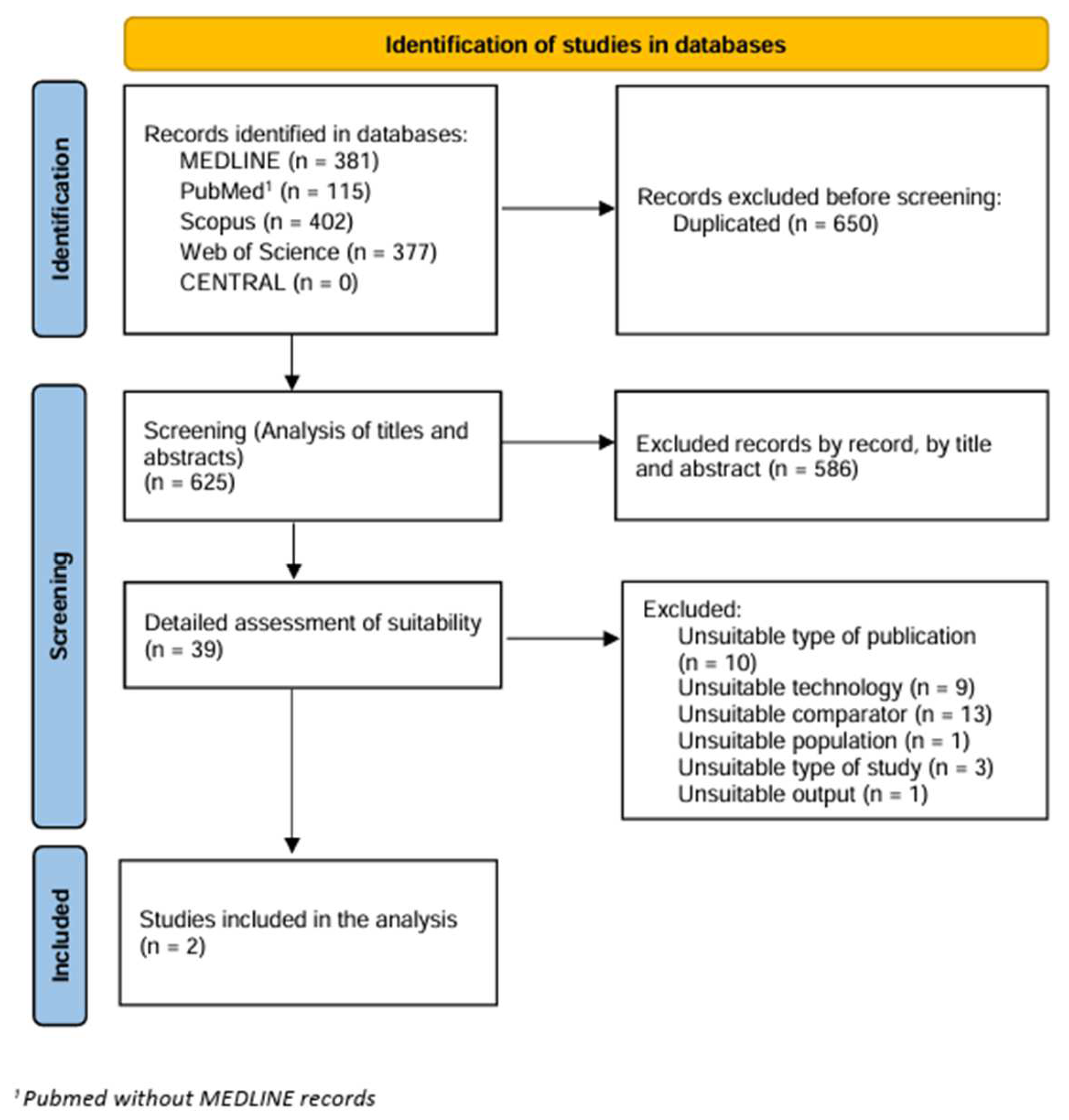
3. Results
3.1. Excluded Studies
3.2. Characteristics of the Studies
3.3. Quality Assessment
4. Discussion
4.1. Limitations
4.2. Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hori, M.; Hagiwara, A.; Goto, M.; Wada, A.; Aoki, S. Low-Field Magnetic Resonance Imaging: Its History and Renaissance. Investig. Radiol. 2021, 56, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.S.; Levin, D.; Parker, L.; Rao, V.M.; Ross-Degnan, D.; Wharam, J.F. Trends in Diagnostic Imaging Utilization among Medicare and Commercially Insured Adults from 2003 through 2016. Radiology 2020, 294, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Marques, J.P.; Simonis, F.F.J.; Webb, A.G. Low-field MRI: An MR physics perspective. J. Magn. Reson. Imaging 2019, 49, 1528–1542. [Google Scholar] [CrossRef] [PubMed]
- Sheth, K.N.; Mazurek, M.H.; Yuen, M.M.; Cahn, B.A.; Shah, J.T.; Ward, A.; Kim, J.A.; Gilmore, E.J.; Falcone, G.J.; Petersen, N.; et al. Assessment of Brain Injury Using Portable, Low-Field Magnetic Resonance Imaging at the Bedside of Critically Ill Patients. JAMA Neurol. 2021, 78, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Anoardo, E.; Rodriguez, G.G. New challenges and opportunities for low-field MRI. J. Magn. Reson. Open 2023, 14, 100086. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Cochrane Handbook for Systematic Reviews of Interventions. Available online: https://training.cochrane.org/handbook (accessed on 29 November 2023).
- Arnold, T.C.; Freeman, C.W.; Litt, B.; Stein, J.M. Low-field MRI: Clinical promise and challenges. J. Magn. Reson. Imaging JMRI 2023, 57, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Fryback, D.G.; Thornbury, J.R. The Efficacy of Diagnostic Imaging. Med. Decis. Making 1991, 11, 88–94. [Google Scholar] [CrossRef]
- Bachmann, L.M.; Estermann, P.; Kronenberg, C.; ter Riet, G. Identifying diagnostic accuracy studies in EMBASE. J. Med. Libr. Assoc. 2003, 91, 341–346. [Google Scholar]
- Whiting, P.; Rutjes, A.W.; Reitsma, J.B.; Bossuyt, P.M.; Kleijnen, J. The development of QUADAS: A tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med. Res. Methodol. 2003, 3, 25. [Google Scholar] [CrossRef]
- Allam, M.F.; Elian, M.M.; Rahman, A.M.; Allam, F.A. The utility of chemical shift imaging and related fat suppression as standalone technique in cryptorchidism using low field MRI. Egypt. J. Radiol. Nucl. Med. 2018, 49, 1140–1144. [Google Scholar] [CrossRef]
- Campbell-Washburn, A.E. 2019 American Thoracic Society BEAR Cage Winning Proposal: Lung Imaging Using High-Performance Low-Field Magnetic Resonance Imaging. Am. J. Respir. Crit. Care Med. 2020, 201, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Montesino, M.D.; Mendoza Mendoza, D. Síndrome del túnel del carpo por tofo: Imagen de resonancia magnética de bajo campo. Reumatol. Clín. 2019, 15, e149–e150. [Google Scholar] [CrossRef] [PubMed]
- Anisimov, N.V.; Pavlova, O.S.; Pirogov, Y.A.; Yarnykh, V.L. Three-dimensional fast single-point macromolecular proton fraction mapping of the human brain at 0.5 Tesla. Quant. Imaging Med. Surg. 2020, 10, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Schröder, F.F.; Post, C.E.; van Raak, S.M.; Simonis, F.F.; Wagenaar, F.-C.; Huis in’t Veld, R.M.; Verdonschot, N. The diagnostic potential of low-field MRI in problematic total knee arthroplasties—A feasibility study. J. Exp. Orthop. 2020, 7, 59. [Google Scholar] [CrossRef] [PubMed]
- Basar, B.; Sonmez, M.; Yildirim, D.K.; Paul, R.; Herzka, D.A.; Kocaturk, O.; Lederman, R.J.; Campbell-Washburn, A.E. Susceptibility artifacts from metallic markers and cardiac catheterization devices on a high-performance 0.55 T MRI system. Magn. Reson. Imaging 2021, 77, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.R.; Cherukuri, V.; O’Reilly, T.; Yu, M.; Mbabazi-Kabachelor, E.; Mulando, R.; Sheth, K.N.; Webb, A.G.; Warf, B.C.; Kulkarni, A.V.; et al. Assessing the utility of low resolution brain imaging: Treatment of infant hydrocephalus. NeuroImage Clin. 2021, 32, 102896. [Google Scholar] [CrossRef] [PubMed]
- Campbell-Washburn, A.E.; Jiang, Y.; Körzdörfer, G.; Nittka, M.; Griswold, M.A. Feasibility of MR fingerprinting using a high-performance 0.55 T MRI system. Magn. Reson. Imaging 2021, 81, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; van Gelderen, P.; de Zwart, J.A.; Campbell-Washburn, A.E.; Duyn, J.H. FMRI based on transition-band balanced SSFP in comparison with EPI on a high-performance 0.55 T scanner. Magn. Reson. Med. 2021, 85, 3196–3210. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Ramasawmy, R.; Javed, A.; Chen, M.Y.; Benkert, T.; Majeed, W.; Lederman, R.J.; Moss, J.; Balaban, R.S.; Campbell-Washburn, A.E. Oxygen-enhanced functional lung imaging using a contemporary 0.55 T MRI system. NMR Biomed. 2021, 34, e4562. [Google Scholar] [CrossRef]
- Self-Gated 3D Stack-of-Spirals UTE Pulmonary Imaging at 0.55T-Javed-2022-Magnetic Resonance in Medicine-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/10.1002/mrm.29079 (accessed on 12 April 2024).
- T2-Weighted Lung Imaging Using a 0.55-T MRI System|Radiology: Cardiothoracic Imaging. Available online: https://pubs.rsna.org/doi/full/10.1148/ryct.2021200611 (accessed on 12 April 2024).
- Campbell-Washburn, A.E.; Suffredini, A.F.; Chen, M.Y. High-Performance 0.55-T Lung MRI in Patient with COVID-19 Infection. Radiology 2021, 299, E246–E247. [Google Scholar] [CrossRef] [PubMed]
- Heiss, R.; Grodzki, D.M.; Horger, W.; Uder, M.; Nagel, A.M.; Bickelhaupt, S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn. Reson. Imaging 2021, 76, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Norris, D.G.; Webb, A. This house proposes that low field and high field MRI are by destiny worst enemies, and can never be the best of friends! Magn. Reson. Mater. Phys. Biol. Med. 2021, 34, 475–477. [Google Scholar] [CrossRef] [PubMed]
- Chiragzada, S.; Satya, P.; Macaluso, J.N., Jr.; Venkataraman, S.S.; Adams, J., Jr.; Nacev, A.N.; Kumar, D. Advantageous Detection of Significant Prostate Cancer Using a Low-Field, Office-Based MRI System. Cureus 2022, 14, e32105. [Google Scholar] [CrossRef] [PubMed]
- Porrelli, D.; Abrami, M.; Pelizzo, P.; Formentin, C.; Ratti, C.; Turco, G.; Grassi, M.; Canton, G.; Grassi, G.; Murena, L. Trabecular bone porosity and pore size distribution in osteoporotic patients—A low field nuclear magnetic resonance and microcomputed tomography investigation. J. Mech. Behav. Biomed. Mater. 2022, 125, 104933. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Bai, H.; Chen, H.; Zhao, Y.; Luo, H.; Wu, Z.; Zhang, Z. Susceptibility-weighted imaging at high-performance 0.5T magnetic resonance imaging system: Protocol considerations and experimental results. Front. Neurosci. 2022, 16, 999240. [Google Scholar] [CrossRef]
- Stamenkovic, B.; Stojanovic, S.; Zivkovic, V.; Djordjevic, D.; Bojanovic, M.; Stankovic, A.; Rancic, N.; Damjanov, N.; Matucci Cerinic, M. Low-Frequency Magnetic Resonance Imaging Identifies Hand Joint Subclinical Inflammation in Systemic Sclerosis. Diagnostics 2022, 12, 2165. [Google Scholar] [CrossRef]
- Bhattacharya, I.; Ramasawmy, R.; Javed, A.; Lowery, M.; Henry, J.; Mancini, C.; Machado, T.; Jones, A.; Julien-Williams, P.; Lederman, R.J.; et al. Assessment of Lung Structure and Regional Function Using 0.55 T MRI in Patients With Lymphangioleiomyomatosis. Investig. Radiol. 2022, 57, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Lévy, S.; Heiss, R.; Grimm, R.; Grodzki, D.; Hadler, D.; Voskrebenzev, A.; Vogel-Claussen, J.; Fuchs, F.; Strauss, R.; Achenbach, S.; et al. Free-Breathing Low-Field MRI of the Lungs Detects Functional Alterations Associated With Persistent Symptoms After COVID-19 Infection. Investig. Radiol. 2022, 57, 742–751. [Google Scholar] [CrossRef]
- Seemann, F.; Javed, A.; Chae, R.; Ramasawmy, R.; O’Brien, K.; Baute, S.; Xue, H.; Lederman, R.J.; Campbell-Washburn, A.E. Imaging gravity-induced lung water redistribution with automated inline processing at 0.55 T cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2022, 24, 35. [Google Scholar] [CrossRef]
- Azour, L.; Condos, R.; Keerthivasan, M.B.; Bruno, M.; Pandit Sood, T.; Landini, N.; Silverglate, Q.; Babb, J.; Chandarana, H.; Moore, W.H. Low-field 0.55 T MRI for assessment of pulmonary groundglass and fibrosis-like opacities: Inter-reader and inter-modality concordance. Eur. J. Radiol. 2022, 156, 110515. [Google Scholar] [CrossRef] [PubMed]
- Wujciak, D. Modern mid-field magnetic resonance imaging in private practice: Field report. Radiologe 2022, 62, 405–409. [Google Scholar] [CrossRef]
- Anzai, Y.; Moy, L. Point-of-Care Low-Field-Strength MRI Is Moving Beyond the Hype. Radiology 2022, 305, 672–673. [Google Scholar] [CrossRef]
- Breit, H.-C.; Bauman, G. Morphologic and Functional Assessment of Sarcoidosis Using Low-Field MRI. Radiology 2022, 303, 255. [Google Scholar] [CrossRef] [PubMed]
- Cawley, P.A.; Nosarti, C.; Edwards, A.D. In-unit neonatal magnetic resonance imaging—New possibilities offered by low-field technology. J. Perinatol. 2022, 42, 843–844. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, M.S.; Griesdale, D.E. Low field magnetic resonance imaging: A “beds-eye-d” view into hypoxic ischemic brain injury after cardiac arrest. Resuscitation 2022, 176, 55–57. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.C.; Tu, D.; Okar, S.V.; Nair, G.; By, S.; Kawatra, K.D.; Robert-Fitzgerald, T.E.; Desiderio, L.M.; Schindler, M.K.; Shinohara, R.T.; et al. Sensitivity of portable low-field magnetic resonance imaging for multiple sclerosis lesions. NeuroImage Clin. 2022, 35, 103101. [Google Scholar] [CrossRef] [PubMed]
- Breit, H.-C.; Vosshenrich, J.; Bach, M.; Merkle, E.M. Neue klinische Anwendungsbereiche der Niederfeld-Magnetresonanztomographie. Radiologe 2022, 62, 394–399. [Google Scholar] [CrossRef] [PubMed]
- New Efforts in Biomedical Imaging|IEEE Journals & Magazine|IEEE Xplore. Available online: https://ieeexplore.ieee.org/document/9870761 (accessed on 12 April 2024).
- Rusche, T.; Vosshenrich, J.; Winkel, D.J.; Donners, R.; Segeroth, M.; Bach, M.; Merkle, E.M.; Breit, H.-C. More Space, Less Noise—New-generation Low-Field Magnetic Resonance Imaging Systems Can Improve Patient Comfort: A Prospective 0.55T–1.5T-Scanner Comparison. J. Clin. Med. 2022, 11, 6705. [Google Scholar] [CrossRef]
- Klippel, E.; Moshagen, V. Neurologische Manifestation einer zerebrotendinösen Xanthomatose—Klinik und kraniale Bildgebung im Niederfeld-MRT. Nervenarzt 2023, 94, 142–144. [Google Scholar] [CrossRef]
- Tian, Y.; Cui, S.X.; Lim, Y.; Lee, N.G.; Zhao, Z.; Nayak, K.S. Contrast-optimal simultaneous multi-slice bSSFP cine cardiac imaging at 0.55 T. Magn. Reson. Med. 2023, 89, 746–755. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lee, N.G.; Cui, S.X.; Nayak, K.S. Lung parenchyma transverse relaxation rates at 0.55 T. Magn. Reson. Med. 2023, 89, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Heiss, R.; Tan, L.; Schmidt, S.; Regensburger, A.P.; Ewert, F.; Mammadova, D.; Buehler, A.; Vogel-Claussen, J.; Voskrebenzev, A.; Rauh, M.; et al. Pulmonary Dysfunction after Pediatric COVID-19. Radiology 2023, 306, e221250. [Google Scholar] [CrossRef]
- Paltiel, H.J. Low-Field-Strength MRI and Ventilation-Perfusion Mismatch after Pediatric COVID-19. Radiology 2023, 306, e222360. [Google Scholar] [CrossRef]
- Osmanodja, F.; Rösch, J.; Knott, M.; Doerfler, A.; Grodzki, D.; Uder, M.; Heiss, R. Diagnostic Performance of 0.55 T MRI for Intracranial Aneurysm Detection. Investig. Radiol. 2023, 58, 121–125. [Google Scholar] [CrossRef]
- Rusche, T.; Breit, H.-C.; Bach, M.; Wasserthal, J.; Gehweiler, J.; Manneck, S.; Lieb, J.M.; De Marchis, G.M.; Psychogios, M.N.; Sporns, P.B. Potential of Stroke Imaging Using a New Prototype of Low-Field MRI: A Prospective Direct 0.55 T/1.5 T Scanner Comparison. J. Clin. Med. 2022, 11, 2798. [Google Scholar] [CrossRef] [PubMed]
- Klein, H.-M. Low-Field Magnetic Resonance Imaging. Fortschr Röntgenstr 2020, 192, 537–548. [Google Scholar] [CrossRef]
- Vosshenrich, J.; Breit, H.-C.; Bach, M.; Merkle, E.M. Economic aspects of low-field magnetic resonance imaging: Acquisition, installation, and maintenance costs of 0.55 T systems. Radiology 2022, 62, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Grist, T.M. The Next Chapter in MRI: Back to the Future? Radiology 2019, 293, 394–395. [Google Scholar] [CrossRef]
| Author of the Study | About the Study | Reason for Exclusion |
|---|---|---|
| Allam 2018 [12] | This study is focused on the evaluation of LF-MRI cryptorchidism. High specificity and diagnostic accuracy were found. | Unsuitable technology: 0.4 T Aperto MRI Hitachi—open |
| Campbell-Washburn 2019 [13] | The study examines lung imaging using a modified MRI system to 0.55 T. According to the results of LF-MRI, the visualisation of the lung parenchyma is improved. | Unsuitable type of publication: Proposal |
| Montesino 2019 [14] | The study evaluated rheumatologic diseases by LF-MRI. LF-MRI may be helpful as a complementary examination. | Unsuitable type of publication: Conference paper |
| Anisimov 2020 [15] | The study focused on imaging of the brain using 0.5 T research MRI using the macromolecular proton fraction (MPF) mapping method. The results demonstrate that MPF is a valuable absolute scale for measuring myelin content using MRI at various magnetic field strengths. | Unsuitable technology:MRI for research purposes Tomikon S50 |
| Schröder 2020 [16] | The study focused on total knee arthroplasty patients. Low-field MRI shows similar diagnostic value to CT and could be a cost-effective alternative without radiation. | Unsuitable technology: 0.25 T Esaote G-scan Brio—open |
| Basar 2021 [17] | The study focused on the evaluation of artefacts. Steel is a source of artefacts independent of the MRI field; an MRI indication <0.55 T may minimise these artefacts. | Unsuitable population: Evaluation of artefact |
| Harper 2021 [18] | Image quality for hydrocephalus treatment planning was evaluated. Images with lower quality than is customarily acceptable can be helpful for hydrocephalus treatment planning. | Unsuitable technology: Prototype |
| Campbell-Washburn 2021 [19] | A 3D FISP MRF sequence was implemented for the 0.55 T system. The feasibility of whole-brain T1 and T2 mapping using MRF in healthy volunteers was demonstrated. | Unsuitable comparator: Phantom MRI |
| Wang 2021 [20] | The study focused on LF-MRI brain imaging, and sensitivity was assessed. It showed that the blood oxygenation level-dependent contrast is sufficiently high and can be used to extend the range of LFMRI applications. | Unsuitable comparator: was no comparator |
| Bhattacharya 2021 [21] | Using high-performance 0.55 T MRI, experts were able to perform simultaneous imaging of pulmonary structure and regional function in patients with lymphangioleiomyomatosis. | Unsuitable comparator: Prototype |
| Javed 2021 [22] | An MRI scan of the lungs was performed. Fast image reconstruction was implemented for diagnostic quality, which was validated and evaluated in our technique in healthy volunteers, patients with lung nodules, and patients with COVID-19 infection. | Unsuitable comparator: Prototype |
| Campbell-Washburn 2021 [23] | An MRI scan of the lungs was performed and compared with CT scans. The MRI scan was of sufficient quality to detect pathologies | Unsuitable comparator: Computed Tomography |
| Campbell-Washburn 2021 [24] | This publication deals with LF-MRI of the lungs in a patient with COVID-19 and focuses on a case report. | Unsuitable type of publication: Reviews and commentary |
| Heiss 2021 [25] | This is a case study of a patient with COVID-19, for which LFMRI was used. LF-MRI enables the precise visualisation of persistent pulmonary changes, which are consistent with CT performed on the same day. | Unsuitable type of publication: Case report |
| Norris 2021 [26] | This publication focuses on expert commentary on the issues surrounding conventional MRI and LFMRI. | Unsuitable type of study: Commentary |
| Chiragzada 2022 [27] | This study is focused on targeted biopsy using MRI-guided biopsy over transrectal ultrasound. The targeted MRI approach significantly benefited the patient by favourably impacting the care. | Unsuitable technology: Transperineal biopsy |
| Porrelli 2022 [28] | The present study demonstrates the synergistic use of LF-NMR and micro-CT in detecting structural morphometric changes in the trabecular bone of osteoporosis specimens compared to osteoarthritis. | Unsuitable technology: Spectrometer—Bruker Mini spec mq 20 |
| Qiu 2022 [29] | A comparison of susceptibility-weighted imaging (SWI) in 0.5 T and 1.5 T was carried out, demonstrating the capability to identify magnetic susceptibility differences between variable tissues, especially the blood veins. | Unsuitable technology: Prototype |
| Stamenkovic 2022 [30] | The study focuses on LF-MRI joint examinations. The findings suggest that MRI should be used in symptomatic patients. | Unsuitable technology: 0.2 T Artroscan—open |
| Bhattacharya 2022 [31] | This study focused on the LF-MRI imaging of lungs with simultaneous imaging of pulmonary structure and regional function in patients with lymphangioleiomyomatosis. | Unsuitable comparator: Computed tomography |
| Lévy 2022 [32] | Functional pulmonary examinations using free-breathing LF-MRI revealed potential quantitative markers of impaired lung function in patients with persistent symptoms after COVID-19 infection, potentially complementing morphologic imaging. | Unsuitable comparator: Computed tomography |
| Seemann 2022 [33] | The study focuses on imaging water in the lungs using cardiovascular magnetic resonance. The results suggest that LF-MRI helps assess changes in the lungs. | Unsuitable comparator: Phantom MRI |
| Azour 2022 [34] | The study evaluated the detection of lung by LF-MRI versus clinically-acquired chest CT images in a cohort of post-COVID patients. LF MRI 0.55 T demonstrates fair-to-moderate inter-reader concordance. | Unsuitable comparator: Computed tomography |
| Wujciak 2022 [35] | Measurement parameters and examination protocols were analysed to see if they met the requirements. The conclusions confirm that LF-MRI can partially replace conventional MRI. | Unsuitable comparator: Compared different scenarios |
| Anzai 2022 [36] | This is an expert commentary on the issues of conventional MRI and LF-MRI. | Unsuitable type of publication: Reviews and commentary |
| Breit 2022 [37] | This is an expert commentary on the issues of conventional MRI and LF-MRI. | Unsuitable type of publication: Reviews and commentary |
| Cawley 2022 [38] | This is an editorial focused on new possibilities offered by low-field technology. | Unsuitable type of publication: Editorial |
| Sekhon 2022 [39] | Editorial about problematic MRI—view into hypoxic-ischemic brain injury after cardiac arrest | Unsuitable type of publication: Editorial |
| Arnold 2022 [40] | The study focused on imaging multiple sclerosis (MS). The authors found that a porTable 64 mT scanner was sensitive in MS patients and that an automated algorithm designed for 3 T image segmentation could be applied. | Unsuitable type of publication: 64 mT MRI vs. 3 T MRI |
| Breit 2022 [41] | This is a review focused on the effects, image acquisition, and diagnostic quality of the examination. | Unsuitable type of study: Review |
| Mertz 2022 [42] | Abstract about New Efforts in Biomedical Imaging. | Unsuitable type of study: Abstract |
| Rusche 2022 [43] | The study was to assess patient comfort when imaged on a newly introduced 0.55 T low-field magnetic resonance (MR) scanner system with a broader bore opening compared to a conventional 1.5 T MR scanner system. | Unsuitable output: Evaluated patient comfort |
| Klippel 2023 [44] | The study focuses on a case report of a neurological patient | Unsuitable technology: 0.4 T Fuji Aperto Lucent Plus- open |
| Tian 2023 [45] | Contemporary LF-MR scanners equipped with high-performance gradient systems allow the use of contrast-optimal flip-angles for multi-slice accelerated examinations without compromising image quality | Unsuitable comparator: Was no comparator |
| Li 2023 [46] | The study focuses on LF-MRI lung imaging. According to the literature, the results are similar to a 1.5 T MRI in the evaluation of lung parenchyma. | Unsuitable comparator: Was no comparator |
| Heiss 2023 [47] | LF-MRI evaluated lung parenchyma in children and adolescents post-COVID-19 compared to healthy controls. LF-MRI showed persistent pulmonary dysfunction in children and adolescents. | Unsuitable comparator: Clinical symptoms and serologic parameter |
| Paltiel 2023 [48] | This is an expert commentary on the issues of LF-MRI. | Unsuitable type of publication: Review and commentary |
| Study | Study Design | Number of Patients | Population | Area |
|---|---|---|---|---|
| Osmanodja 2023 [49] | Cross-over, single-centre, retro-prospective | 9 patients | Patients with suspected intracranial aneurysm | Brain |
| Rusche 2022 [50] | Cross-over and case-control, single-centre, prospective | A total of 27 patients were included in the study (17 in the stroke group and 10 in the control group) | Patients with suspected stroke or transient ischemic attack | Brain |
| Study | Outputs | Measurements | Results |
|---|---|---|---|
| Osmanodja 2023 [49] | Size of aneurysm | TOF MRA Two unblinded raters | Insignificant differences in aneurysm size |
| Rusche 2022 [50] |
| DWI/ADC and FLAIR sequences
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mašková, B.; Rožánek, M.; Gajdoš, O.; Karnoub, E.; Kamenský, V.; Donin, G. Assessment of the Diagnostic Efficacy of Low-Field Magnetic Resonance Imaging: A Systematic Review. Diagnostics 2024, 14, 1564. https://doi.org/10.3390/diagnostics14141564
Mašková B, Rožánek M, Gajdoš O, Karnoub E, Kamenský V, Donin G. Assessment of the Diagnostic Efficacy of Low-Field Magnetic Resonance Imaging: A Systematic Review. Diagnostics. 2024; 14(14):1564. https://doi.org/10.3390/diagnostics14141564
Chicago/Turabian StyleMašková, Barbora, Martin Rožánek, Ondřej Gajdoš, Evgeniia Karnoub, Vojtěch Kamenský, and Gleb Donin. 2024. "Assessment of the Diagnostic Efficacy of Low-Field Magnetic Resonance Imaging: A Systematic Review" Diagnostics 14, no. 14: 1564. https://doi.org/10.3390/diagnostics14141564
APA StyleMašková, B., Rožánek, M., Gajdoš, O., Karnoub, E., Kamenský, V., & Donin, G. (2024). Assessment of the Diagnostic Efficacy of Low-Field Magnetic Resonance Imaging: A Systematic Review. Diagnostics, 14(14), 1564. https://doi.org/10.3390/diagnostics14141564







