Comprehensive Study of the IBMP ELISA IgA/IgM/IgG COVID-19 Kit for SARS-CoV-2 Antibody Detection
Abstract
1. Introduction
2. Materials and Methods
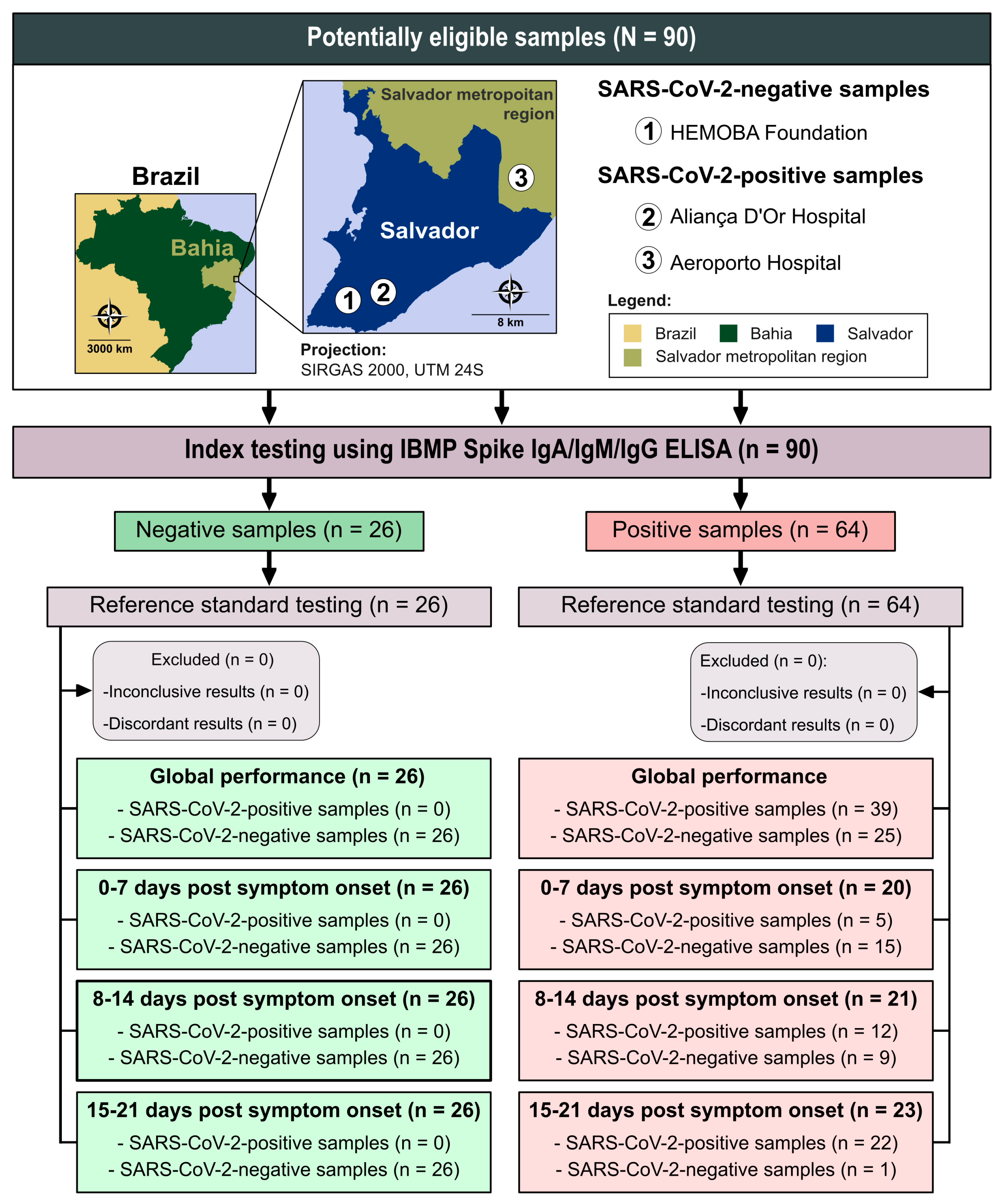
2.1. Study Design
2.2. IBMP ELISA IgA/IgM/IgG COVID-19
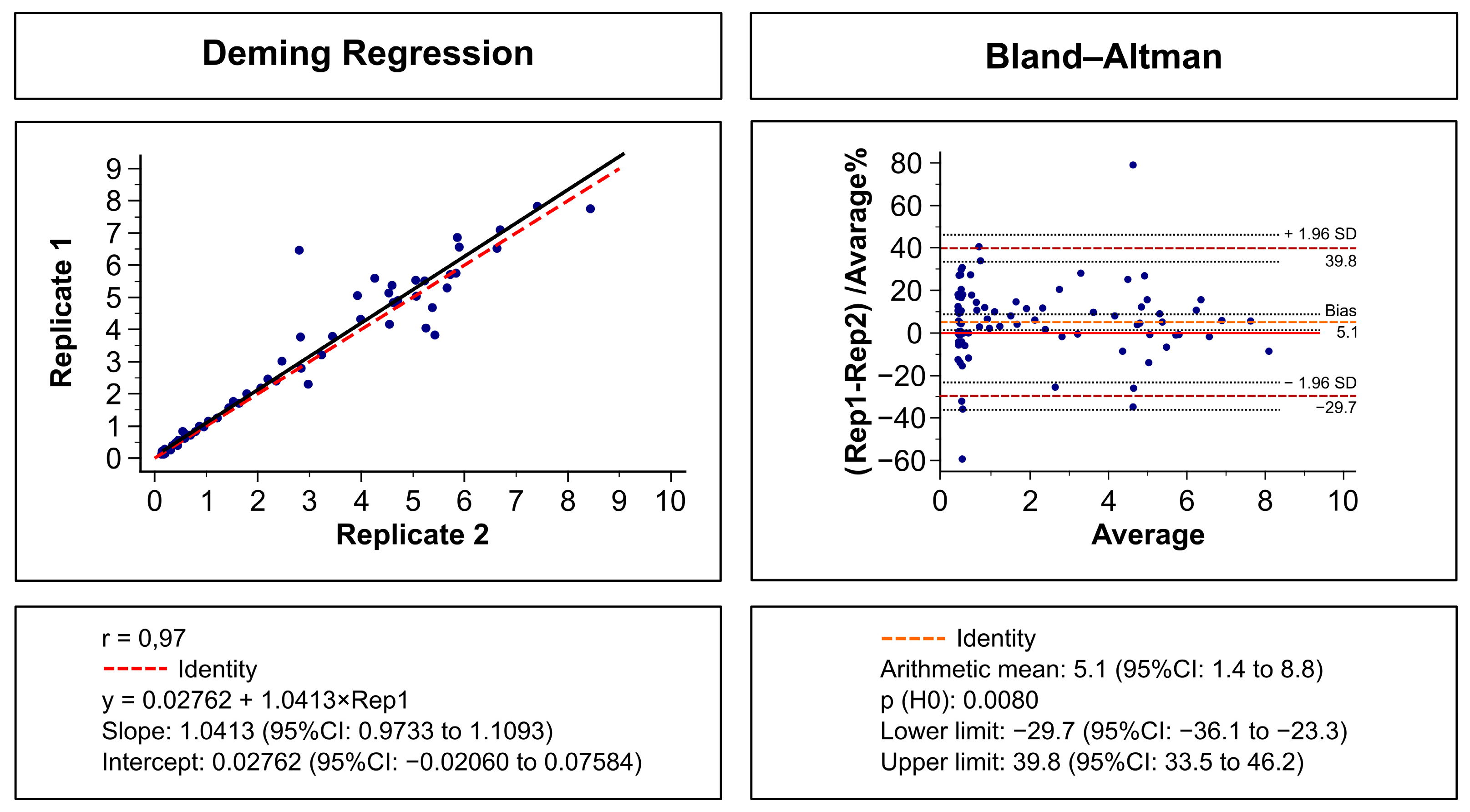
2.3. Imprecision Assessment
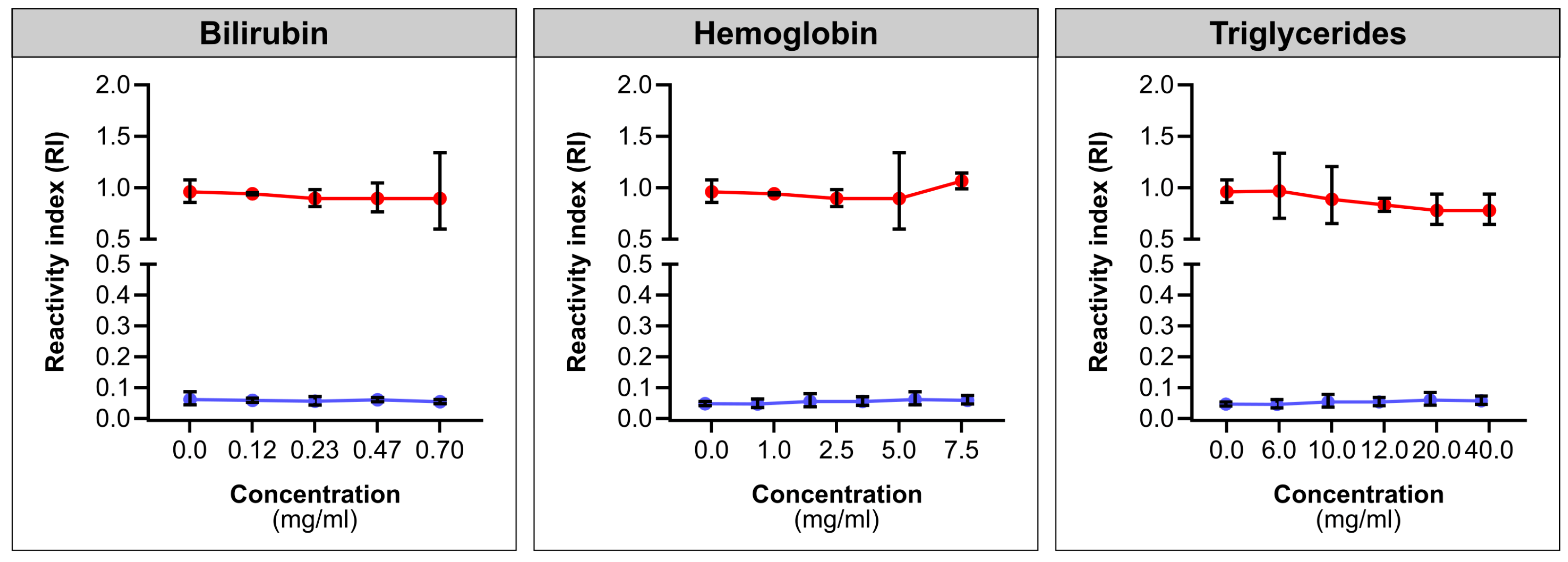
2.4. Interferants Analysis
2.5. Commercial ELISA Test Comparison
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Participants
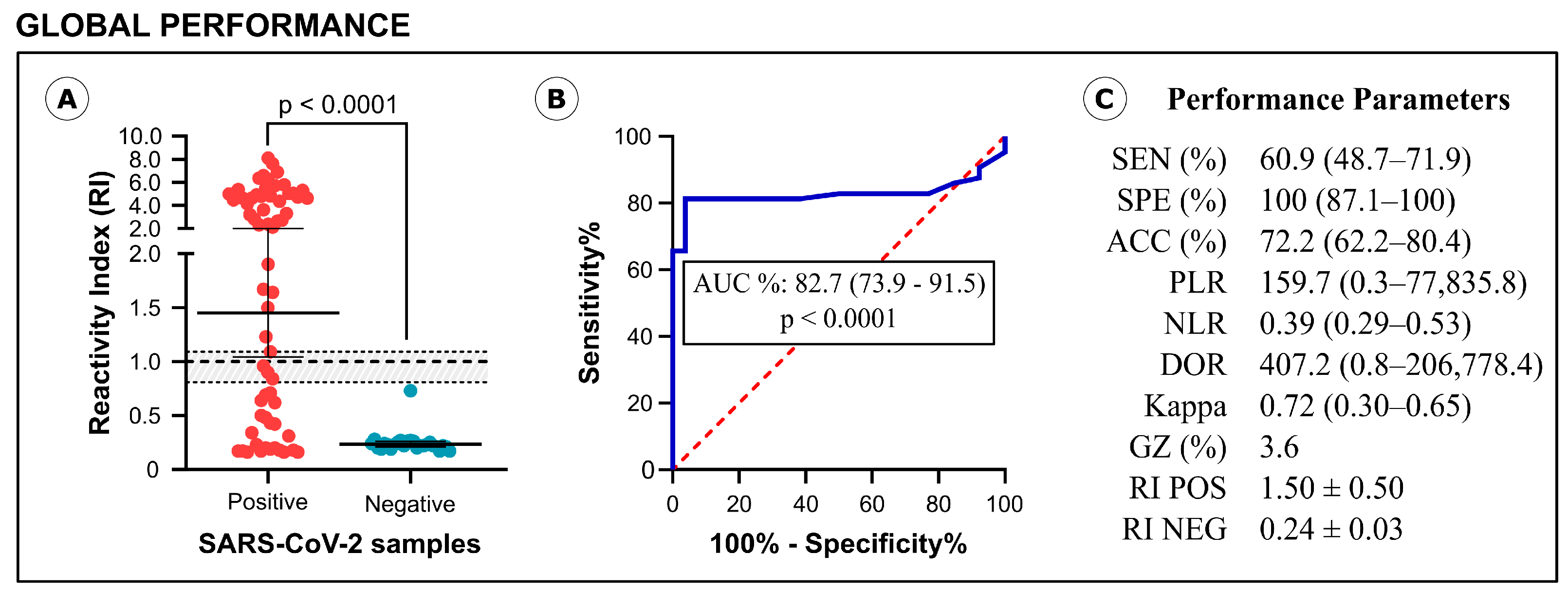
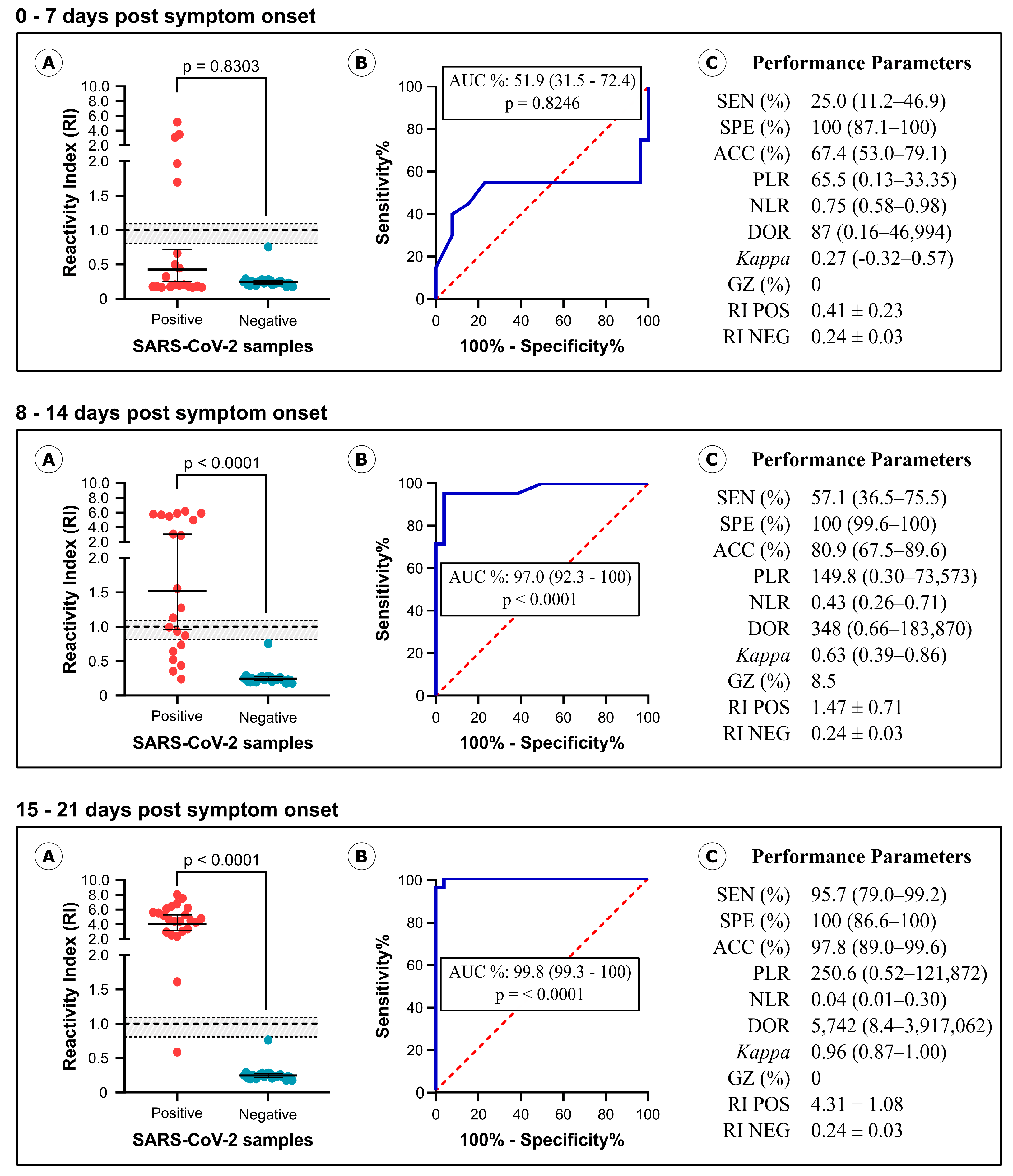
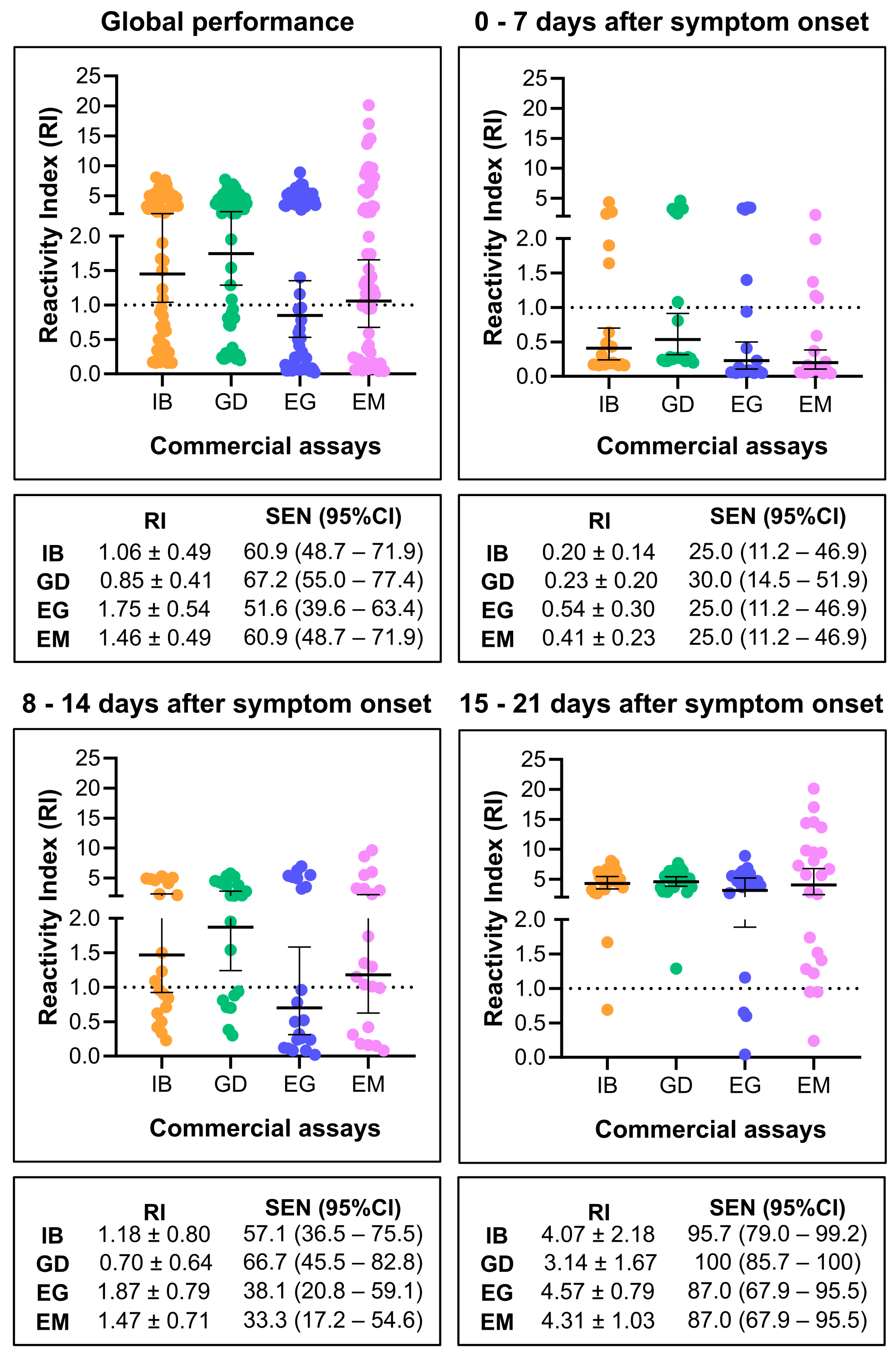
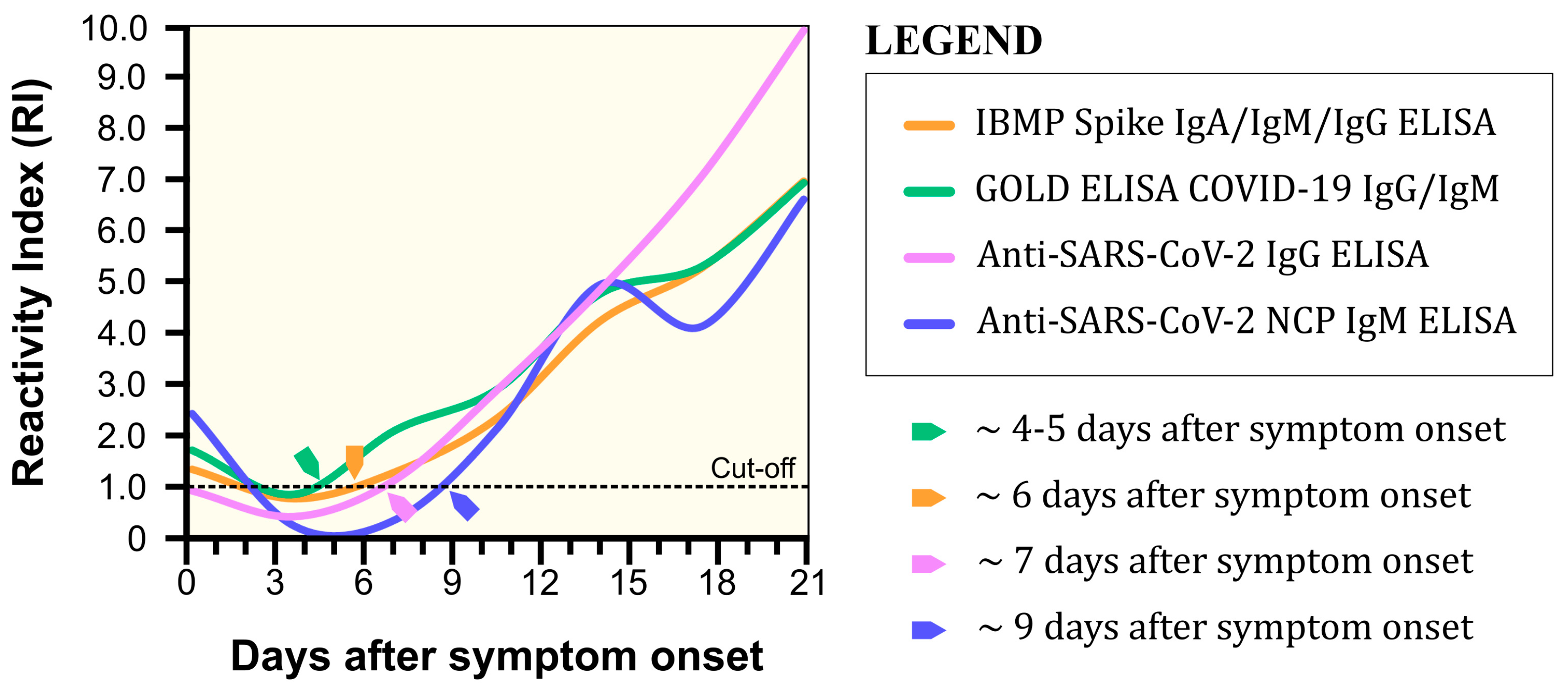
3.2. Diagnostic Performance of IBMP Spike IgA/IgM/IgG ELISA
4. Discussion
5. Strengths and Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.; Zhai, J.; Feng, Y.; Zhou, N.; Zhang, X.; Zou, J.-J.; Li, N.; Guo, Y.; Li, X.; Shen, X.; et al. Isolation of SARS-CoV-2-Related Coronavirus from Malayan Pangolins. Nature 2020, 583, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.T.-Y.; Jia, N.; Zhang, Y.-W.; Shum, M.H.-H.; Jiang, J.-F.; Zhu, H.-C.; Tong, Y.-G.; Shi, Y.-X.; Ni, X.-B.; Liao, Y.-S.; et al. Identifying SARS-CoV-2-Related Coronaviruses in Malayan Pangolins. Nature 2020, 583, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cui, W.; Tian, B.-P. The Potentiali Intermediate Hosts for SARS-CoV-2. Front. Microbiol. 2020, 11, 580137. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and Clinical Characteristics of 99 Cases of 2019 Novel Coronavirus Pneumonia in Wuhan, China: A Descriptive Study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with SARS-CoV-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.; Ni, Z.; Hu, Y.Y.Y.-H.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.C.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A New Coronavirus Associated with Human Respiratory Disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef]
- Cucinotta, D.; Vanelli, M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020, 91, 157–160. [Google Scholar] [CrossRef]
- Lenharo, M. WHO Declares End to COVID-19’s Emergency Phase. Nature 2023, 882. [Google Scholar] [CrossRef]
- Kevadiya, B.D.; Machhi, J.; Herskovitz, J.; Oleynikov, M.D.; Blomberg, W.R.; Bajwa, N.; Soni, D.; Das, S.; Hasan, M.; Patel, M.; et al. Diagnostics for SARS-CoV-2 Infections. Nat. Mater. 2021, 20, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Khatami, F.; Saatchi, M.; Zadeh, S.S.T.; Aghamir, Z.S.; Shabestari, A.N.; Reis, L.O.; Aghamir, S.M.K. A Meta-Analysis of Accuracy and Sensitivity of Chest CT and RT-PCR in COVID-19 Diagnosis. Sci. Rep. 2020, 10, 22402. [Google Scholar] [CrossRef] [PubMed]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, Point-of-Care Antigen and Molecular-Based Tests for Diagnosis of SARS-CoV-2 Infection. Cochrane Database Syst. Rev. 2021, 3, CD013705. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Wikramaratna, P.S.; Paton, R.S.; Ghafari, M.; Lourenço, J. Estimating the False-Negative Test Probability of SARS-CoV-2 by RT-PCR. Eurosurveillance 2020, 25, 2000568. [Google Scholar] [CrossRef]
- Cocherie, T.; Bastide, M.; Sakhi, S.; Zafilaza, K.; Flandre, P.; Leducq, V.; Jary, A.; Burrel, S.; Louet, M.; Calvez, V.; et al. Decreased Sensitivity of Rapid Antigen Test Is Associated with a Lower Viral Load of Omicron than Delta SARS-CoV-2 Variant. Microbiol. Spectr. 2022, 10, e0192222. [Google Scholar] [CrossRef] [PubMed]
- Wagenhäuser, I.; Knies, K.; Hofmann, D.; Rauschenberger, V.; Eisenmann, M.; Reusch, J.; Gabel, A.; Flemming, S.; Andres, O.; Petri, N.; et al. Virus Variant-Specific Clinical Performance of SARS Coronavirus Two Rapid Antigen Tests in Point-of-Care Use, from November 2020 to January 2022. Clin. Microbiol. Infect. 2023, 29, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Petherick, A. Developing Antibody Tests for SARS-CoV-2. Lancet 2020, 395, 1101–1102. [Google Scholar] [CrossRef] [PubMed]
- Fox, T.; Geppert, J.; Dinnes, J.; Scandrett, K.; Bigio, J.; Sulis, G.; Hettiarachchi, D.; Mathangasinghe, Y.; Weeratunga, P.; Wickramasinghe, D.; et al. Antibody Tests for Identification of Current and Past Infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2022, 11, CD013652. [Google Scholar] [CrossRef]
- Wheeler, S.E.; Shurin, G.V.; Yost, M.; Anderson, A.; Pinto, L.; Wells, A.; Shurin, M.R. Differential Antibody Response to MRNA COVID-19 Vaccines in Healthy Subjects. Microbiol. Spectr. 2021, 9, e0034121. [Google Scholar] [CrossRef]
- Vandeberg, P.; Cruz, M.; Diez, J.M.; Merritt, W.K.; Santos, B.; Trukawinski, S.; Wellhouse, A.; Jose, M.; Willis, T. Production of Anti-SARS-CoV-2 Hyperimmune Globulin from Convalescent Plasma. Transfusion 2021, 61, 1705–1709. [Google Scholar] [CrossRef] [PubMed]
- Ong, D.S.Y.; Fragkou, P.C.; Schweitzer, V.A.; Chemaly, R.F.; Moschopoulos, C.D.; Skevaki, C. European Society of Clinical Microbiology and Infectious Diseases (ESCMID) Study Group for Respiratory Viruses (ESGREV) How to Interpret and Use COVID-19 Serology and Immunology Tests. Clin. Microbiol. Infect. 2021, 27, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and Clinical Application of a Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Dean, A.G.; Sullivan, K.M.; Soe, M.M.; Mir, R.A. OpenEpi: Open Source Epidemiologic Statistics for Public Health. Available online: www.OpenEpi.com (accessed on 20 February 2024).
- Clinical and Laboratory Standards Institute (CLSI). Evaluation of Precision Performance of Quantitative Measurement Methods–Approved Guideline, 3rd ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2014; Volume 24, ISBN 1-56238-968-8. [Google Scholar]
- Swets, J.A. Measuring the Accuracy of Diagnostic Systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef] [PubMed]
- Ouchchane, L.; Rabilloud, M.; Boire, J.-Y. Sensibilité, spécificité et valeurs prédictives. In Évaluation des Méthodes D’analyse Appliquées aux Sciences de la vie et de la Santé—Biostatistique; Beuscart, R., Bénichou, J., Roy, P., Quantin, C., Eds.; Omniscience: Paris, France, 2009; Volume 2, pp. 49–78. ISBN 978-2-916097-18-3. [Google Scholar]
- Sackett, D.L.; Straus, S. On Some Clinically Useful Measures of the Accuracy of Diagnostic Tests. ACP J. Club 1998, 129, A17–A19. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Artalejo, F.; Banegas Banegas, J.R.; González Enríquez, J.; Martín Moreno, J.M.; Villar Alvarez, F. Analysis of Clinical Decisions. Med. Clin. 1990, 94, 348–354. [Google Scholar]
- Santos, F.L.N.; Celedon, P.A.; Zanchin, N.I.; Souza, W.V.; Silva, E.D.; Foti, L.; Krieger, M.A.; Gomes, Y.M. Accuracy of Chimeric Proteins in the Serological Diagnosis of Chronic Chagas Disease—A Phase II Study. PLoS Negl. Trop. Dis. 2017, 11, e0005433. [Google Scholar] [CrossRef] [PubMed]
- Glas, A.S.; Lijmer, J.G.; Prins, M.H.; Bonsel, G.J.; Bossuyt, P.M.M. The Diagnostic Odds Ratio: A Single Indicator of Test Performance. J. Clin. Epidemiol. 2003, 56, 1129–1135. [Google Scholar] [CrossRef]
- Payton, M.E.; Greenstone, M.H.; Schenker, N. Overlapping Confidence Intervals or Standard Error Intervals: What Do They Mean in Terms of Statistical Significance? J. Insect Sci. 2003, 3, 34. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Bland, J.M.; Altman, D.G. Statistical Methods for Assessing Agreement between Two Methods of Clinical Measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.F.; Korevaar, D.A.; Altman, D.G.; Bruns, D.E.; Gatsonis, C.A.; Hooft, L.; Irwig, L.; Levine, D.; Reitsma, J.B.; de Vet, H.C.W.; et al. STARD 2015 Guidelines for Reporting Diagnostic Accuracy Studies: Explanation and Elaboration. BMJ Open 2016, 6, e012799. [Google Scholar] [CrossRef] [PubMed]
- Whitman, J.D.; Hiatt, J.; Mowery, C.T.; Shy, B.R.; Yu, R.; Yamamoto, T.N.; Rathore, U.; Goldgof, G.M.; Whitty, C.; Woo, J.M.; et al. Evaluation of SARS-CoV-2 Serology Assays Reveals a Range of Test Performance. Nat. Biotechnol. 2020, 38, 1174–1183. [Google Scholar] [CrossRef] [PubMed]
- Fox, A.; Marino, J.; Amanat, F.; Krammer, F.; Hahn-Holbrook, J.; Zolla-Pazner, S.; Powell, R.L. Robust and Specific Secretory IgA against SARS-CoV-2 Detected in Human Milk. iScience 2020, 23, 101735. [Google Scholar] [CrossRef] [PubMed]
- Rio-Aige, K.; Azagra-Boronat, I.; Castell, M.; Selma-Royo, M.; Collado, M.C.; Rodríguez-Lagunas, M.J.; Pérez-Cano, F.J. The Breast Milk Immunoglobulinome. Nutrients 2021, 13, 1810. [Google Scholar] [CrossRef] [PubMed]
- Van Elslande, J.; Decru, B.; Jonckheere, S.; Van Wijngaerden, E.; Houben, E.; Vandecandelaere, P.; Indevuyst, C.; Depypere, M.; Desmet, S.; André, E.; et al. Antibody Response against SARS-CoV-2 Spike Protein and Nucleoprotein Evaluated by Four Automated Immunoassays and Three ELISAs. Clin. Microbiol. Infect. 2020, 26, 1557.e1–e1557.e7. [Google Scholar] [CrossRef] [PubMed]
- Tuaillon, E.; Bolloré, K.; Pisoni, A.; Debiesse, S.; Renault, C.; Marie, S.; Groc, S.; Niels, C.; Pansu, N.; Dupuy, A.M.; et al. Detection of SARS-CoV-2 Antibodies Using Commercial Assays and Seroconversion Patterns in Hospitalized Patients. J. Infect. 2020, 81, e39–e45. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, A.C.; Holm, D.K.; Justesen, U.S.; Gorm-Jensen, T.; Andersen, N.S.; Øvrehus, A.; Johansen, I.S.; Michelsen, J.; Sprogøe, U.; Lillevang, S.T. Comparison of Six Commercially Available SARS-CoV-2 Antibody Assays—Choice of Assay Depends on Intended Use. Int. J. Infect. Dis. 2021, 103, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Cobos, A.; Gómez de Frutos, S.; Domingo García, D.; Navarro Lara, E.; Yarci Carrión, A.; Fontán García-Rodrigo, L.; Fraile Torres, A.M.; Cardeñoso Domingo, L. Evaluation of Diagnostic Accuracy of 10 Serological Assays for Detection of SARS-CoV-2 Antibodies. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 955–961. [Google Scholar] [CrossRef]
- Santos, L.A.d.O.; Campelo, Y.D.M.; Beltrão, R.P.L.; Mendonça, G.d.S.; Silva, V.A.d.; Campelo, V.M.d.B. Analysis of the Effectiveness Rate of Rapid Serological Tests for COVID-19 Registered in ANVISA, a Systematic Review in the Literature. Res. Soc. Dev. 2021, 10, e264101119615. [Google Scholar] [CrossRef]
- Akobeng, A.K. Understanding Diagnostic Tests 2: Likelihood Ratios, Pre- and Post-Test Probabilities and Their Use in Clinical Practice. Acta Paediatr. 2007, 96, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, R.H. Validation of Serological Assays for Diagnosis of Infectious Diseases. Rev. Sci. Tech. 1998, 17, 469–526. [Google Scholar] [CrossRef] [PubMed]
| Samples | Repeatability | Between Runs | Within Days | Within Lab | |||||
|---|---|---|---|---|---|---|---|---|---|
| SD | CV% | SD | CV% | SD | CV% | SD | CV% | ||
| Batch 1 | #1 (RI = 0.16) | <0.01 | 2.90 | 0.06 | 29.68 | 0.70 | 30.48 | 0.06 | 25.62 |
| #2 (RI = 0.16) | <0.01 | 4.19 | 0.08 | 36.32 | 0.07 | 27.67 | 0.06 | 28.94 | |
| #3 (RI = 1.30) | 0.03 | 10.69 | 0.12 | 8.57 | 0.16 | 11.48 | 0.13 | 9.52 | |
| #4 (RI = 1.50) | 0.02 | 5.74 | 0.11 | 7.35 | 0.16 | 10.03 | 0.25 | 14.69 | |
| #5 (RI = 2.17) | 0.02 | 3.02 | 0.13 | 5.81 | 0.10 | 4.23 | 0.25 | 10.59 | |
| #6 (RI = 4.46) | 0.07 | 6.60 | 0.02 | 0.50 | 0.18 | 3.88 | 0.57 | 12.17 | |
| Batch 2 | #1 (RI = 0.16) | <0.01 | 0.26 | 0.08 | 47.11 | 0.07 | 35.22 | 0.10 | 40.98 |
| #2 (RI = 0.16) | <0.01 | 2.51 | 0.07 | 41.51 | 0.07 | 37.35 | 0.10 | 41.73 | |
| #3 (RI = 1.30) | 0.01 | 2.41 | 0.05 | 3.99 | 0.05 | 3.89 | 0.06 | 5.40 | |
| #4 (RI = 1.50) | 0.02 | 5.01 | 0.21 | 13.69 | 0.27 | 16.04 | 0.29 | 15.55 | |
| #5 (RI = 2.17) | 0.03 | 4.97 | 0.42 | 19.59 | 0.42 | 18.29 | 0.44 | 17.61 | |
| #6 (RI = 4.46) | 0.08 | 7.86 | 0.89 | 22.44 | 1.03 | 23.07 | 0.83 | 17.82 | |
| Batch 3 | #1 (RI = 0.16) | <0.01 | 1.11 | 0.03 | 19.24 | 0.08 | 36.98 | 0.06 | 27.38 |
| #2 (RI = 0.16) | <0.01 | 1.94 | 0.02 | 12.23 | 0.06 | 28.24 | 0.06 | 25.61 | |
| #3 (RI = 1.30) | 0.01 | 5.37 | 0.17 | 12.93 | 0.15 | 11.47 | 0.12 | 9.49 | |
| #4 (RI = 1.50) | 0.02 | 6.55 | 0.42 | 23.93 | 0.36 | 19.14 | 0.34 | 18.17 | |
| #5 (RI = 2.17) | <0.01 | 0.11 | 0.39 | 16.86 | 0.36 | 14.69 | 0.37 | 15.92 | |
| #6 (RI = 4.46) | 0.01 | 0.62 | 0.85 | 18.70 | 0.92 | 18.75 | 0.75 | 15.41 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattar, S.B.; Celedon, P.A.F.; Leony, L.M.; Vasconcelos, L.d.C.M.; Sampaio, D.D.; Marchini, F.K.; Morello, L.G.; Lin, V.H.; Crestani, S.; Camelier, A.A.; et al. Comprehensive Study of the IBMP ELISA IgA/IgM/IgG COVID-19 Kit for SARS-CoV-2 Antibody Detection. Diagnostics 2024, 14, 1514. https://doi.org/10.3390/diagnostics14141514
Mattar SB, Celedon PAF, Leony LM, Vasconcelos LdCM, Sampaio DD, Marchini FK, Morello LG, Lin VH, Crestani S, Camelier AA, et al. Comprehensive Study of the IBMP ELISA IgA/IgM/IgG COVID-19 Kit for SARS-CoV-2 Antibody Detection. Diagnostics. 2024; 14(14):1514. https://doi.org/10.3390/diagnostics14141514
Chicago/Turabian StyleMattar, Sibelle Botogosque, Paola Alejandra Fiorani Celedon, Leonardo Maia Leony, Larissa de Carvalho Medrado Vasconcelos, Daniel Dias Sampaio, Fabricio Klerynton Marchini, Luis Gustavo Morello, Vanessa Hoysan Lin, Sandra Crestani, Aquiles Assunção Camelier, and et al. 2024. "Comprehensive Study of the IBMP ELISA IgA/IgM/IgG COVID-19 Kit for SARS-CoV-2 Antibody Detection" Diagnostics 14, no. 14: 1514. https://doi.org/10.3390/diagnostics14141514
APA StyleMattar, S. B., Celedon, P. A. F., Leony, L. M., Vasconcelos, L. d. C. M., Sampaio, D. D., Marchini, F. K., Morello, L. G., Lin, V. H., Crestani, S., Camelier, A. A., Meireles, A. C., de Oliveira Junior, A. L. F., Bandeira, A. C., Macedo, Y. S. F., Duarte, A. O., Pavan, T. B. S., de Siqueira, I. C., & Santos, F. L. N. (2024). Comprehensive Study of the IBMP ELISA IgA/IgM/IgG COVID-19 Kit for SARS-CoV-2 Antibody Detection. Diagnostics, 14(14), 1514. https://doi.org/10.3390/diagnostics14141514









