Evaluation of Plasma Atherogenic Index, Triglyceride-Glucose Index and Other Lipid Ratios as Predictive Biomarkers of Coronary Artery Disease in Different Age Groups
Abstract
1. Introduction
2. Materials and Methods
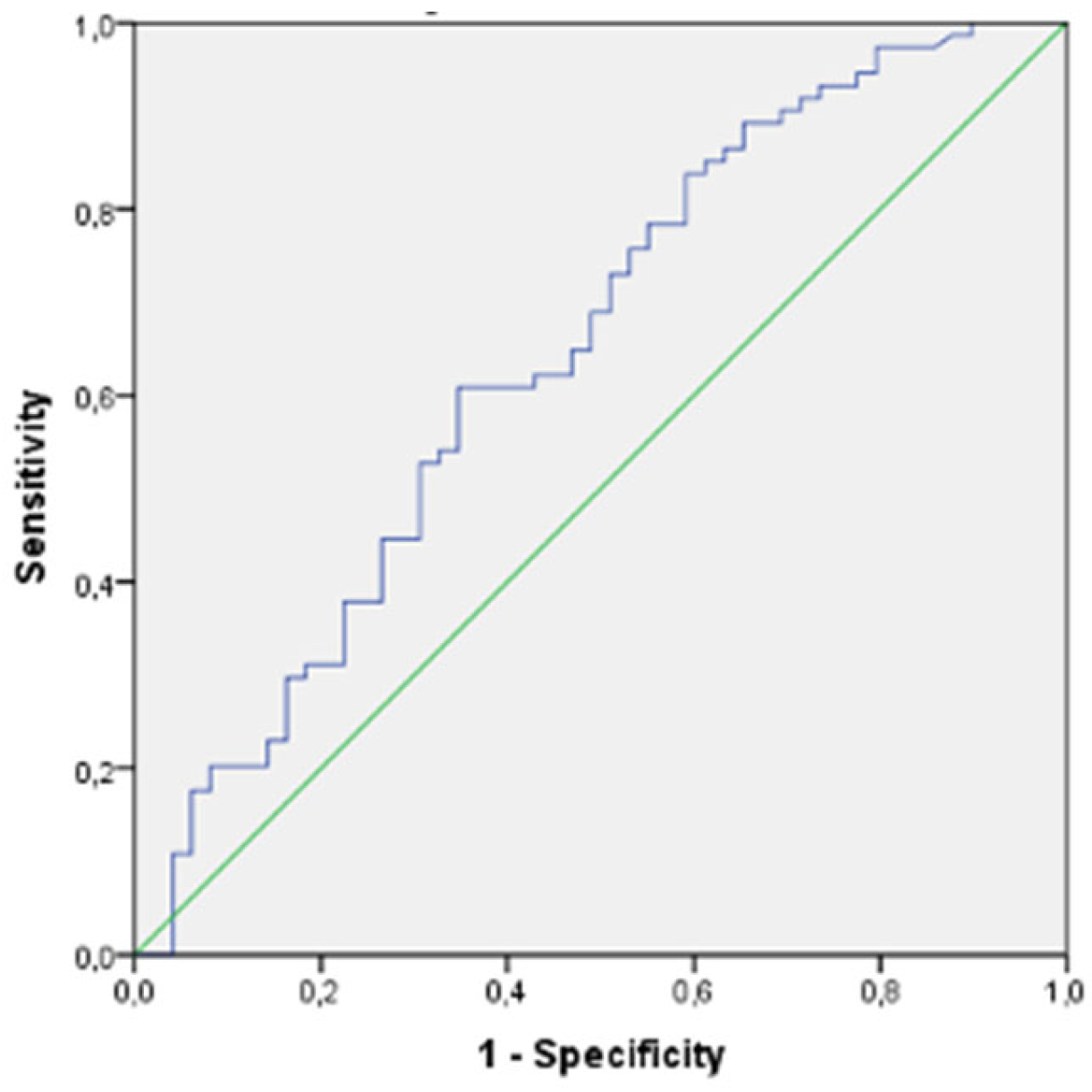
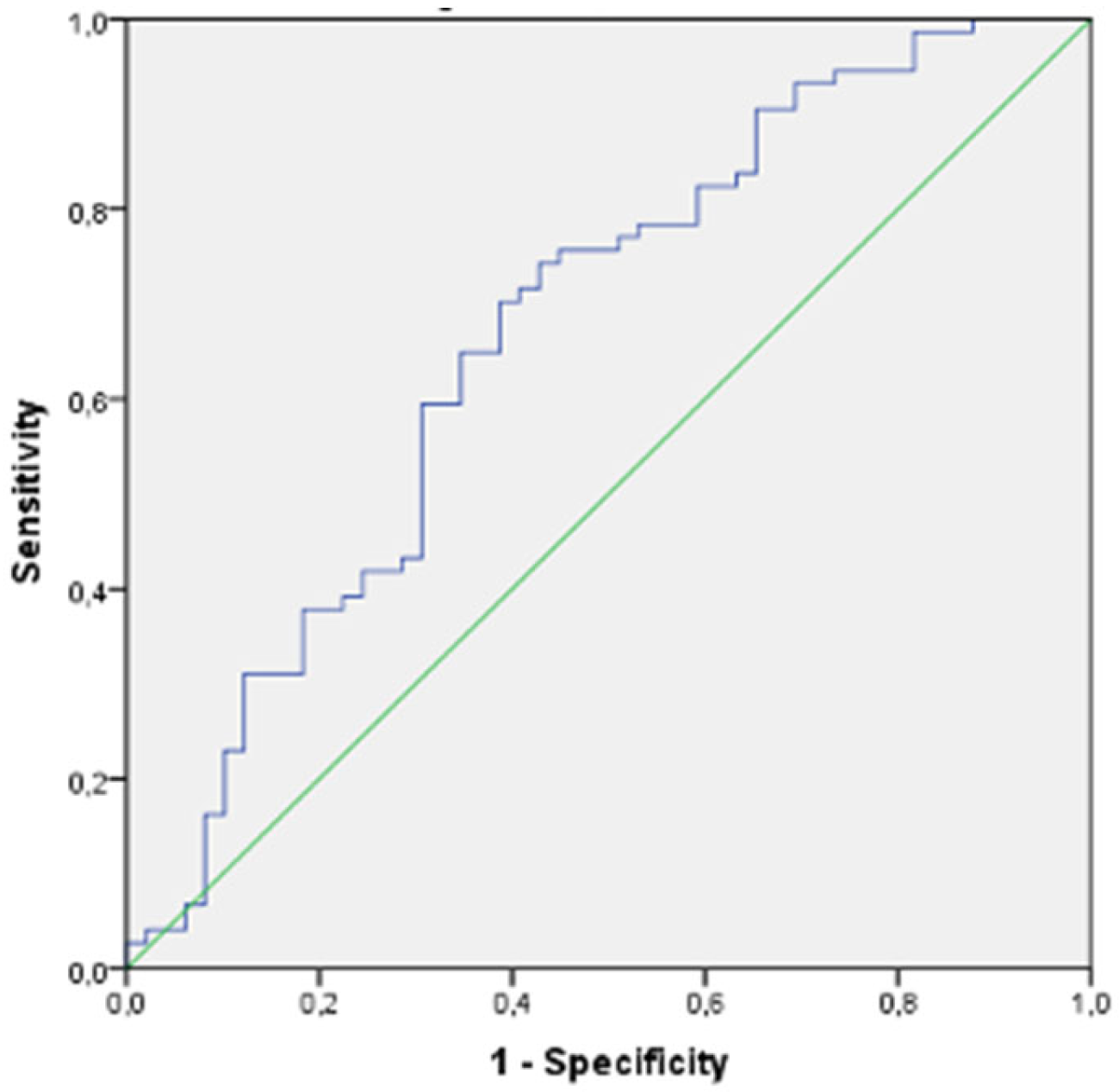
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. ESC Scientific Document Group. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Ulloque-Badaracco, J.R.; Hernandez-Bustamante, E.A.; Alarcon-Braga, E.A.; Mosquera-Rojas, M.D.; Campos-Aspajo, A.; Salazar-Valdivia, F.E.; Valdez-Cornejo, V.A.; Benites-Zapata, V.A.; Herrera-Añazco, P.; Valenzuela-Rodríguez, G.; et al. Atherogenic index of plasma and coronary artery disease: A systematic review. Open Med. 2022, 17, 1915–1926. [Google Scholar] [CrossRef]
- Attiq, A.; Afzal, S.; Ahmad, W.; Kandeel, M. Hegemony of inflammation in atherosclerosis and coronary artery disease. Eur. J. Pharmacol. 2024, 966, 176338. [Google Scholar] [CrossRef] [PubMed]
- Sudhir, K. Lipoprotein-associated phospholipase A2, vascular inflammation and cardiovascular risk prediction. Vasc. Health Risk Manag. 2006, 2, 153–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okan, T.; Topaloglu, C. Association of ratios of monocyte/high-density lipoprotein cholesterol and neutrophil/high-density lipoprotein cholesterol with atherosclerotic plaque type on coronary computed tomography. Cardiovasc. J. Afr. 2024, 34, 1–6. [Google Scholar]
- Matsuura, Y.; Kanter, J.E.; Bornfeldt, K.E. Highlighting Residual Atherosclerotic Cardiovascular Disease Risk. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e1–e9. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Ridker, P.M.; Rifai, N.; Rose, L.; Buring, J.E.; Cook, N.R. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N. Engl. J. Med. 2002, 347, 1557–1565. [Google Scholar] [CrossRef]
- Zhu, L.; Lu, Z.; Zhu, L.; Ouyang, X.; Yang, Y.; He, W.; Feng, Y.; Yi, F.; Song, Y. Lipoprotein ratios are better than conventional lipid parameters in predicting coronary heart disease in Chinese Han people. Kardiol. Pol. 2015, 73, 931–938. [Google Scholar] [CrossRef]
- Zhou, K.; Qin, Z.; Tian, J.; Cui, K.; Yan, Y.; Lyu, S. The Atherogenic Index of Plasma: A Powerful and Reliable Predictor for Coronary Artery Disease in Patients with Type 2 Diabetes. Angiology 2021, 72, 934–941. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Li, S.; Ma, Y.; Lin, J.; Wan, J.; Zhao, M. The atherogenic index of plasma (PA-I) is a predictor for the severity of coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1140215. [Google Scholar]
- Kurklu, H.A.; Tan, T.S.; Ozyuncu, N.; Baskovski, E.; Ozdol, C. Atherogenic Index of Plasma Predicts Obstructive Coronary Artery Disease in Patients with Stable Angina Pectoris. Diagnostics 2023, 13, 3249. [Google Scholar] [CrossRef] [PubMed]
- Demirtola, A.İ.; Erdöl, M.A.; Mammadli, A.; Göktuğ Ertem, A.; Yayla, Ç.; Akçay, A.B. Predicting coronary artery severity in patients undergoing coronary computed tomographic angiography: Insights from pan-immune inflammation value and atherogenic index of plasma. Nutr. Metab. Cardiovasc. Dis. 2024, in press. [CrossRef] [PubMed]
- Wu, X.; Qiu, W.; Yang, H.; Chen, Y.J.; Liu, J.; Zhao, G. Associations of the triglyceride-glucose index and atherogenic index of plasma with the severity of new-onset coronary artery disease in different glucose metabolic states. Cardiovasc. Diabetol. 2024, 23, 76. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Tan, Z.; Huang, Y.; Zhao, H.; Liu, M.; Yu, P.; Ma, J.; Zhao, Y.; Zhu, W.; Wang, J. Relationship between the triglyceride-glucose index and risk of cardiovascular diseases and mortality in the general population: A systematic review and meta-analysis. Cardiovasc. Diabetol. 2022, 21, 124. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [PubMed]
- Ormazabal, V.; Nair, S.; Elfeky, O.; Aguayom, C.; Salomon, C.; Zuñiga, F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018, 17, 122. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Angelopoulos, A.; Tsioufis, K.; Antoniades, C.; Tousoulis, D. Cardiovascular risk stratification by coronary computed tomography angiography imaging: Current state-of-the-art. Eur. J. Prev. Cardiol. 2022, 29, 608–624. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, J.E.; Schuijf, J.D.; de Graaf, F.R.; Boersma, E.; Pundziute, G.; Spanó, F.; Boogers, M.J.; Schalij, M.J.; Kroft, L.J.; de Roos, A.; et al. Diagnostic performance of non-invasive multidetector computed tomography coronary angiography to detect coronary artery disease using different endpoints: Detection of significant stenosis vs. detection of atherosclerosis. Eur. Heart J. 2011, 32, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Hong, L.; Han, Y.; Deng, C.; Chen, A. Correlation between atherogenic index of plasma and coronary artery disease in males of different ages: A retrospective study. BMC Cardiovasc. Disord. 2022, 22, 440. [Google Scholar] [CrossRef]
- Huang, H.; Yu, X.; Li, L.; Shi, G.; Li, F.; Xiao, J.; Yun, Z.; Cai, G. Atherogenic index of plasma is related to coronary atherosclerotic disease in elderly individuals: A cross-sectional study. Lipids Health Dis. 2021, 20, 68. [Google Scholar] [CrossRef]
- Wu, T.T.; Gao, Y.; Zheng, Y.Y.; Ma, Y.T.; Xie, X. Atherogenic index of plasma (PA-I): A novel predictive indicator for the coronary artery disease in postmenopausal women. Lipids Health Dis. 2018, 17, 197. [Google Scholar] [CrossRef] [PubMed]
- Özen, Y.; Özbay, B.M.; Yakut, I.; Kanal, Y.; Abdelmottelaeb, W.; Nriagu, B.N.; Salmon, J.T.S.; Quintanilla, B.; Munguía, C.; Reyes Castro, T.; et al. Sherpal Atherogenic index of plasma and triglyceride-glucose index to predict more advanced coronary artery diseases in patients with the first diagnosis of acute coronary syndrome. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3993–4005. [Google Scholar] [PubMed]
- Quispe, R.; Manalac, R.J.; Faridi, K.F.; Blaha, M.J.; Toth, P.P.; Kulkarni, K.R.; Nasir, K.; Virani, S.S.; Banach, M.; Blumenthal, R.S.; et al. Relationship of the triglyceride to high-density lipoprotein cholesterol (TG/HDLC) ratio to the remainder of the lipid profile: The Very Large Database of Lipids-4 (VLDL-4) study. Atherosclerosis 2015, 242, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Qi, J.; Mao, H.; Wang, N.; Ye, X.; Zhou, L.; Tong, G.; Yang, J.; Pan, H.; Huang, J. Coronary plaque tissue characterization in patients with premature coronary artery disease. Int. J. Cardiovasc. Imaging 2020, 36, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Yang, L.; Peng, Y.; Zhang, Z. The predictive value of triglyceride-glucose index for assessing the severity and MACE of premature coronary artery disease. Cardiovasc. J. Afr. 2024, 34, 1–6. [Google Scholar]
- Ivanova, E.A.; Myasoedova, V.A.; Melnichenko, A.A.; Grechko, A.V.; Orekhov, A.N. Small Dense Low-Density Lipoprotein as Biomarker for Atherosclerotic Diseases. Oxid. Med. Cell Longev. 2017, 2017, 1273042. [Google Scholar] [CrossRef] [PubMed]
- Arai, H.; Kokubo, Y.; Watanabe, M.; Sawamura, T.; Ito, Y.; Minagawa, A.; Okamura, T.; Miyamato, Y. Small dense low-density lipoproteins cholesterol can predict incident cardiovascular disease in an urban Japanese cohort: The Suita study. J. Atheroscler. Thromb. 2013, 20, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Dobiásová, M.; Frohlich, J. The plasma parameter log (TG/HDLC) as an atherogenic index: Correlation with lipoprotein particle size and esterification rate in apoB-lipoprotein-depleted plasma (FER(HDL)). Clin. Biochem. 2001, 34, 583–588. [Google Scholar] [CrossRef]
- Cai, G.; Shi, G.; Xue, S.; Lu, W. The atherogenic index of plasma is a strong and independent predictor for coronary artery disease in the Chinese Han population. Medicine 2017, 96, e8058. [Google Scholar] [CrossRef] [PubMed]
- Varbo, A.; Benn, M.; Tybjærg-Hansen, A.; Nordestgaard, B.G. Elevated remnant cholesterol causes both low-grade inflammation and ischemic heart disease, whereas elevated low-density lipoprotein cholesterol causes ischemic heart disease without inflammation. Circulation 2013, 128, 1298–1309. [Google Scholar] [CrossRef]
- Guo, X.; Ma, L. Inflammation in coronary artery disease-clinical implications of novel HDLCholesterol-related inflammatory parameters as predictors. Coron. Artery Dis. 2023, 34, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Saely, C.H.; Aczel, S.; Marte, T.; Langer, P.; Hoefle, G.; Drexel, H. The metabolic syndrome, insulin resistance, and cardiovascular risk in diabetic and nondiabetic patients. J. Clin. Endocrinol. Metab. 2005, 90, 5698–5703. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Vijayakumar, A.; Kahn, B.B. Metabolites as regulators of insulin sensitivity and metabolism. Nat. Rev. Mol. Cell Biol. 2018, 19, 654–672. [Google Scholar] [CrossRef] [PubMed]
- Razani, B.; Chakravarthy, M.V.; Semenkovich, C.F. Insulin resistance and atherosclerosis. Endocrinol. Metab. Clin. N. Am. 2008, 37, 603–621. [Google Scholar] [CrossRef]
- Scott, D.A.; Ponir, C.; Shapiro, M.D.; Chevli, P.A. Associations between insulin resistance indices and subclinical atherosclerosis: A contemporary review. Am. J. Prev. Cardiol. 2024, 18, 100676. [Google Scholar] [CrossRef]
- Minh, H.V.; Tien, H.A.; Sinh, C.T.; Thang, D.C.; Chen, C.H.; Tay, J.C.; Siddique, S.; Wang, T.D.; Sogunuru, G.P.; Chia, Y.C.; et al. Assessment of preferred methods to measure insulin resistance in Asian patients with hypertension. J. Clin. Hypertens. 2021, 23, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wu, K.; Lin, Y.; Huang, M.; Xie, S. Association of triglyceride glucose index with all-cause and cardiovascular mortality in the general population. Cardiovasc. Diabetol. 2023, 22, 320. [Google Scholar] [CrossRef] [PubMed]
- Packard, C.J. Triglyceride lowering 2.0: Back to the future? Eur. Heart J. 2020, 41, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Beverly, J.K.; Budoff, M.J. Atherosclerosis: Pathophysiology of insulin resistance, hyperglycemia, hyperlipidemia, and inflammation. J. Diabetes 2020, 12, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Collet, J.P.; Zeitouni, M.; Procopi, N.; Hulot, J.S.; Silvain, J.; Kerneis, M.; Thomas, D.; Lattuca, B.; Barthelemy, O.; Lavie-Badie, Y.; et al. ACTION Study Group. Long-Term Evolution of Premature Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 74, 1868–1878. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Liu, J.; Li, B.; Li, C.; Liu, Z.; Yang, M.; Zhang, Q.; Zhong, W.; Zhao, P. Serum lipid profiles in patients with acute myocardial infarction in Hakka population in southern China. Lipids Health Dis. 2017, 16, 246. [Google Scholar] [CrossRef] [PubMed]
- Khoja, A.; Andraweera, P.H.; Lassi, Z.S.; Ali, A.; Zheng, M.; Pathirana, M.M.; Aldridge, E.; Wittwer, M.R.; Chaudhuri, D.D.; Tavella, R.; et al. Risk Factors for Early-Onset versus Late-Onset Coronary Heart Disease (CHD): Systematic Review and Meta-Analysis. Heart Lung Circ. 2023, 32, 1277–1311. [Google Scholar] [CrossRef]
- Tian, X.; Chen, S.; Zuo, Y.; Zhang, Y.; Zhang, X.; Xu, Q.; Luo, Y.; Wu, S.; Wang, A. Association of lipid, inflammatory, and metabolic biomarkers with age at onset for incident cardiovascular disease. BMC Med. 2022, 20, 383. [Google Scholar] [CrossRef]
| Index | Formulae |
|---|---|
| Atherogenic Index (AI) | Non-HDLC/HDL C |
| Plasma Atherogenic Index (PA-I) | log (TG/HDLC) |
| Triglyceride/Glucose index (TGI) | Ln (TG × glucose/2) |
| Castelli Risk index-I | TC/HDLC |
| Castelli Risk index-II | LDLC/HDLC |
| Triglyceride/Glucose index (TGI) | Ln (TG × glucose/2) |
| Lipoprotein Combined Index (LCI) | (TC × Tg × LDLC)/HDLC |
| Remnant Lipoprotein Cholesterol (RLPC) | TC − (HDLC) − (LDLC) |
| Non HDL Cholesterol | TC − HDLC |
| Characteristics | Non-CAD Group n = 49 | CAD Group n = 74 | p-Value |
|---|---|---|---|
| Age (years) | 54.12 ± 5.67 | 52.45 ± 5.86 | 0.121 |
| Gender (female %) | 29/49 (59%) | 23/74 (31.1%) | 0.003 |
| Smoking | 12/49 (24.5%) | 33/74 (44.6%) | 0.035 |
| BMI (kg/m2) | 26.7 8 ± 4.62 | 28.15 ± 3.92 | 0.080 |
| AGATSTON | 0 | 194 ± 310.17 | 0.001 |
| Sytolic Blood Pressure (mmHg) | 130.51 ± 15.09 | 133.89 ±14.93 | 0.65 |
| Diastolic Blood Pressure (mmHg) | 78.65 ± 9.50 | 82.45 ± 10.63 | 0.138 |
| Fasting Blood Glucose (mmol/L) | 5.48 ± 1.01 | 5.77 ± 0.82 | 0.407 |
| Total Cholesterol (mmol/L) | 12.16 ± 2.15 | 13.16 ± 3.19 | 0.040 |
| Triglyceride (mmol/L) | 8.23 ± 5.12 | 10.67 ± 5.63 | 0.016 |
| LDLCholesterol (mmol/L) | 7.45 ± 2.08 | 8.45 ± 2.84 | 0.037 |
| HDL Cholesterol (mmol/L) | 3.11 ± 1.01 | 2.97 ± 2.00 | 0.642 |
| Non-HDL Cholesterol (mmol/L) | 9.05 ± 2.27 | 10.19 ± 3.94 | 0.069 |
| Total-C/HDLC (CR-I) | 4.26 ± 1.43 | 4.99 ± 1.72 | 0.015 |
| LDLC/HDLC (CR-II) | 2.62 ± 1.14 | 3.17 ± 1.28 | 0.018 |
| Non-HDLC/HDLC. (Atherogenic index) | 3.26 ± 1.43 | 3.99 ± 1.72 | 0.015 |
| Triglyceride/HDLC. | 3.22 ± 2.97 | 4.19 ± 2.81 | 0.072 |
| Lipoprotein combine index (LCI) = (TC × TG × LDL)/HDLC | 334.92 ± 445.64 | 527.17 ± 498.61 | 0.031 |
| Plasma Atherogenic index (PA-I) = log (TG/HDL C) | 0.37 ± 0.33 | 0.53 ± 0.27 | 0.005 |
| RLPC = TC − (HDLC) − (LDLC) | 1.59 ± 1.43 | 1.74 ± 3.18 | 0.764 |
| Triglyceride/Glucose Index = TGI = Ln (TG × glucose/2) | 8.71 ± 0.64 | 9.05 ± 0.60 | 0.004 |
| Characteristics | Non-CAD Group n = 41 | CAD Group n = 59 | p-Value |
|---|---|---|---|
| Age (years) | 65.63 ± 4.94 | 64.96 ± 5.56 | 0.121 |
| Gender (female %) | 22/41 (53.7%) | 13/59 (22%) | 0.001 |
| Smoking | 9/41 (22%) | 20/59 (33.9%) | 0.263 |
| BMI (kg/m2) | 29.12 ± 4.65 | 26.75 ± 3.26 | 0.004 |
| AGATSTON | 0 | 334.78 ± 405.05 | 0.001 |
| Systolic Blood Pressure (mmHg) | 136.78 ± 13.65 | 134.83 ± 17.09 | 0.545 |
| Diastolic Blood Pressure (mmHg) | 82.19 ± 11.18 | 79.01 ± 11.35 | 0.169 |
| Fasting Blood Glucose (mmol/L) | 5.55 ± 0.72 | 6.10 ± 0.85 | 0.166 |
| Total Cholesterol (mmol/L) | 12.68 ± 2.75 | 12.45 ± 2.48 | 0.665 |
| Triglyceride (mmol/L) | 9.02 ± 3.70 | 8.98 ± 4.98 | 0.970 |
| LDLCholesterol (mmol/L) | 8.26 ± 2.17 | 7.79 ± 2.22 | 0.298 |
| HDL Cholesterol (mmol/L) | 2.78 ± 0.58 | 2.82 ± 0.70 | 0.733 |
| Non-HDLCholesterol (mmol/L) | 9.89 ± 2.66 | 9.62 ± 2.38 | 0.589 |
| Total-C/HDLC (CR-I) | 4.71 ± 1.33 | 4.61 ± 1.32 | 0.708 |
| LDLC/HDLC (CR-II) | 3.08 ± 0.97 | 2.87 ± 0.93 | 0.282 |
| Non-HDLC/HDLC. (Atherogenic index) | 3.71 ± 1.33 | 3.61 ± 1.32 | 0.708 |
| Triglyceride/HDLC. | 3.55 ± 2.17 | 3.62 ± 2.87 | 0.903 |
| Lipoprotein combined index (LCI) = (TC × TG × LDL)/HDLC | 395.23 ± 318.88 | 369.22 ± 343.61 | 0.702 |
| RLPC = TC − (HDLC) − (LDLC) | 1.63 ± 1.32 | 1.82 ± 1.11 | 0.431 |
| Plasma Atherogenic Index (PA-I) = log (TG/HDL C) | 0.48 ± 0.22 | 0.45 ± 0.28 | 0.591 |
| Triglyceride/GlucoseIndex = Ln (TG × glucose/2) | 8.89 ± 0.44 | 8.92 ± 0.67 | 0.840 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okan, T.; Doruk, M.; Ozturk, A.; Topaloglu, C.; Dogdus, M.; Yilmaz, M.B. Evaluation of Plasma Atherogenic Index, Triglyceride-Glucose Index and Other Lipid Ratios as Predictive Biomarkers of Coronary Artery Disease in Different Age Groups. Diagnostics 2024, 14, 1495. https://doi.org/10.3390/diagnostics14141495
Okan T, Doruk M, Ozturk A, Topaloglu C, Dogdus M, Yilmaz MB. Evaluation of Plasma Atherogenic Index, Triglyceride-Glucose Index and Other Lipid Ratios as Predictive Biomarkers of Coronary Artery Disease in Different Age Groups. Diagnostics. 2024; 14(14):1495. https://doi.org/10.3390/diagnostics14141495
Chicago/Turabian StyleOkan, Taha, Mehmet Doruk, Ali Ozturk, Caner Topaloglu, Mustafa Dogdus, and Mehmet Birhan Yilmaz. 2024. "Evaluation of Plasma Atherogenic Index, Triglyceride-Glucose Index and Other Lipid Ratios as Predictive Biomarkers of Coronary Artery Disease in Different Age Groups" Diagnostics 14, no. 14: 1495. https://doi.org/10.3390/diagnostics14141495
APA StyleOkan, T., Doruk, M., Ozturk, A., Topaloglu, C., Dogdus, M., & Yilmaz, M. B. (2024). Evaluation of Plasma Atherogenic Index, Triglyceride-Glucose Index and Other Lipid Ratios as Predictive Biomarkers of Coronary Artery Disease in Different Age Groups. Diagnostics, 14(14), 1495. https://doi.org/10.3390/diagnostics14141495








