The Rationale and Design of the KOSovan Acute Coronary Syndrome (KOS-ACS) Registry
Abstract
1. Introduction
2. Description of KOS-ACS Registry
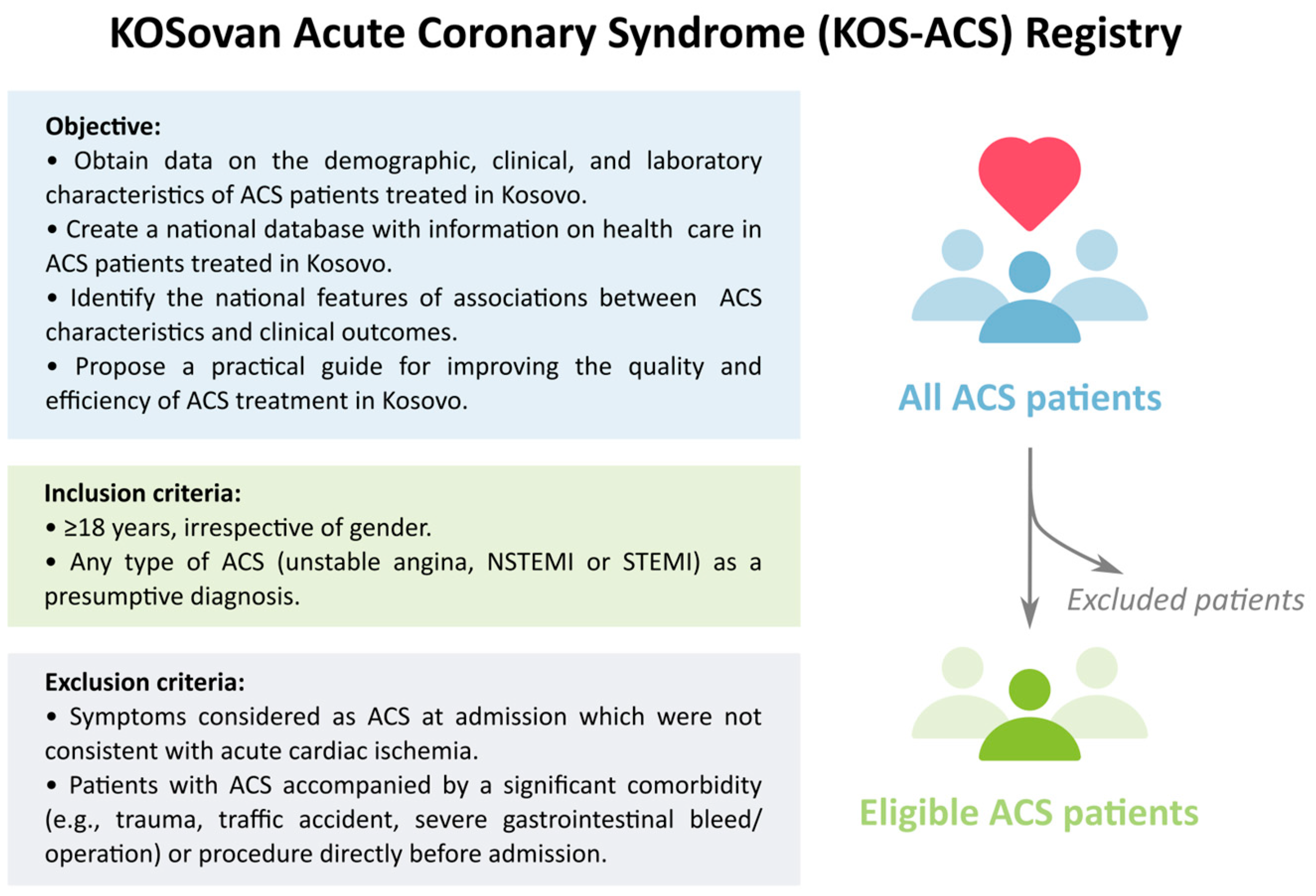
2.1. Objectives
2.2. Desing of the KOS-ACS Registry
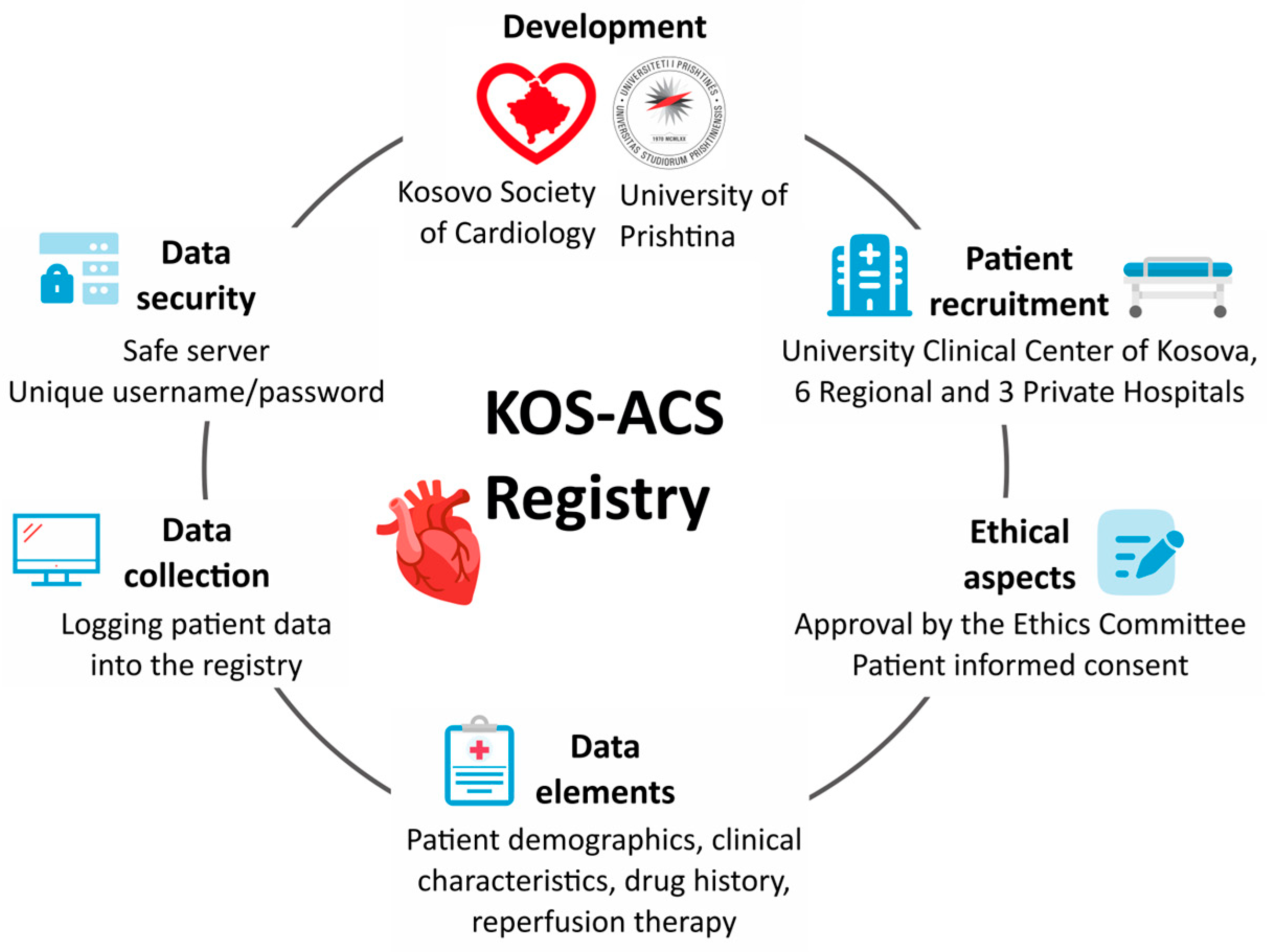
2.3. The Developers of the KOS-ACS Registry
2.4. Participation
2.5. Patients
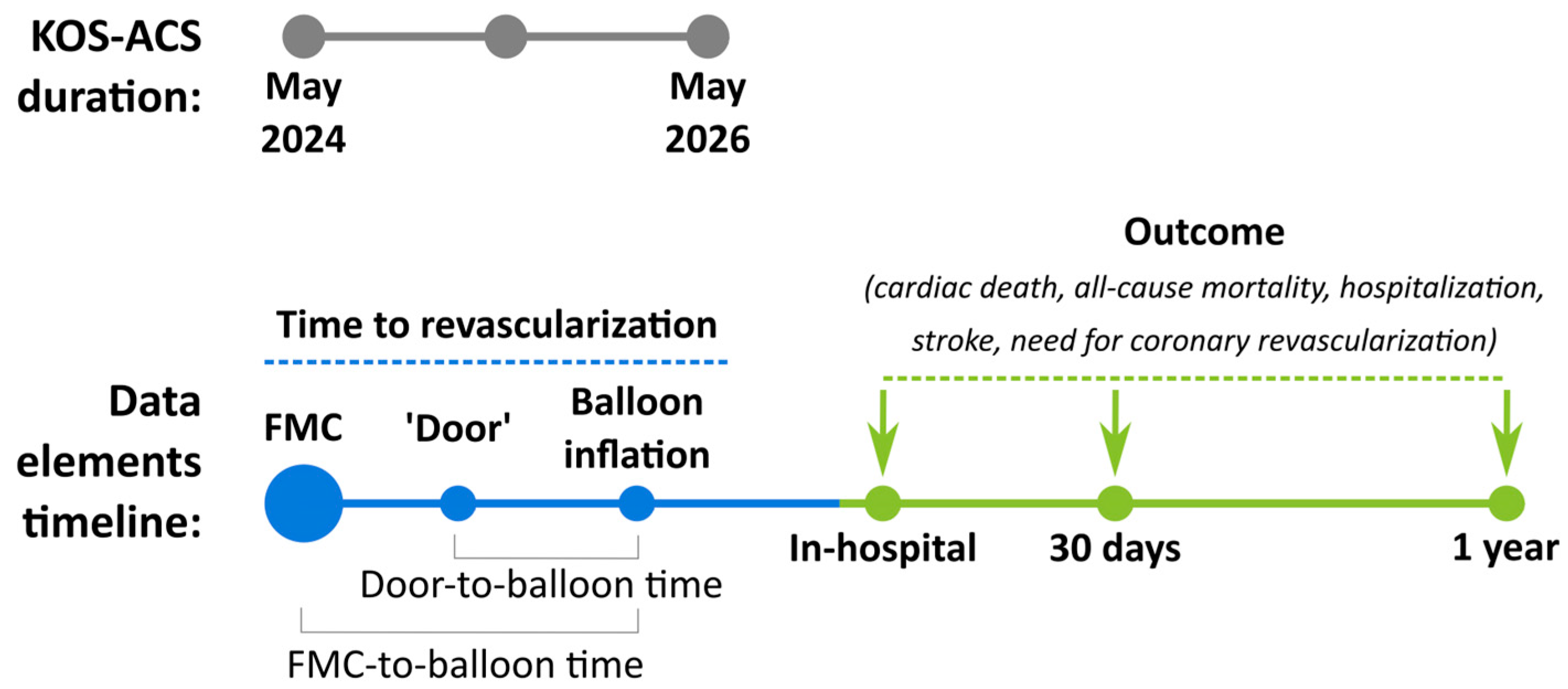
2.6. Data Elements
2.7. Data Collection
2.8. Data Security
2.9. Ethical Aspects
3. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chew, D.P.; Hyun, K.; Morton, E.; Horsfall, M.; Hillis, G.S.; Chow, C.K.; Quinn, S.; D’Souza, M.; Yan, A.T.; Gale, C.P.; et al. Objective Risk Assessment vs Standard Care for Acute Coronary Syndromes: A Randomized Clinical Trial. JAMA Cardiol. 2021, 6, 304–313. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.; Jones, D.; Wolfe, R. State-wide reduction in in-hospital cardiac complications in association with the introduction of a national standard for recognizing deteriorating patients. Resuscitation 2017, 121, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Bonello, L.; Laine, M.; Puymirat, E.; Lemesle, G.; Thuny, F.; Paganelli, F.; Michelet, P.; Roch, A.; Kerbaul, F.; Boyer, L. Timing of Coronary Invasive Strategy in Non-ST-Segment Elevation Acute Coronary Syndromes and Clinical Outcomes: An Updated Meta-Analysis. JACC Cardiovasc. Interv. 2016, 9, 2267–2276. [Google Scholar] [CrossRef]
- Kheifets, M.; Vaknin-Assa, H.; Greenberg, G.; Orvin, K.; Assali, A.; Kornowski, R.; Perl, L. Trends in ST-elevation myocardial infarction. Coron. Artery Dis. 2022, 31, e026811. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.A.; Eagle, K.A.; Gore, J.M.; Steg, P.G.; Anderson, F.A.; GRACE and GRACE2 Investigators. The Global Registry of Acute Coronary Events, 1999 to 2009—GRACE. Heart 2010, 96, 1095–1101. [Google Scholar] [CrossRef]
- Herrett, E.; Smeeth, L.; Walker, L.; Weston, C.; MINAP Academic Group. The Myocardial Ischaemia National Audit Project (MINAP). Heart 2010, 96, 1264–1267. [Google Scholar] [CrossRef]
- Leurent, G.; Garlantézec, R.; Auffret, V.; Hacot, J.P.; Coudert, I.; Filippi, E.; Rialan, A.; Moquet, B.; Rouault, G.; Gilard, M.; et al. Gender differences in presentation, management and in-hospital outcome in patients with ST-segment elevation myocardial infarction: Data from 5000 patients included in the ORBI prospective French regional registry. Arch. Cardiovasc. Dis. 2014, 107, 291–298. [Google Scholar] [CrossRef]
- Shaw, C.; Nitsch, D.; Steenkamp, R.; Junghans, C.; Shah, S.; O’donoghue, D.; Fogarty, D.; Weston, C.; Sharpe, C.C. Inpatient coronary angiography and revascularisation following non–ST-elevation acute coronary syndrome in patients with renal impairment: A cohort study using the Myocardial Ischaemia National Audit Project. PLoS ONE 2014, 9, e99925. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, P.; Gierlotka, M.; Gąsior, M.; Osadnik, T.; Hawranek, M.; Lekston, A.; Zembala, M.; Poloński, L. In-hospital and 12-month outcomes after acute coronary syndrome treatment in patients aged <40 years of age (from the Polish Registry of Acute Coronary Syndromes). Am. J. Cardiol. 2014, 114, 175–180. [Google Scholar]
- Gutierrez, J.A.; Karwatowska-Prokopczuk, E.; Murphy, S.A.; Belardinelli, L.; Farzaneh-Far, R.; Walker, G.; Morrow, D.A.; Scirica, B.M. Effects of ranolazine in patients with chronic angina in patients with and without percutaneous coronary intervention for acute coronary syndrome: Observations from the MERLIN-TIMI 36 trial. Clin. Cardiol. 2015, 38, 469–475. [Google Scholar] [CrossRef]
- Huo, Y.; Lee, S.W.; Sawhney, J.P.; Kim, H.S.; Krittayaphong, R.; Nhan, V.T.; Alonso-Garcia, A.; Han, Y.L.; Ge, J.; Chin, C.T.; et al. Rationale, design, and baseline characteristics of the EPICOR Asia study (Long-term Follow-up of Antithrombotic Management Patterns in Acute Coronary Syndrome Patients in Asia). Clin. Cardiol. 2015, 38, 511–519. [Google Scholar] [CrossRef]
- Reibis, R.; Völler, H.; Gitt, A.; Jannowitz, C.; Halle, M.; Pittrow, D.; Hildemann, S. Management of patients with ST-segment elevation or non–ST-segment elevation acute coronary syndromes in cardiac rehabilitation centers. Clin. Cardiol. 2014, 37, 213–221. [Google Scholar] [CrossRef]
- Thukkani, A.K.; Fonarow, G.C.; Cannon, C.P.; Cox, M.; Hernandez, A.F.; Peterson, E.D.; Peacock, W.F.; Laskey, W.K.; Schwamm, L.H.; Bhatt, D.L.; et al. Get with the Guidelines Steering Committee and Investigators. Quality of care for patients with acute coronary syndromes as a function of hospital revascularization capability: Insights from Get with the Guidelines-CAD. Clin. Cardiol. 2014, 37, 285–292. [Google Scholar] [CrossRef]
- Chan Pin Yin, D.R.P.P.; Vos, G.A.; van der Sangen, N.M.R.; Walhout, R.; Tjon Joe Gin, R.M.; Nicastia, D.M.; Langerveld, J.; Claassens, D.M.F.; Gimbel, M.E.; Azzahhafi, J.; et al. Rationale and Design of the Future Optimal Research and Care Evaluation in Patients with Acute Coronary Syndrome (FORCE-ACS) Registry: Towards “Personalized Medicine” in Daily Clinical Practice. J. Clin. Med. 2020, 9, 3173. [Google Scholar] [CrossRef]
- Wang, G.; Chen, X.H.; Li, S.Y.; Zhang, Z.K.; Gong, W.; Yan, Y.; Nie, S.P.; Henriques, J.P. Effect of complete revascularization in acute coronary syndrome after 75 years old: Insights from the BleeMACS registry. J. Geriatr. Cardiol. 2023, 20, 728–736. [Google Scholar] [CrossRef]
- Fox, K.A. Registries and surveys in acute coronary syndrome. Eur. Heart J. 2006, 27, 2260–2262. [Google Scholar] [CrossRef]
- Gitt, A.K.; Bueno, H.; Danchin, N.; Fox, K.; Hochadel, M.; Kearney, P.; Maggioni, A.P.; Opolski, G.; Seabra-Gomes, R.; Weidinger, F. The role of cardiac registries in evidence-based medicine. Eur. Heart J. 2010, 31, 525–529. [Google Scholar] [CrossRef] [PubMed]
- Bradley, E.H.; Nallamothu, B.K.; Herrin, J.; Ting, H.H.; Stern, A.F.; Nembhard, I.M.; Yuan, C.T.; Green, J.C.; Kline-Rogers, E.; Wang, Y.; et al. National efforts to improve door-to-balloon time: Results from the Door-to-Balloon Alliance. J. Am. Coll. Cardiol. 2009, 54, 2423–2429. [Google Scholar] [CrossRef] [PubMed]
- Widimsky, P.; Wijns, W.; Fajadet, J.; de Belder, M.; Knot, J.; Aaberge, L.; Andrikopoulos, G.; Baz, J.A.; Betriu, A.; Claeys, M.; et al. Reperfusion therapy for ST-elevation acute myocardial infarction in Europe: Description of the current situation in 30 countries. Eur. Heart J. 2010, 31, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Bar, O.; Elias, A.; Halhal, B.; Marcusohn, E. Time to coronary catheterization in patients with non-ST-segment elevation acute coronary syndrome and high Global Registry of Acute Coronary Events score. J. Cardiovasc. Med. 2024, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Eggers, K.M.; James, S.K.; Jernberg, T.; Lindahl, B. Timing of coronary angiography in patients with non-ST-elevation acute coronary syndrome: Long-term clinical outcomes from the nationwide SWEDEHEART registry. EuroIntervention 2022, 18, 582–589. [Google Scholar] [CrossRef]
- Popova, Y.V.; Kiselev, A.R.; Sagaydak, O.V.; Posnenkova, O.M.; Gridnev, V.I.; Oshchepkova, E.V. Application of the Appropriate Use Criteria for Coronary Revascularization in Patients with Acute Coronary Syndrome in the Russian Federation: Data from the Federal Registry. Eurasian J. Med. 2021, 53, 96–101. [Google Scholar] [CrossRef]
- Sawano, M.; Kohsaka, S.; Ishii, H.; Numasawa, Y.; Yamaji, K.; Inohara, T.; Amano, T.; Ikari, Y.; Nakamura, M. One-Year Outcome After Percutaneous Coronary Intervention for Acute Coronary Syndrome—An Analysis of 20,042 Patients from a Japanese Nationwide Registry. Circ. J. 2021, 85, 1756–1767. [Google Scholar] [CrossRef]
- Hoedemaker, N.P.G.; de Winter, R.J.; Hof, A.V.; Kolkman, E.; Damman, P. Optimal Medical Therapy Prescription in Patients with Acute Coronary Syndrome in the Netherlands: A Multicenter Pilot Registry. Am. J. Cardiovasc. Drugs 2021, 21, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.; Paradkar, A.; Goldenberg, I.; Shlomo, N.; Cohen, T.; Kornowski, R.; Eisen, A. Temporal Trends Analysis of the Characteristics, Management, and Outcomes of Women With Acute Coronary Syndrome (ACS): ACS Israeli Survey Registry 2000–2016. J. Am. Heart Assoc. 2020, 9, e014721. [Google Scholar] [CrossRef]
- Hoedemaker, N.P.G.; Damman, P.; Bosker, H.A.; Danse, P.W.; Liem, A.H.; Geerdes, B.; van Laarhoven, H.; de Winter, R.J.; NVVC NSTEMI-ACS Project Group. Treatment patterns of non-ST-elevation acute coronary syndrome patients presenting at non-PCI centres in the Netherlands and possible logistical consequences of adopting same-day transfer to PCI centres: A registry-based evaluation. Neth Heart J. 2019, 27, 191–199. [Google Scholar] [CrossRef]
- Ludman, P.; Zeymer, U.; Danchin, N.; Kala, P.; Laroche, C.; Sadeghi, M.; Caporale, R.; Shaheen, S.M.; Legutko, J.; Iakobishvili, Z.; et al. Care of patients with ST-elevation myocardial infarction: An international analysis of quality indicators in the acute coronary syndrome STEMI Registry of the EURObservational Research Programme and ACVC and EAPCI Associations of the European Society of Cardiology in 11,462 patients. Eur. Heart J. Acute Cardiovasc. Care 2023, 12, 22–37. [Google Scholar]
- Bajraktari, G.; Thaqi, K.; Pacolli, S.; Gjoka, S.; Rexhepaj, N.; Daullxhiu, I.; Sylejmani, X.; Elezi, S. In-hospital mortality following acute myocardial infarction in Kosovo: A single center study. Ann. Saudi Med. 2008, 28, 430–434. [Google Scholar] [PubMed]
- Gashi, M.; Bajraktari, G.; Gashi, S.; Ahmeti, H.; Degoricija, V. Correlates of in-hospital mortality in patients with acute coronary syndrome in Kosovo. Acta Clin. Croat. 2022, 61, 19–29. [Google Scholar] [CrossRef]
- Poniku, A.; Batalli, A.; Shita, D.; Rexhaj, Z.; Ferati, A.; Leka, R.; Bajraktari, A.; Abdyli, G.; Haliti, E.; Ibrahimi, P.; et al. Smoking and hypertriglyceridemia predict STEMI in Kosovo patients with acute myocardial infarction. Preprint 2023, 2023090861. [Google Scholar] [CrossRef]
- Byrne, R.A.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. 2023, 44, 3720–3826. [Google Scholar] [CrossRef]
- Zeymer, U.; Ludman, P.; Danchin, N.; Kala, P.; Laroche, C.; Sadeghi, M.; Caporale, R.; Shaheen, S.M.; Legutko, J.; Iakobsishvili, Z.; et al. Reperfusion therapies and in-hospital outcomes for ST-elevation myocardial infarction in Europe: The ACVC-EAPCI EORP STEMI Registry of the European Society of Cardiology. Eur. Heart J. 2021, 42, 4536–4549. [Google Scholar] [CrossRef]
- Askanas, Z.; Rywik, S.; Szczypiorowski, B.; Kapuścińska, E.; Korewicki, J. The registration program of cases of recent myocardial infarction conducted within the framework of medical care of the Warsaw emergency service. Cor Vasa 1970, 12, 169–177. [Google Scholar]
- Soufiani, A.; Chraibi, H.; Asfalou, I.; Ouafi, N.E.; Hattaoui, M.E.; Habbal, R.; Chaib, A.; Fellat, R.; Akoudad, H.; Benyass, A.; et al. The national moroccan registry of ST-elevation myocardial infarction (MR-MI). BMC Cardiovasc. Disord. 2023, 23, 419. [Google Scholar] [CrossRef]
- Bugiardini, R.; Ricci, B.; Cenko, E.; Vasiljevic, Z.; Kedev, S.; Davidovic, G.; Zdravkovic, M.; Miličić, D.; Dilic, M.; Manfrini, O.; et al. Delayed Care and Mortality Among Women and Men with Myocardial Infarction. J. Am. Heart Assoc. 2017, 6, e005968. [Google Scholar] [CrossRef]
- Cenko, E.; Ricci, B.; Kedev, S.; Vasiljevic, Z.; Dorobantu, M.; Gustiene, O.; Knežević, B.; Miličić, D.; Dilic, M.; Trninic, D.; et al. Reperfusion therapy for ST-elevation acute myocardial infarction in Eastern Europe: The ISACS-TC registry. Eur. Heart J. Qual. Care Clin. Outcomes 2016, 2, 45–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bajraktari, G.; Elezi, S.; Bytyci, I.; Ibrahimi, P.; Abdyli, G.; Pllana-Pruthi, E.; Karahoda, R.; Batalli, A.; Poniku, A.; Shatri, M.; et al. The Rationale and Design of the KOSovan Acute Coronary Syndrome (KOS-ACS) Registry. Diagnostics 2024, 14, 1486. https://doi.org/10.3390/diagnostics14141486
Bajraktari G, Elezi S, Bytyci I, Ibrahimi P, Abdyli G, Pllana-Pruthi E, Karahoda R, Batalli A, Poniku A, Shatri M, et al. The Rationale and Design of the KOSovan Acute Coronary Syndrome (KOS-ACS) Registry. Diagnostics. 2024; 14(14):1486. https://doi.org/10.3390/diagnostics14141486
Chicago/Turabian StyleBajraktari, Gani, Shpend Elezi, Ibadete Bytyci, Pranvera Ibrahimi, Genc Abdyli, Edita Pllana-Pruthi, Rona Karahoda, Arlind Batalli, Afrim Poniku, Mentor Shatri, and et al. 2024. "The Rationale and Design of the KOSovan Acute Coronary Syndrome (KOS-ACS) Registry" Diagnostics 14, no. 14: 1486. https://doi.org/10.3390/diagnostics14141486
APA StyleBajraktari, G., Elezi, S., Bytyci, I., Ibrahimi, P., Abdyli, G., Pllana-Pruthi, E., Karahoda, R., Batalli, A., Poniku, A., Shatri, M., Gashi, D., Bajraktari, A., Shatri, F., & Henein, M. Y., on behalf of KOS-ACS Investigators. (2024). The Rationale and Design of the KOSovan Acute Coronary Syndrome (KOS-ACS) Registry. Diagnostics, 14(14), 1486. https://doi.org/10.3390/diagnostics14141486







