Understanding the Dynamics of Inflammatory Cytokines in Endodontic Diagnosis: A Systematic Review
Abstract
1. Introduction
1.1. Inflammation
1.2. Immune System
1.3. Cytokines
1.4. Diagnosis
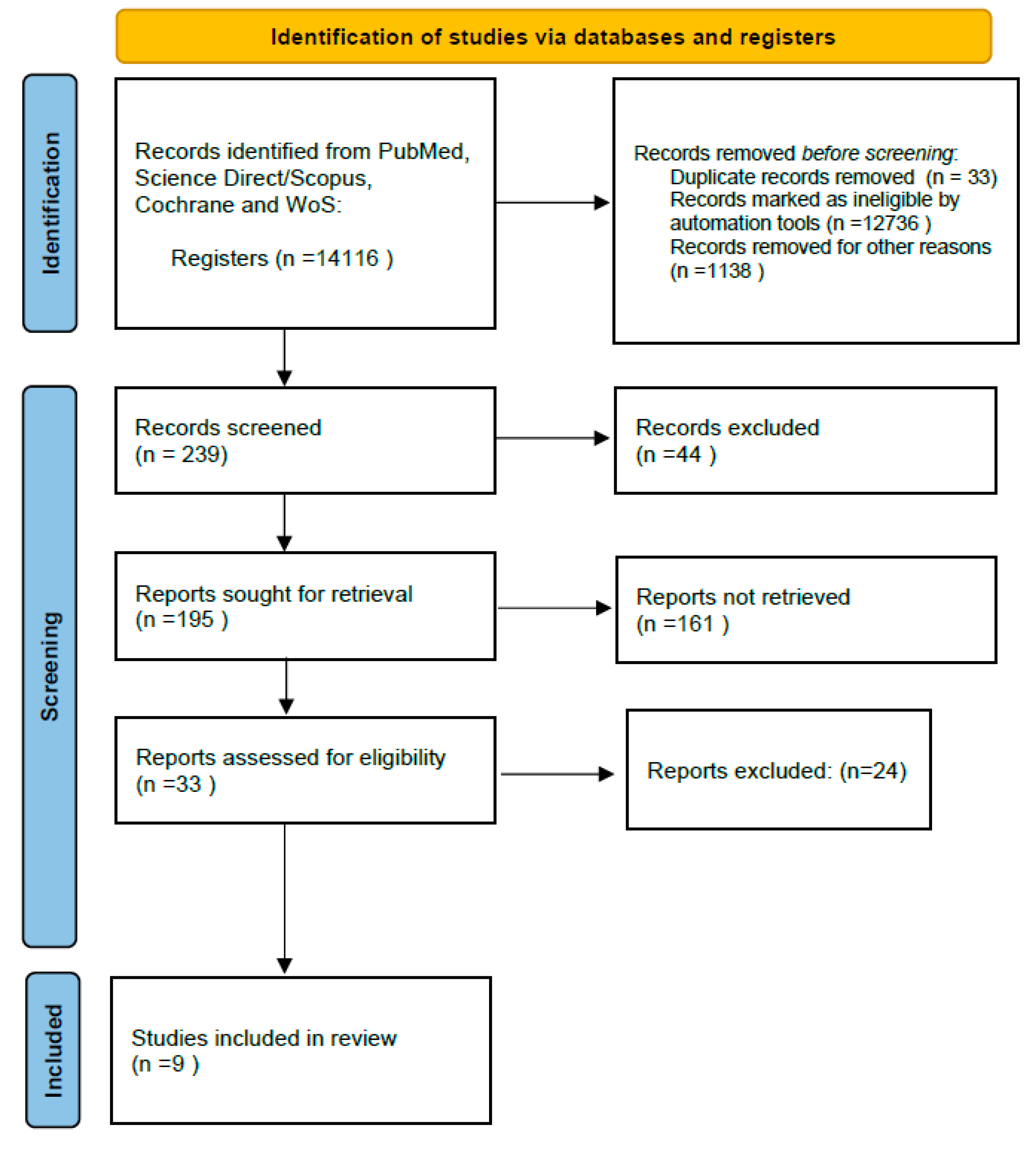
2. Materials and Methods
2.1. Criteria for Considering the Selected Studies
- -
- Patient or Population: children with permanent dentition and adults of all ages presenting reversible or irreversible pulpitis, symptomatic apical periodontitis, asymptomatic apical periodontitis, and/or reinfection of a previous root canal treatment.
- -
- Type of intervention: diagnosis based on the identification of cytokines (type and quantity), pathology of the pulp and symptomatology in a review on dental examination, emergency, and additional tests such as radiographs.
- -
- Comparison: how studies analyse inflammation, cytokines, and pathology to reach a diagnosis: systematic reviews, meta-analysis, randomised controlled studies, and controlled clinical trials. These analyse the extirpated pulp by extracting it partially and treating the rest or by extracting the tooth.
- -
- Outcome measures: modification of the endodontic diagnosis in daily practice. Different approach to investigate an inflamed pulp.
2.2. Search Strategy and Databases
- PubMed: “root canal therapy” or “root canal debridement” or “root canal treatment” or “endodon*” AND “inflamm*” AND “cytokin*”.
- Other combinations in other databases: “root canal therapy” AND “inflammation” OR “cytokine” AND “endodontic*”.
- (“pulp inflammation” OR “dental pulp”) AND (“inflam*” AND “cytokin*”) AND “diagnosis”.
- (“pulp NEXT inflammation” OR “dental pulp”) AND (“inflam*” AND “cytokin*”) AND “TREATMENT”
2.3. Study Selection
2.3.1. Included Studies
- -
- Articles that are Level A or B of evidence-based systematic reviews, meta-analysis, randomised controlled trials, controlled clinical trials, and cohort studies. Articles published in peer-reviewed scientific journals in the English language, published between 2014 and 2023.
- -
- Articles will be related and relevant to the title of the systematic review: “Influence of inflammatory cytokines in the endodontic diagnosis”.
- -
- These articles need to be related to inflammation (including pulpitis and apical periodontitis).
- -
- Articles found by citation chaining and relevant to the topic will be considered.
2.3.2. Excluded Studies
- -
- Studies that do not meet the inclusion criteria. Low-level evidence studies (Levels C, D, E): case reports, case series, case–control studies, cross-sectional studies, and expert opinions.
- -
- Articles related to systemic inflammation and diseases (non-endodontic related such as Arthritis).
- -
- Articles related to inflammation and periodontitis (commonly known as “gum disease”).
2.4. Data Extraction and Management
2.5. Quality Assessment
- -
- Two reviewers made this stage following the JBI (The Joanna Briggs Institute) appraisal tool. It evaluates the quality of several types of studies, such as cohort studies or systematic reviews [31].
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, C.; Abbott, P. An overview of the dental pulp: Its functions and responses to injury. Aust. Dent. J. 2007, 52, S4–S6. [Google Scholar] [CrossRef] [PubMed]
- Bergenholtz, G.; Mjör, I.; Cotton, W.; Hanks, C.; Kim, S.; Torneck, C.; Trowbridge, H. Consensus Report. J. Dent. Res. 1985, 64, 631–633. [Google Scholar] [CrossRef] [PubMed]
- Colombo, J.S.; Moore, A.N.; Hartgerink, J.D.; D’souza, R.N. Scaffolds to control inflammation and facilitate dental pulp regeneration. J. Endod. 2014, 40, S6–S12. [Google Scholar] [CrossRef] [PubMed]
- Henriques, L.C.F.; de Brito, L.C.N.; Tavares, W.L.F.; Vieira, L.Q.; Sobrinho, A.P.R. cytokine analysis in lesions refractory to endodontic treatment. J. Endod. 2011, 37, 1659–1662. [Google Scholar] [CrossRef] [PubMed]
- Dal-Fabbro, R.; Swanson, W.B.; Capalbo, L.C.; Sasaki, H.; Bottino, M.C. Next-generation biomaterials for dental pulp tissue immunomodulation. Dent. Mater. 2023, 39, 333–349. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.B.; Neto, A.P.d.S.; Maia, S.M.A.S.; Guimaraes, C.d.S.; Quidute, I.L.; Carvalho, A.d.A.T.; Junior, S.A.; Leao, J.C. The Role of TNF-α as a Proinflammatory Cytokine in Pathological Processes. Open Dent. J. 2019, 13, 332–338. [Google Scholar] [CrossRef]
- Maia, L.; Antonio, A. Systematic Reviews in Dental Research. A Guideline. J. Clin. Pediatr. Dent. 2012, 37, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Ajuz, N.C.; Antunes, H.; Mendonca, T.A.; Pires, F.R.; Siqueira, J.F., Jr.; Armada, L. Immunoexpression of interleukin 17 in apical periodontitis lesions. J. Endod. 2014, 40, 1400–1403. [Google Scholar] [CrossRef] [PubMed]
- Andrade, A.L.; Nonaka, C.F.; Gordon-Nunez, M.A.; Freitas Rde, A.; Galvao, H.C. Immunoexpression of interleukin 17, transforming growth factor beta1, and forkhead box P3 in periapical granulomas, radicular cysts, and residual radicular cysts. J. Endod. 2013, 39, 990–994. [Google Scholar] [CrossRef]
- Meschi, N.; Patel, B.; Ruparel, N.B. Material Pulp Cells and Tissue Interactions. J. Endod. 2020, 46, S150–S160. [Google Scholar] [CrossRef]
- Xiong, H.; Wei, L.; Peng, B. The Presence and involvement of interleukin-17 in apical periodontitis. Int. Endod. J. 2019, 52, 1128–1137. [Google Scholar] [CrossRef]
- Braz-Silva, P.H.; Bergamini, M.L.; Mardegan, A.P.; De Rosa, C.S.; Hasseus, B.; Jonasson, P. Inflammatory profile of chronic apical periodontitis: A literature review. Acta Odontol. Scand. 2019, 77, 173–180. [Google Scholar] [CrossRef]
- Rechenberg, D.-K.; Galicia, J.C.; Peters, O.A. Biological markers for pulpal inflammation: A systematic review. PLoS ONE 2016, 11, e0167289. [Google Scholar] [CrossRef]
- Marinho, A.C.; Martinho, F.C.; Leite, F.R.; Nascimento, G.G.; Gomes, B.P. Proinflammatory activity of primarily infected endodontic content against macrophages after different phases of the root canal therapy. J. Endod. 2015, 41, 817–823. [Google Scholar] [CrossRef]
- Wang, L.; Yang, F.; Qiu, Y.; Ye, L.; Song, D.; Huang, D. The Potential Roles of T Cells in Periapical Lesions. J. Endod. 2022, 48, 70–79. [Google Scholar] [CrossRef]
- Hussein, H.; Kishen, A. Local Immunomodulatory Effects of Intracanal Medications in Apical Periodontitis. J. Endod. 2022, 48, 430–456. [Google Scholar] [CrossRef]
- Martinho, F.C.; Nascimento, G.G.; Leite, F.R.; Gomes, A.P.; Freitas, L.F.; Camões, I.C. Clinical influence of different intracanal medications on th1-type and th2-type cytokine responses in apical periodontitis. J. Endod. 2015, 41, 169–175. [Google Scholar] [CrossRef]
- Soh, J.A.; Sheriff, S.O.; Ramar, N.A.; Pulikkotil, S.J.; Nagendrababu, V.; Neelakantan, P.; Amalraj, F.D. Effect of root canal debridement on inflammatory cytokine levels. Aust. Endod. J. 2019, 45, 171–176. [Google Scholar] [CrossRef]
- Rechenberg, D.-K.; Bostanci, N.; Zehnder, M.; Belibasakis, G.N. Periapical fluid RANKL and IL-8 are differentially regulated in pulpitis and apical periodontitis. Cytokine 2014, 69, 116–119. [Google Scholar] [CrossRef]
- Gomes, B.P.F.d.A.; Herrera, D.R. Etiologic role of root canal infection in apical periodontitis and its relationship with clinical symptomatology. Braz. Oral Res. 2018, 32, 82–110. [Google Scholar] [CrossRef]
- Azuma, M.M.; Samuel, R.O.; Gomes-Filho, J.E.; Dezan-Junior, E.; Cintra, L.T.A. The role of IL-6 on apical periodontitis: A systematic review. Int. Endod. J. 2014, 47, 615–621. [Google Scholar] [CrossRef]
- Sabeti, M.A.; Nikghalb, K.D.; Pakzad, R.; Fouad, A.F. Expression of selected inflammatory mediators with different clinical characteristics of pulpal inflammation. J. Endod. 2024, 50, 336–343. [Google Scholar] [CrossRef]
- Kahler, B.; Taha, N.; Lu, J.; Saoud, T. Vital pulp therapy for permanent teeth with diagnosis of irreversible pulpitis: Biological basis and outcome. Aust. Dent. J. 2023, 68, S110–S122. [Google Scholar] [CrossRef]
- Philip, N.; Suneja, B. Minimally invasive endodontics: A new era for pulpotomy in mature permanent teeth. Br. Dent. J. 2022, 233, 1035–1041. [Google Scholar] [CrossRef]
- Hahn, C.-L.; Liewehr, F.R. Relationships between Caries Bacteria, Host Responses, and Clinical Signs and Symptoms of Pulpitis. J. Endod. 2007, 33, 213–219. [Google Scholar] [CrossRef]
- Lancaster, P.E.; Craddock, H.L.; Carmichael, F.A. Estimation of remaining dentine thickness below deep lesions of caries. Br. Dent. J. 2011, 211, E20. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (Updated February 2022). Cochrane. 2022. Available online: https://www.training.cochrane.org/handbook (accessed on 24 January 2024).
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef]
- Boland, A.; Cherry, M.G.; Dickson, R. Doing a Systematic Review: A Student’s Guide, 2nd ed.; SAGE: Oaks, CA, USA, 2017. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.; Holly, C.; Kahlil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an Umbrella review approach. Int. J. Evid. Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Cooper, P.R.; Holder, M.J.; Smith, A.J. Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. J. Endod. 2014, 40 (Suppl. S4), S46–S51. [Google Scholar] [CrossRef]
- Khorasani, M.M.Y.; Hassanshahi, G.; Brodzikowska, A.; Khorramdelazad, H. Role(s) of cytokines in pulpitis: Latest evidence and therapeutic approaches. Cytokine 2020, 126, 154896. [Google Scholar] [CrossRef]
- Zanini, M.; Meyer, E.; Simon, S. Pulp Inflammation Diagnosis from Clinical to Inflammatory Mediators: A Systematic Review. J. Endod. 2017, 43, 1033–1051. [Google Scholar] [CrossRef]
- Hirsch, V.; Wolgin, M.; Mitronin, A.V.; Kielbassa, A.M. Inflammatory cytokines in normal and irreversibly inflamed pulps: A systematic review. Arch. Oral Biol. 2017, 82, 38–46. [Google Scholar] [CrossRef]
- Arora, S.; Cooper, P.R.; Friedlander, L.T.; Rizwan, S.; Seo, B.; Rich, A.M.; Hussaini, H.M. Potential application of immunotherapy for modulation of pulp inflammation: Opportunities for vital pulp treatment. Int. Endod. J. 2021, 54, 1263–1274. [Google Scholar] [CrossRef]
- Arruda-Vasconcelos, R.; Louzada, L.M.; Feres, M.; Tomson, P.L.; Cooper, P.R.; Gomes, B.P.F.A. Investigation of microbialprofile, levels of endotoxin and lipoteichoic acid in teethwith symptomatic irreversible pulpitis: A clinical study. Int. Endod. J. 2020, 54, 46–60. [Google Scholar] [CrossRef]
- Neri, D. Antibody-cytokine fusions: Versatile productsfor the modulation of anticancer immunity. Cancer Immunol. Res. 2019, 7, 348–354. [Google Scholar] [CrossRef]
| Article, Year, Author | Cytokines Studied | Objective | Results |
|---|---|---|---|
| The role of IL-6 on apical periodontitis: A systematic review. (2014) Azuma et al. [26]. | IL-6 | Examine the current knowledge of the role of IL-6 in apical periodontitis and if it could serve as a measure for differential diagnosis or as a biomarker that can further predict the progression of bone resorption. | IL-6 may increase levels of inflammation and reabsorbing bone in the presence of infection. IL-1 and TNF-α also induce bone resorptions. Further studies are needed to assess the relationship between specific cytokines and apical periodontitis. |
| Inflammatory profile of chronic apical periodontitis: a literature review. (2009, Braz Silva et al.) [12] | IL-17, TGF-β, IL-8, IL-6, TNF-α. | Review the inflammatory biomarkers related to apical periodontitis. | Different inflammatory cells and their byproducts are involved in the creation of apical periodontitis. The most important cytokines identified: IL-17, TGF-β, IL-8, IL-6, TNF-α. |
| Inflammation and regeneration in the dentin-pulp complex: A double-edged sword. (2014, Cooper et al.) [32] | Summarise and clarify the complex signalling during the invasion of bacteria, inflammatory cells, and immune system activation | The effects mediators are temporal context dependent. Further research is needed between inflammation and regenerative responses. | |
| Role(s) of cytokines in pulpitis: Latest evidence and therapeutic approaches. (2017, Khorasani et al.) [33] | IL-8, IL-6, IL-1, IL-2. | Describe the role of cytokines in pulpitis. | Inflammatory cytokines play an important role and regulate the intensity of the immune response against infection. Some mentioned: IL-8, IL-6, IL-1, IL-2. |
| The Presence and involvement of interleukin-17 in apical periodontitis. (2019, Xiong et al.) [11]. | IL 17 | Reviews recent studies regarding the collective in vitro, in vivo, and clinical evidence of the presence and involvement of IL-17 in AP | Evidence for the presence of IL-17 in AP from human and animal models is clear. However, there is relatively little information currently available that would highlight the specific role of IL-17 in AP |
| Proinflammatory activity of primarily infected endodontic content against macrophages after different phases of the root canal therapy. (2015, Marinho et al.) [14] | IL-1β and TNF-α | Investigate the levels of endotoxins in teeth with apical periodontitis and determine the inflammatory mediators. | IL-1β and TNF-α were reduced when the levels of endotoxins decreased. These were minimised because of chemo-mechanical debridement of the root canal and, consequently, less activation of the proinflammatory cells such as macrophages. |
| Periapical fluid RANKL and IL-8 are differentially regulated in pulpitis and apical periodontitis. (Rechenberg et al., 2014) [19] | IL-8 | Research the levels of RANKL, OPG, and IL-8 in periapical tissue fluid of human teeth diagnosed with irreversible pulpitis and apical periodontitis. | Results suggest that periapical bone resorption, determined by RANKL, occurs before inflammatory cell recruitment signalling, determined by IL-8. |
| Pulp Inflammation Diagnosis from Clinical to Inflammatory Mediators: A Systematic Review. (Zanini et al., 2017) [34] | Review inflammatory mediator expression in the context of clinical diagnosis to evaluate pulp inflammation severity. | Clinical irreversible pulpitis is related to specific levels of inflammatory mediator expression. The difference between reversible and irreversible is both quantitative and qualitative. | |
| Inflammatory cytokines in normal and irreversibly inflamed pulps: A systematic review. (2017, Hirsch et al.) [35] | IL-6, IL-8, IL-2, TNF- α. | Review literature regarding the inflammatory process and pulpitis | inconsistencies between studies exist and therefore, it is difficult to select just one specific cytokine suitable for testing. Some cytokines mentioned: IL-6, IL-8, IL-2, TNF-α. |
| Healthy Pulp/Reversible Pulpitis | Irreversible Pulpitis (Increased Levels) | Pulpal Necrosis/Apical Periodontitis (Increased Levels) |
|---|---|---|
| IL-4 | IL-1 β | IL-1 |
| IL-10 | IL-2 * | IL-6 |
| Low levels of IL-2 * | IL-6 | IL-8 |
| Low levels of IL-8 | IL-8 | IL-17 |
| Low levels of TNF-α | TNF-α | TNF-α |
| MMP-3 | INF-y | TFG-β |
| MMP-9 | RANK-L/OPG system | |
| MMP-9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero-Navarro, I.; Irigoyen-Camacho, M.E.; Zepeda-Zepeda, M.A.; Ribas-Perez, D.; Castaño-Seiquer, A.; Sofian-Pauliuc, I. Understanding the Dynamics of Inflammatory Cytokines in Endodontic Diagnosis: A Systematic Review. Diagnostics 2024, 14, 1099. https://doi.org/10.3390/diagnostics14111099
Barbero-Navarro I, Irigoyen-Camacho ME, Zepeda-Zepeda MA, Ribas-Perez D, Castaño-Seiquer A, Sofian-Pauliuc I. Understanding the Dynamics of Inflammatory Cytokines in Endodontic Diagnosis: A Systematic Review. Diagnostics. 2024; 14(11):1099. https://doi.org/10.3390/diagnostics14111099
Chicago/Turabian StyleBarbero-Navarro, Ignacio, Maria Esther Irigoyen-Camacho, Marco Antonio Zepeda-Zepeda, David Ribas-Perez, Antonio Castaño-Seiquer, and Iuliana Sofian-Pauliuc. 2024. "Understanding the Dynamics of Inflammatory Cytokines in Endodontic Diagnosis: A Systematic Review" Diagnostics 14, no. 11: 1099. https://doi.org/10.3390/diagnostics14111099
APA StyleBarbero-Navarro, I., Irigoyen-Camacho, M. E., Zepeda-Zepeda, M. A., Ribas-Perez, D., Castaño-Seiquer, A., & Sofian-Pauliuc, I. (2024). Understanding the Dynamics of Inflammatory Cytokines in Endodontic Diagnosis: A Systematic Review. Diagnostics, 14(11), 1099. https://doi.org/10.3390/diagnostics14111099








