Scoping Review of Experimental and Clinical Evidence and Its Influence on Development of the Suction Ureteral Access Sheath
Abstract
1. Introduction
1.1. Historical Role of Ureteral Access Sheaths in Retrograde Intrarenal Surgery
1.2. The Utility of UAS and Recommendations in Guidelines
1.3. Role of Suction in Endourology and RIRS
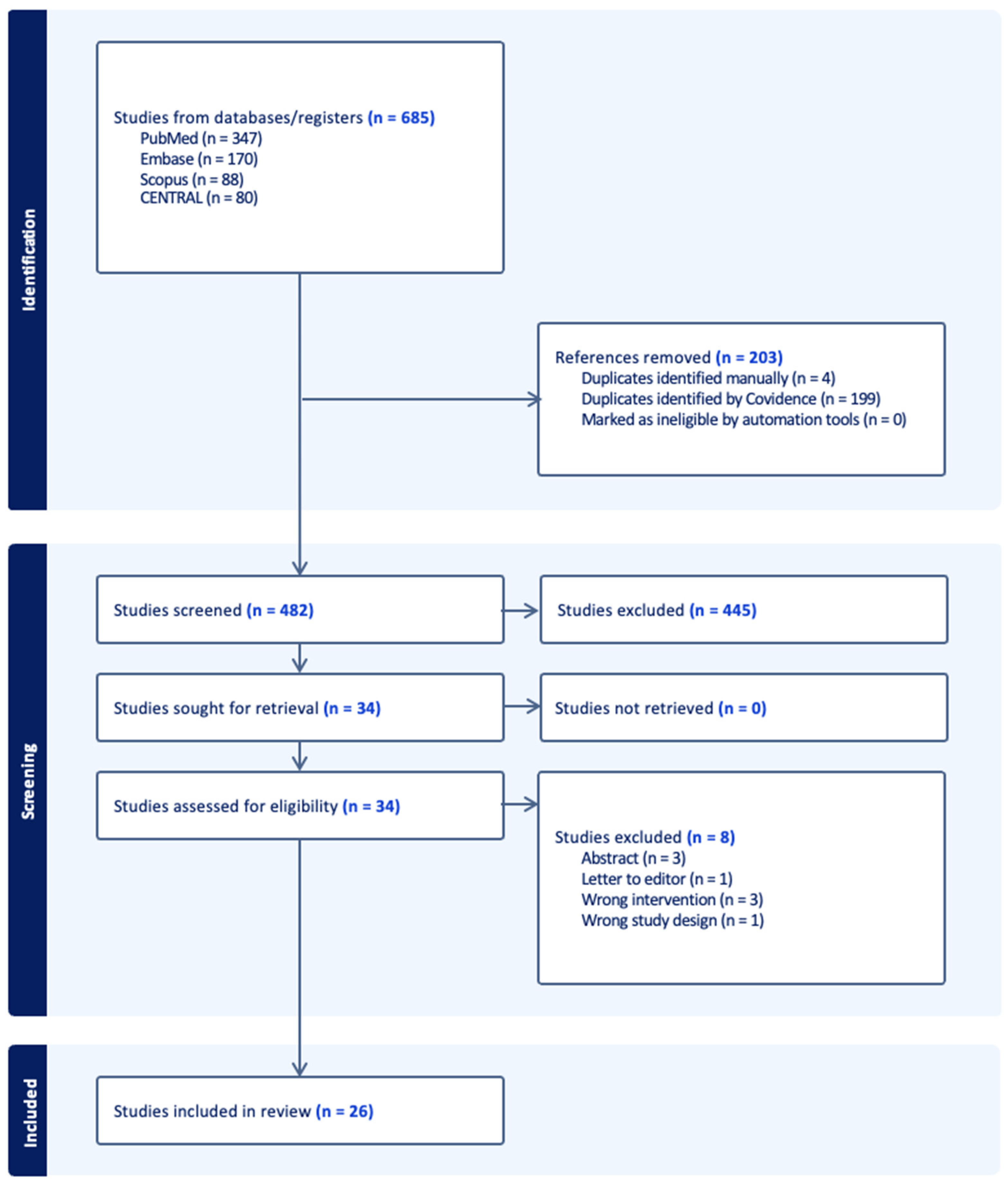
2. Material and Methods
3. Results
3.1. Results of Review of Experimental Studies (Table 1)
| Author | Year | Country | Study Type | Sample Size | Suction Modality | Outcome |
|---|---|---|---|---|---|---|
| Zhu et al. [36] | 2016 | China | in vivo | 9 live pigs (18 kidneys units) | SUAS with IRP measure and suctioning/working channel | IRPs were similar to those recorded by the invasive blood pressure monitor. IRP from the renal pelvic outlet were similar to those from the upper calyceal area |
| Chen et al. [35] | 2022 | China | ex vivo | 20 porcine cadaveric kidneys | 12/14 Fr FANS, 46 cm vs. UAS Pressure sensor was placed in the renal calix by renal puncture | FANS vs. UAS group: Operative time 44.2 vs. 39.7 p = 0.1 Residual stone volume 33.7 vs. 92.5 p = 0.017 Stone clearance 98.5% vs. 95.9% p = 0.017 Complete SFR: 70% FANS |
| Wang et al. [37] | 2022 | China | ex vivo | 12 adult porcine fresh harvested kidneys | 12/14 Fr SUAS vs. UAS 6 Fr pressure monitor catheters via renal puncture | SUAS maintains lower IRP than UAS under same parameters. Both SUAS and UAS can be used when irrigation is ≤50 cc/min; SUAS showed clear advantages over UAS in maintaining lower pressure when irrigation rate is ≥100 cc/min. |
| Ostergar et al. [38] | 2022 | USA | in vivo | 3 female porcine cadaveric kidneys | SUAS connected to wall suction at 0–300 mmHg with 11, 12 Fr sheath sizes, varying irrigation pressure IRP was monitored with a Swan–Ganz balloon catheter placed in the calix and connected to a transducer. | Use of SUAS during RIRS can lower mean IRP; however, this effect could reverse with extended suctioning, especially under conditions of high vacuum (>200 mmHg) owing to outflow-tract collapse. |
3.2. Results of Review of Clinical Studies (Table 2, Table 3 and Table 4)
3.2.1. Operative Time
| Author | Suction Modality | Laser Modality | Operative Time | Stone Free Rate | Definition of Stone Free Rate |
| Zeng et al. [40] | First report of SUAS modified from 12/14 Fr UAS (Well Lead Medical, Guangzhou, China) Negative pressure aspirator set to continuous mode at 150–200 mmHg; semirigid scope passes through it | Ho:YAG 0.5–0.6 J 30–35 Hz | 27.3 min | 97.3% (immediate SFR); 100% (1 month) | no visual stone fragment on fluoroscopy and KUB |
| Zhu et al. [41] | 12/14 Fr SUAS (KYB, Wuxi, China) | Ho:YAG 12–20 W 14–20 Hz | 49.7 min (16.3) | 82.4%(1 Day), 88.8% (30 Days) | radiological residual <2 mm on KUB or NCCT |
| Qian et al. [42] | 12/14 Fr SUAS (Cook Medical, Bloomington, IN, USA), connected with the negative pressure pump at 0.01 MPa. | Ho:YAG 12–20 W 14–20 Hz | 72.9 min (28.1) | Day 1 post-op: 86.4% vs. 71.6%; p = 0.034 1-month post-op: 88.9% vs. 82.7%; p = 0.368 | complete absence of RF or asymptomatic RF < 4 mm at KUB or NCCT at 1 month |
| Tang et al. [50] | 11/13 Fr SUAS (Well Lead Medical, China) | Ho:YAG 200-micron fibre 0.8–1.0 J 15–20 Hz | 61.4 ± 5.2 versus 60.3 ± 5.6 (p = 0.183) | 1 day 73.2% versus 86.2% (p = 0.034); 2 week 82.6% versus 90.8% (p = 0.110); 4 week 94.2% versus 95.4% (p = 0.719) | no radiological evidence of stones or the presence of <= 2 mm asymptomatic fragments on KUB or NCCT |
| Gauhar et al. [51] | SUAS (ClearPetra, Guangzhou, China) vs. DISS by aspiration | TFL | NR | FANS:66.7% vs. DISS: 64.3% | RF < 4 mm not stone free |
| Lai et al. [52] | 14/16 Fr SUAS (ClearPetra, Well Lead Medical, China) | Ho:YAG 1–1.5 J 15–20 Hz | 72.4 (21.3) | 14 (50) | absence of any fragments by low-dose NCCT |
| Wang et al. [53] | 35-cm or 25-cm SUAS 12/14 Fr (ClearPetra, China) connected to a vacuum at 30–40 pKa. semirigid scope passes through it. Inflow irrigation with mechanical pump at 60 c.c./minute. The aspiration pressure was set at 200 mmHg. | Ho:YAG 550-micron fibre 0.5–0.6 W 5–30 Hz | 33.7 min ± 12.2 | 33/35 (94.3%) | No stones seen on KUB (immediate postop); No stones on NCCT or KUB (3 months post-op). |
| Author | Sepsis Rate | Ureteric Injury Rate | Other Complications | Outcome | |
| Zeng et al. [40] | Fever 1.9% (Clavien I) | - | Successful SUAS insertion: 77.1% ureteral false passage 0.9% (Clavien IIIa) | Novel technique with modification to the common UAS. Less retropulsion of stone fragments and improved immediate SFR. Continuous irrigation and aspiration yield better visualization and possibly reduce IRP; minimal learning curve and no special equipment required. | |
| Zhu et al. [41] | 5.50% | 0 | ureteral stricture 0.6%, septic shock 0.6% | Suctioning UAS has advantages of higher SFR one day postoperatively, fewer infectious complications, and shorter operative time. | |
| Qian et al. [42] | fever: 3.70% vs. 14.8%; p = 0.030; SIRS: 1.23% vs. 12.3%; p = 0.012) | - | - | The application of suctioning UAS during FURS was associated with higher SFR on day 1 after surgery and a lower incidence of postoperative fever or SIRS. | |
| Tang et al. [50] | urosepsis: 0% versus 4.6% (p = 0.044); fever: 2.4% versus 10.3% (p = 0.031) | NR | bleeding: 1.2% versus 3.4% (p = 0.317); pain: 2.4% versus 14.9% (p = 0.003) | RIRS with SUAS, a new partnership to treat 1–2 cm infectious upper ureteral stones, was satisfying as it achieved a high SFR rate and a low rate of infectious complications. This method was safe and reproducible in clinical practice. | |
| Gauhar et al. [51] | 2 vs. 0 | 0 vs. 1 | Pelvicalyceal system bleeding (7 vs. 1) | Both had high SFR, minimal complications, suction-enhanced vision, 1st comparative study | |
| Lai et al. [52] | Infection 1 (4); Fever 2 (7) | 1 (4) | Steinstrasse: 1(4); Emesis: 1(4) | SUAS improves the stone-free rate in patients with 2–4 cm kidney stones, reducing the comorbidities. | |
| Wang et al. [53] | Fever—3/35 Sepsis 1/35 | 0 | Haematuria (requiring catheter drainage)—3/35 | SUAS in the treatment of complex steinstrasse is safe and effective | |
| Author | Accrual Year | Country | Study Type | Groups of Comparison | Sample Size |
| Huang et al. [43] | Nov 2013–Aug 2015 | China | prospective | - | 40 patients with solitary kidney |
| Du et al. [44] | Dec 2014–Jun 2017 | China | prospective | negative pressure suctioning system vs. FURS | 122 |
| Chen et al. [54] | 2014 | China | retrospective | FURS with SUAS vs. MPCNL | 91 |
| Gao et al. [45] | Jul 2020–Aug 2021 | China | retrospective | no | 278 patients. 310 kidney units |
| Deng et al. [55] | Jun 2015–Oct 2020 | China | retrospective | FURS with SUAS vs. MPCNL | 127 (57 FURS, 70 MPCNL) |
| Author | Suction Modality | Laser Modality | Operative Time | Stone Free Rate | Definition of Stone Free Rate |
| Huang et al. [43] | patented irrigation and suctioning platform with UAS | Ho:YAG. 0.8 J 20–30 Hz | 25.2 ± 14.5 min | 87.5% (4 weeks); 92.5% (12 weeks) | no residual stone and residual stone < 4 mm in size on KUB |
| Du et al. [44] | SUAS 12/14 Fr 30–45 cm with semirigid URS | Ho:YAG 550 micron fibre 0.6–0.8 J 25–30 Hz | 25.3 min (5.6) | 100% | residual < 4 mm on KUB |
| Chen et al. [54] | integrated pressure-measuring SUAS 12/14 Fr | Ho:YAG 0.8 J 30 Hz | 65.62 min (22.54) | 93.10% | residual < 3 mm on KUB |
| Gao et al. [45] | patented irrigation and suctioning platform with UAS | Ho:YAG 0.8–1.6 J 20–30 Hz | 75 min (60–110 min) | One-session SFR and one-month SFR were 80.65% and 82.26%. | no residual stone or residual stone < 4 mm in size by KUB |
| Deng et al. [55] | UAS 12/14 Fr with pressure measuring and suctioning | Ho:YAG 0.8 J 20 Hz | 61.8 ± 21.1 min SUAS group. 43.4 ± 18.9 min MPCNL group | 3 m 91.2 (52/57) SUAS group. 95.7 (67/70)MPCNL Group | <2 mm on NCCT |
| Author | Sepsis Rate | Ureteric Injury Rate | Other Complications | Outcome | |
| Huang et al. [43] | fever 5% | - | - | intelligently pressure-controlled flexible URS in treating upper urinary tract calculi for patients with a solitary kidney with advantages of high lithotripsy efficacy and low complication rate. | |
| Du et al. [44] | 1.60% | 0 | - | treatment of large urinary tract stone >1.5 cm with system shows shorter operative time, lower incidence of postoperative fever and secondary surgery, and higher stone clearance rate | |
| Chen et al. [54] | 10.80% | 0 | - | for single kidney stone with a diameter of 2–3 cm, intelligent pressure-controlled FURS and MPCNL are both effective treatment methods, but the FURS has advantages, such as fewer complications, shorter hospital stay, and less bleeding. | |
| Gao et al. [45] | nil | - | CD reporting. 8 patients had Clavien–Dindo Grade II, and 2 patients had Grade III complications (ureter lesions) | good safety and efficacy of patented irrigation and suctioning platform with UAS, with one-session SFR of 80.65% (250/310) and a low complication rate (3.26%). Patients with stone size < 40 mm or Guy’s stone score of Grade I had a significantly higher potential to reach stone-free after treatment. | |
| Deng et al. [55] | SUAS Group 5 (8.8%). MPCNL group 6 (8.6%) | - | Ascites SUAS 1 (1.8%) MPCNL 2 (2.9%) | FURS with SUAS and MPCL with 2–3 cm renal stones in solitary kidneys are effective. MPCNL shorter operative time, FURS with SUAS has less bleeding, shorter hospital stay and less damage to kidney function | |
| Author | Accrual Year | Country | Study Type | Groups of Comparison | Sample Size |
| Chen et al. [46] | Aug 2021–Jan 2022 | China | prospective | - | 53 |
| Gauhar et al. [47] | Nov 2021–Oct 2022 | Multi-centre | retrospective | FANS 10/12 vs. 12/14 | 35 |
| Zhong et al. [48] | Jun 2016–Jan 2018 | China | retrospective | No | 52 |
| Huang et al. [49] | Feb 2022–Feb 2023 | China | retrospective | Yes | 371 |
| Chen et al. [56] | Jan 2022–Nov 2022 | China | retrospective | FURS with FANS versus miniPCNL | 96 pts FURS with FANS versus 96 miniPCNL |
| Zhang et al. [57] | Aug 2021–Apr 2022 | China | retrospective | FANS vs. UAS | 214 pts (102 FANS versus 112 UAS) |
| Liang et al. [58] | Oct 2021–Nov 2022 | China | retrospective | no | 224 |
| Yu et al. [59] | Jan 2021–Sep 2022 | China | retrospective, matched-pair analysis | FANS vs. UAS | FANS 152/conventional 152 |
| Wang et al. [60] | Jul 2017–Jul 2018 | China | retrospective | vacuum UAS vs. miniPCNL | 28 vs. 56 |
| Gauhar et al. [61] | Sep 2022–Mar 2023 | Multi-centre | retrospective | No | 45 |
| Author | Suction Modality | Laser Modality | Operative Time | Stone Free Rate | Definition of Stone Free Rate |
| Chen et al. [46] | FANS (Zhangjiagang, China) 12/14 Fr female: 36 cm; male: 46 cm (negative pressure: 50–150 cmH2O) | Ho:YAG 20–40 Hz 0.6–1.2 J | 70.8 (26.9) | 29 (69.8%) | stone volume clearance rate = 1 − (residual stone volume/preoperative stone volume) × 100% |
| Gauhar et al. [47] | FANS Elephant II first or second generation (Zhejiang YiGao Medical Technology Co., Ltd., Hangzhou, China) 10/12 Fr and 12/14 Fr 40 to 55 cm (negative pressure: 0.02 MPa) | TFL 0.2–0.4 J 200–400 Hz and HP Ho:YAG 0.4 J 40 Hz | 76 vs. 63 min | 94.7% vs. 68.8% | Stone-free status was defined as the absence of a single RF > 2 mm on NCCT. |
| Zhong et al. [48] | Flexible pressure-measuring ureteroscopic sheath 12–14 Fr | Ho:YAG | 34.5 ± 18.3 min | 95.7% at Day 1–2, 100% at one month | Residual stone < 4 mm at KUB X-ray or CT-scan if X-ray was negative |
| Huang et al. [49] | FANS 11/13 or 12/14 Fr | Ho:YAG | 40.3 ± 18.9 min in traditional FURS group, 37.7 ± 20.1 min in suction group | 52 (50.5%) and 81 (78.6%) in traditional FURS and suction group respectively at 1 day, 78 (75.7%) and 97 (94.2%) in traditional FURS and suction group respectively at 30 days | Residual fragments < 3 mm at CT scan |
| Chen et al. [56] | 12/14 Fr, 36 cm for female, 46 cm for males, FANS (Woek, Nanchang, China): negative pressure value to 2–7 Kpa. The irrigation volume was adjusted to a range of 80–200 mL/min. | Ho:YAG 1.0–1.2 J 15–30 Hz. | 49.3 (11.9), 25–74 versus 50.6 (11.4), 25–71 (p = 0.06) | 85.4% versus 90.6% (0.266) | NCCT showing zero stone fragments |
| Zhang et al. [57] | 12/14 Fr UAS (Shenzhen Kang Yi Bo Technology Development Co., Ltd., Shenzhen, China) 45 for male 35 for female; FANS 12/14 Fr (Zhangjiagang Huamei MedicalEquipment Co., Ltd., Zhangjiagang, China) 45 cm for male 35 cm for female negative pressure was set at −20 to −60 kPa. | Ho:YAG 0.6–1.2 J 5–20 Hz for fragmentation, and the dusting mode using 0.2–0.6 J 20–30 Hz. | 1 d 86.3% versus 75.0% (p = 0.038); 30 d 91.2% versus 81.3% (p = 0.037) | no residual stone or radiological residue fragment < 2 mm | |
| Liang et al. [58] | FANS 12/14 Fr (Elephant II, Zhejiang YiGao Medical Technology Co., Ltd., Hangzhou, China) | Ho:YAG 1–1.5 J 15–20 Hz. | 69.2 ± 65.2 min | postoperative day 1: 172/224 (76.8%); postoperative day 30: 218/224 (97.3%); | absence of any stones or residual fragments ≤ 2 mm under non-contrast CT |
| Yu et al. [59] | FANS–12/14 Fr; Zhangjiagang, China. Conventional UAS–12/14 Fr; Zhangjiagang, China | Ho:YAG 1.0–1.2 J; 15–30 Hz | FANS 56.5 ± 13.9/UAS 59.9 ± 16.2 | FANS 116 (76.3%) UAS 11 (7.2%) p < 0.001) at 1 day postoperatively No difference at 1 month | zero stone fragments at CT scans on 1st day and 1 month after the surgery. |
| Wang et al. [60] | FANS (ClearPetra, Well Lead Medical, China) | Ho:YAG | RIRS 72.4 (21.3), 42–106 miniPCNL 67.4 (25), 44–114 | RIRS 25 (89.3%) miniPCNL 52 (92.9%) | zero fragments on low-dose CT on postoperative day 1. |
| Gauhar et al. [61] | FANS Clearpetra 12/14 Fr | Not mentioned | 65 min | 93.3% at three months | Absence of stone fragments at CT scan |
| Author | Sepsis Rate | Ureteric Injury Rate | Other Complications | Outcome | |
| Chen et al. [46] | 2 (4) | 0 | Emesis: 2 (3.8%) | intelligently pressure-controlled flexible URS in treating upper urinary tract calculi for patients with a solitary kidney with advantages of high lithotripsy efficacy and low complication rate. | |
| Gauhar et al. [47] | 4 vs. 0 | 0 | 1 fornix rupture | treatment of large urinary tract stone > 1.5 cm with system shows shorter operative time, lower incidence of postoperative fever and secondary surgery, and higher stone clearance rate | |
| Zhong et al. [48] | Fever 1/52 | 1 Ureteral extravasation | 8/52 Haematuria without transfusions | Ureteroscopic lithotripsy with intelligent pressure control improves the efficiency of the lithotripsy and rate of stone clearance | |
| Huang et al. [49] | Fever rate: 3.6% vs. 6.3% in traditional FURS and suction groups, respectively. Absence of sepsis | Non-reported | Not reported | vacuum-assisted dedusting lithotripsy (VADL) technique can significantly improve the postoperative SFR for the patients with kidney or proximal ureteral stones less than 3 cm in size treated by flexible ureteroscope. | |
| Chen et al. [56] | infection: 0% versus 3.1%; fever 4.2% versus 7.3% | NR | total complications: 5.2% versus 13.5% (p = 0.048); emesis: 1% versus 4.2%; transfusion: 0% versus 1%; interventional embolization 0% versus 1% | In the treatment of 2–3 cm renal stones, FURS with a novel FANS may provide a superior alternative to mini-PCNL, potentially challenging its established status | |
| Zhang et al. [57] | infectious: 8.8% versus 18.8% (p = 0.037) (fever: 3.9% versus 9.8%) (urosepsis 3.9% versus 6.3%) (septic shock 1% versus 2.7%) | 0 versus 0 | overall complications: 11.8% versus 22.3% (p = 0.041); Hb loss −0.54 +/− 0.69 g/dl versus −0.83 +/− 0.66 g/dl, p = 0.002); steinstrasse: 0% versus 1.8% | Compared to UAS combined with flexible ureteroscope for treating unilateral renal calculi, FANS had superiority in higher SFR on 1 day and 30 days postoperatively. Shorter operation time, lower haemoglobin loss, and lower incidences of overall and infectious CR were observed in FANS group. | |
| Liang et al. [58] | Fever–2/224 | 0 | 0 | for single kidney stone with a diameter of 2–3 cm, intelligent pressure-controlled FURS and MPCNL are both effective treatment methods, but the FURS has advantages, such as fewer complications, shorter hospital stay, and less bleeding. | |
| Yu et al. [59] | FANS 9 (5.9%)/conv UAS 28 (11.9%) | no | no | FANS has a higher SFR 1 day postoperatively. In addition, FANS has contributed to shorter operative time and fewer complications. | |
| Wang et al. [60] | FANS-2. miniPCNL-5 | no | no | In the treatment of 2–4 cm renal stone, using FANS in RIRS can improve surgical efficiency with lower postoperative early pain scores. | |
| Gauhar et al. [61] | Fever 16/45 (35.6%), Sepsis 0/45 | 3/45 (6.7%) | Reintervention for residual fragments 3/45 | FANS improves single-session SFR and reduces the need for a ureteric stent or catheter | |
3.2.2. Stone Free Rates
3.2.3. Complications
4. Discussion
4.1. History of Development of the UAS (Figure 2)
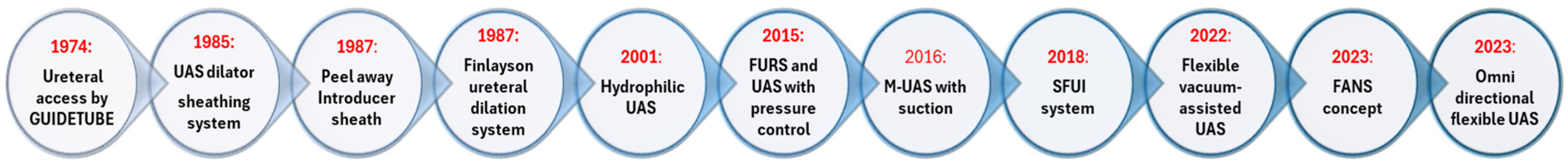
4.2. The Influence of Pressure with the Use of SUAS and FANS in RIRS
4.3. Future Direction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Takayasu, H.; Aso, Y. Recent development for pyeloureteroscopy: Guide tube method for its introduction into the ureter. J. Urol. 1974, 112, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Traxer, O.; Zhong, W.; Osther, P.; Pearle, M.S.; Preminger, G.M.; Mazzon, G.; Seitz, C.; Geavlete, P.; Fiori, C.; et al. International Alliance of Urolithiasis guideline on retrograde intrarenal surgery. BJU Int. 2023, 131, 153–164. [Google Scholar] [CrossRef]
- Wong, V.K.; Aminoltejari, K.; Almutairi, K.; Lange, D.; Chew, B.H. Controversies associated with ureteral access sheath placement during ureteroscopy. Investig. Clin. Urol. 2020, 61, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Stern, J.M.; Yiee, J.; Park, S. Safety and efficacy of ureteral access sheaths. J. Endourol. 2007, 21, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Yitgin, Y.; Yitgin, E.; Verep, S.; Gasimov, K.; Tefik, T.; Karakose, A. Is Access Sheath Essential for Safety and Effective Retrograde Intrarenal Stone Surgery? J. Coll. Physicians Surg. Pak. 2021, 31, 1202–1206. [Google Scholar] [PubMed]
- Meier, K.; Hiller, S.; Dauw, C.; Hollingsworth, J.; Kim, T.; Qi, J.; Telang, J.; Ghani, K.R.; Jafri, S.M.A. Understanding Ureteral Access Sheath Use Within a Statewide Collaborative and Its Effect on Surgical and Clinical Outcomes. J. Endourol. 2021, 35, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Bapir, R.; Bhatti, K.H.; Eliwa, A.; Garcia-Perdomo, H.A.; Gherabi, N.; Hennessey, D.; Mourmouris, P.; Ouattara, A.; Perletti, G.; Philipraj, J.; et al. Infectious complications of endourological treatment of kidney stones: A meta-analysis of randomized clinical trials. Arch. Ital. Urol. Androl. 2022, 94, 97–106. [Google Scholar] [CrossRef] [PubMed]
- Ecer, G.; Sonmez, M.G.; Aydin, A.; Topcu, C.; Alalam, H.N.I.; Guven, S.; Balasar, M. Comparison of retrograde intrarenal stone surgery with and without a ureteral access sheath using kidney injury molecule-1 (KIM-1) levels: A prospective randomized study. Urolithiasis 2022, 50, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Lane, J.; Whitehurst, L.; Hameed, B.M.Z.; Tokas, T.; Somani, B.K. Correlation of Operative Time with Outcomes of Ureteroscopy and Stone Treatment: A Systematic Review of Literature. Curr. Urol. Rep. 2020, 21, 17. [Google Scholar] [CrossRef]
- Miernik, A.; Wilhelm, K.; Ardelt, P.U.; Adams, F.; Kuehhas, F.E.; Schoenthaler, M. Standardized flexible ureteroscopic technique to improve stone-free rates. Urology 2012, 80, 1198–1202. [Google Scholar] [CrossRef]
- Chung, J.H.; Baek, M.; Park, S.S.; Han, D.H. The Feasibility of Pop-Dusting Using High-Power Laser (2 J x 50 Hz) in Retrograde Intrarenal Surgery for Renal Stones: Retrospective Single-Center Experience. J. Endourol. 2021, 35, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Castellani, D.; Traxer, O.; Ragoori, D.; Galosi, A.B.; De Stefano, V.; Gadzhiev, N.; Tanidir, Y.; Inoue, T.; Emiliani, E.; Bin Hamri, S.; et al. Improving Outcomes of Same-sitting Bilateral Flexible Ureteroscopy for Renal Stones in Real-world Practice-Lessons Learnt from Global Multicenter Experience of 1250 Patients. Eur. Urol. Open Sci. 2023, 52, 51–59. [Google Scholar] [CrossRef]
- Quiroz, Y.; Somani, B.K.; Tanidir, Y.; Tekgul, S.; Silay, S.; Castellani, D.; Lim, E.J.; Fong, K.Y.; Rojo, E.G.; Corrales, M.; et al. Retrograde Intrarenal Surgery in Children: Evolution, Current Status, and Future Trends. J. Endourol. 2022, 36, 1511–1521. [Google Scholar] [CrossRef] [PubMed]
- Damar, E.; Senocak, C.; Ozbek, R.; Haberal, H.B.; Sadioglu, F.E.; Yordam, M.; Bozkurt, O.F. Does ureteral access sheath affect the outcomes of retrograde intrarenal surgery: A prospective study. Minim. Invasive Ther. Allied Technol. 2022, 31, 777–781. [Google Scholar] [CrossRef]
- Traxer, O.; Thomas, A. Prospective evaluation and classification of ureteral wall injuries resulting from insertion of a ureteral access sheath during retrograde intrarenal surgery. J. Urol. 2013, 189, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Kaler, K.S.; Lama, D.J.; Safiullah, S.; Cooper, V.; Valley, Z.A.; O’Leary, M.L.; Patel, R.M.; Klopfer, M.J.; Li, G.-P.; Landman, J.; et al. Ureteral Access Sheath Deployment: How Much Force Is Too Much? Initial Studies with a Novel Ureteral Access Sheath Force Sensor in the Porcine Ureter. J. Endourol. 2019, 33, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, K.; Cho, S.Y.; Ng, A.C.; Usawachintachit, M.; Tan, Y.K.; Deng, Y.L.; Shen, C.; Gyawali, P.; Alenezi, H.; Basiri, A.; et al. The Urological Association of Asia clinical guideline for urinary stone disease. Int. J. Urol. 2019, 26, 688–709. [Google Scholar] [CrossRef]
- Quhal, F.; Seitz, C. Guideline of the guidelines: Urolithiasis. Curr. Opin. Urol. 2021, 31, 125–129. [Google Scholar] [CrossRef]
- EAU Guidelines on Urolithiasis. GUIDELINES-Uroweb Uroweb-European Association of Urology. Available online: https://uroweb.org/guidelines/urolithiasis/chapter/guidelines (accessed on 2 March 2024).
- Assimos, D.; Krambeck, A.; Miller, N.L.; Monga, M.; Murad, M.H.; Nelson, C.P.; Pace, K.T.; Pais, V.M., Jr.; Pearle, M.S.; Preminger, G.M.; et al. Surgical Management of Stones: American Urological Association/Endourological Society Guideline, PART II. J. Urol. 2016, 196, 1161–1169. [Google Scholar] [CrossRef]
- Komeya, M.; Odaka, H.; Watanabe, T.; Kiuchi, H.; Ogawa, T.; Yao, M.; Matsuzaki, J. Gap between UAS and ureteroscope predicts renal stone-free rate after flexible ureteroscopy with the fragmentation technique. World J. Urol. 2021, 39, 2733–2739. [Google Scholar] [CrossRef]
- Shi, J.; Huang, T.; Song, B.; Liu, W.; Cheng, Y.; Fang, L. The optimal ratio of endoscope-sheath diameter with negative-pressure ureteral access sheath: An in vitro research. World J. Urol. 2024, 42, 122. [Google Scholar] [CrossRef]
- Fang, L.; Xie, G.; Zheng, Z.; Liu, W.; Zhu, J.; Huang, T.; Lu, Y.; Cheng, Y. The Effect of Ratio of Endoscope-Sheath Diameter on Intrapelvic Pressure During Flexible Ureteroscopic Lasertripsy. J. Endourol. 2019, 33, 132–139. [Google Scholar] [CrossRef]
- Kaplan, A.G.; Lipkin, M.E.; Scales, C.D., Jr.; Preminger, G.M. Use of ureteral access sheaths in ureteroscopy. Nat. Rev. Urol. 2016, 13, 135–140. [Google Scholar] [CrossRef]
- Ozman, O.; Basatac, C.; Akgul, M.; Cakir, H.; Cinar, O.; Simsekoglu, F.; Yazıcı, C.M.; Sancak, E.B.; Baseskioglu, B.; Akpınar, H.; et al. The Effect of Ureteral Access Sheath Use/Caliber Change on Outcomes of Retrograde Intrarenal Surgery, Short-Term Kidney Functions, Radiation Exposure, Ureteroscope Lifetime, and Factors Predicting Insertion Failure: A RIRSearch Study. J. Laparoendosc. Adv. Surg. Tech. 2024, 34, 33–38. [Google Scholar] [CrossRef]
- Ozman, O.; Akgul, H.M.; Basatac, C.; Cinar, O.; Sancak, E.B.; Yazici, C.M.; Önal, B.; Akpınar, H. Multi-aspect analysis of ureteral access sheath usage in retrograde intrarenal surgery: A RIRSearch group study. Asian J. Urol. 2024, 11, 80–85. [Google Scholar] [CrossRef]
- Giulioni, C.; Castellani, D.; Traxer, O.; Gadzhiev, N.; Pirola, G.M.; Tanidir, Y.; da Silva, R.; Glover, X.; Giusti, G.; Proietti, S.; et al. Experimental and clinical applications and outcomes of using different forms of suction in retrograde intrarenal surgery. Results from a systematic review. Actas Urol. Esp. (Engl. Ed.) 2024, 48, 57–70. [Google Scholar] [CrossRef]
- Negrete-Pulido, O.; Gutierrez-Aceves, J. Management of infectious complications in percutaneous nephrolithotomy. J. Endourol. 2009, 23, 1757–1762. [Google Scholar] [CrossRef]
- Quhal, F.; Zeng, G.; Seitz, C. Current evidence for suction in endourological procedures: Comprehensive review of literature. Curr. Opin. Urol. 2023, 33, 77–83. [Google Scholar] [CrossRef]
- Johnson, G.B.; Portela, D.; Grasso, M. Advanced ureteroscopy: Wireless and sheathless. J. Endourol. 2006, 20, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Cheng, Y.; Lu, Y.; Rong, H.; Huang, T.; Shi, J.; Fang, L. Factors affecting the intraoperative calculi excretion during flexible ureteroscopy lithotripsy: An in vitro analysis. World J. Urol. 2024, 42, 130. [Google Scholar] [CrossRef] [PubMed]
- Gauhar, V.; Castellani, D.; Teoh, J.Y.; Nedbal, C.; Chiacchio, G.; Gabrielson, A.T.; Heldwein, F.L.; Wroclawski, M.L.; de la Rosette, J.; da Silva, R.D.; et al. Catheter-Associated Urinary Infections and Consequences of Using Coated versus Non-Coated Urethral Catheters-Outcomes of a Systematic Review and Meta-Analysis of Randomized Trials. J. Clin. Med. 2022, 11, 4463. [Google Scholar] [CrossRef]
- Patel, N.; Akhavein, A.; Hinck, B.; Jain, R.; Monga, M. Tipless Nitinol Stone Baskets: Comparison of Penetration Force, Radial Dilation Force, Opening Dynamics, and Deflection. Urology 2017, 103, 256–260. [Google Scholar] [CrossRef]
- Phukan, C.; Nirmal, T.J.; Wann, C.V.; Chandrasingh, J.; Kumar, S.; Kekre, N.S.; Devasia, A. Can we predict the need for intervention in steinstrasse following shock wave lithotripsy? Urol. Ann. 2017, 9, 51–54. [Google Scholar] [CrossRef]
- Chen, Y.; Li, C.; Gao, L.; Lin, L.; Zheng, L.; Ke, L.; Chen, J.; Kuang, R. Novel Flexible Vacuum-Assisted Ureteral Access Sheath Can Actively Control Intrarenal Pressure and Obtain a Complete Stone-Free Status. J. Endourol. 2022, 36, 1143–1148. [Google Scholar] [CrossRef]
- Zhu, X.; Song, L.; Xie, D.; Peng, Z.; Guo, S.; Deng, X.; Liu, S.; Fan, D.; Huang, J.; Liu, T.; et al. Animal Experimental Study to Test Application of Intelligent Pressure Control Device in Monitoring and Control of Renal Pelvic Pressure During Flexible Ureteroscopy. Urology 2016, 91, 242.e11–242.e15. [Google Scholar] [CrossRef]
- Wang, D.; Han, Z.; Bi, Y.; Ma, G.; Xu, G.; Hu, Q.; Xi, H. Comparison of intrarenal pressure between convention and vacuum-assisted ureteral access sheath using an ex vivo porcine kidney model. World J. Urol. 2022, 40, 3055–3060. [Google Scholar] [CrossRef]
- Ostergar, A.; Wong, D.; Shiang, A.; Ngo, S.; Venkatesh, R.; Desai, A.; Sands, K.G. Intrarenal Pressure with Vacuum-Assisted Ureteral Access Sheaths Using an In Situ Cadaveric Porcine Model. J. Endourol. 2023, 37, 353–357. [Google Scholar] [CrossRef]
- Dybowski, B.; Bres-Niewada, E.; Rzeszutko, M.; Tkaczyk, A.; Wozniak, B.; Wojcik, M.; Znajdek, Z. Risk factors for infectious complications after retrograde intrarenal surgery—A systematic review and narrative synthesis. Cent. Eur. J. Urol. 2021, 74, 437–445. [Google Scholar]
- Zeng, G.; Wang, D.; Zhang, T.; Wan, S.P. Modified Access Sheath for Continuous Flow Ureteroscopic Lithotripsy: A Preliminary Report of a Novel Concept and Technique. J. Endourol. 2016, 30, 992–996. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Cui, Y.; Zeng, F.; Li, Y.; Chen, Z.; Hequn, C. Comparison of suctioning and traditional ureteral access sheath during flexible ureteroscopy in the treatment of renal stones. World J. Urol. 2019, 37, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Liu, C.; Hong, S.; Xu, J.; Qian, C.; Zhu, J.; Wang, S.; Zhang, J. Application of Suctioning Ureteral Access Sheath during Flexible Ureteroscopy for Renal Stones Decreases the Risk of Postoperative Systemic Inflammatory Response Syndrome. Int. J. Clin. Pract. 2022, 2022, 9354714. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xie, D.; Xiong, R.; Deng, X.; Huang, C.; Fan, D.; Peng, Z.; Qin, W.; Zeng, M.; Song, L. The Application of Suctioning Flexible Ureteroscopy with Intelligent Pressure Control in Treating Upper Urinary Tract Calculi on Patients with a Solitary Kidney. Urology 2018, 111, 44–47. [Google Scholar] [CrossRef]
- Du, C.; Song, L.; Wu, X.; Deng, X.; Yang, Z.; Zhu, X.; Zhu, L.; He, J. A study on the clinical application of a patented perfusion and suctioning platform and ureteral access sheath in the treatment of large ureteral stones below L4 level. Int. Urol. Nephrol. 2019, 51, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhang, Z.; Li, X.; Cai, W.; Zheng, B.; Lu, Y.; Zhao, H.; You, J.; Zheng, G.; Bao, W.; et al. High stone-free rate immediately after suctioning flexible ureteroscopy with Intelligent pressure-control in treating upper urinary tract calculi. BMC Urol. 2022, 22, 180. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zheng, L.; Lin, L.; Li, C.; Gao, L.; Ke, L.; Kuang, R.; Chen, J. A novel flexible vacuum-assisted ureteric access sheath in retrograde intrarenal surgery. BJU Int. 2022, 130, 586–588. [Google Scholar] [CrossRef]
- Gauhar, V.; Traxer, O.; Castellani, D.; Ragoori, D.; Heng, C.T.; Chew, B.H.; Somani, B.K.; Bin Hamri, S. A Feasibility Study on Clinical Utility, Efficacy and Limitations of 2 Types of Flexible and Navigable Suction Ureteral Access Sheaths in Retrograde Intrarenal Surgery for Renal Stones. Urology 2023, 178, 173–179. [Google Scholar] [CrossRef]
- Zhong, Y.; Xie, D.; Luo, C.; Liao, X.; Liu, T.; Deng, X.; Zhu, L.; Song, L. Clinical application of flexible ureteroscopic sheath with controllable intraluminal pressure in treating ureteral stones. Asian J. Urol. 2023, 10, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yang, Y.; Xie, H.; Fu, Z.; Zhu, F.; Xie, L.; Liu, C. Vacuum-assisted dedusting lithotripsy in the treatment of kidney and proximal ureteral stones less than 3 cm in size. World J. Urol. 2023, 41, 3097–3103. [Google Scholar] [CrossRef]
- Tang, Q.L.; Liang, P.; Ding, Y.F.; Zhou, X.Z.; Tao, R.Z. Comparative efficacy between retrograde intrarenal surgery with vacuum-assisted ureteral access sheath and minimally invasive percutaneous nephrolithotomy for 1–2 cm infectious upper ureteral stones: A prospective, randomized controlled study. Front. Surg. 2023, 10, 1200717. [Google Scholar] [CrossRef]
- Gauhar, V.; Traxer, O.; Mohammed, S.; Hamri, S.; Lim, E.; Fong, K. MP074-Three Different Techniques to show utility and outcomes of suction in Retrograde Intrarenal Surgery. Eur. Urol. Open Sci. 2022, 39, S131–S132. [Google Scholar] [CrossRef]
- Lai, D.; He, Y.; Li, X.; Chen, M.; Zeng, X. RIRS with Vacuum-Assisted Ureteral Access Sheath versus MPCNL for the Treatment of 2-4 cm Renal Stone. BioMed Res. Int. 2020, 2020, 8052013. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Xu, Y.; Liu, Z.; Liang, J.; Lai, D.; Guan, W.; Xu, G. Using vacuum-assisted ureteral access sheath in the treatment of complex steinstrasse. Urolithiasis 2023, 51, 89. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Qiu, X.; Du, C.; Xie, D.; Liu, T.; Wang, G.; Song, L. The Comparison Study of Flexible Ureteroscopic Suctioning Lithotripsy with Intelligent Pressure Control versus Minimally Invasive Percutaneous Suctioning Nephrolithotomy in Treating Renal Calculi of 2 to 3 cm in Size. Surg. Innov. 2019, 26, 528–535. [Google Scholar] [CrossRef]
- Deng, X.; Xie, D.; Huang, X.; Huang, J.; Song, L.; Du, C. Suctioning Flexible Ureteroscopy with Automatic Control of Renal Pelvic Pressure versus Mini PCNL for the Treatment of 2–3-cm Kidney Stones in Patients with a Solitary Kidney. Urol. Int. 2022, 106, 1293–1297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xi, H.; Yu, Y.; Cheng, X.; Yang, H.; Deng, W.; Liu, W.; Wang, G.; Zhou, X. Flexible ureteroscopy with novel flexible ureteral access sheath versus mini-percutaneous nephrolithotomy for treatment of 2–3 cm renal stones. Int. J. Urol. 2024, 31, 281–286. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, T.; Li, F.; Wang, X.; Liu, F.; Jiang, B.; Zou, X.; Zhang, G.; Yuan, Y.; Xiao, R.; et al. Comparison of traditional and novel tip-flexible suctioning ureteral access sheath combined with flexible ureteroscope to treat unilateral renal calculi. World J. Urol. 2023, 41, 3619–3627. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Liang, L.; Lin, Y.; Yu, Y.; Xu, X.; Liang, Z.; Sheng, J.; Shen, B. Application of tip-bendable ureteral access sheath in flexible ureteroscopic lithotripsy: An initial experience of 224 cases. BMC Urol. 2023, 23, 175. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Chen, Y.; Zhou, X.; Li, X.; Liu, W.; Cheng, X.; Chen, L.; Yang, H.; Wang, G.; Xi, H. Comparison of novel flexible and traditional ureteral access sheath in retrograde intrarenal surgery. World J. Urol. 2024, 42, 7. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.J.; Liang, P.; Yang, T.X.; Liu, Y.Q.; Tang, Q.L.; Zhou, X.Z.; Tao, R.Z. RIRS with FV-UAS vs. MPCNL for 2–3-cm upper urinary tract stones: A prospective study. Urolithiasis 2024, 52, 31. [Google Scholar] [CrossRef]
- Gauhar, V.; Ong, C.S.H.; Traxer, O.; Chew, B.H.; Gadzhiev, N.; Teoh, J.Y.C.; Hamri, S.B.; Heng, C.T.; Castellani, D.; Somani, B.K.; et al. Step-by-step guide to flexible and navigable suction ureteric access sheath (FANS). Urol. Video J. 2023, 20, 100250. [Google Scholar] [CrossRef]
- Newman, R.C.; Hunter, P.T.; Hawkins, I.F.; Finlayson, B. A general ureteral dilator-sheathing system. Urology 1985, 25, 287–288. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.; Lee, W.J.; Smith, A.D. Applications of the peel-away introducer sheath. J. Urol. 1987, 137, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.C.; Hunter, P.T.; Hawkins, I.F.; Finlayson, B. The ureteral access system: A review of the immediate results in 43 cases. J. Urol. 1987, 137, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Kourambas, J.; Byrne, R.R.; Preminger, G.M. Does a ureteral access sheath facilitate ureteroscopy? J. Urol. 2001, 165, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Auge, B.K.; Pietrow, P.K.; Lallas, C.D.; Raj, G.V.; Santa-Cruz, R.W.; Preminger, G.M. Ureteral access sheath provides protection against elevated renal pressures during routine flexible ureteroscopic stone manipulation. J. Endourol. 2004, 18, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Rehman, J.; Monga, M.; Landman, J.; Lee, D.I.; Felfela, T.; Conradie, M.C.; Srinivas, R.; Sundaram, C.P.; Clayman, R.V. Characterization of intrapelvic pressure during ureteropyeloscopy with ureteral access sheaths. Urology 2003, 61, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Pietrow, P.K.; Auge, B.K.; Delvecchio, F.C.; Silverstein, A.D.; Weizer, A.Z.; Albala, D.M.; Preminger, G.M. Techniques to maximize flexible ureteroscope longevity. Urology 2002, 60, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Dauw, C.A.; Simeon, L.; Alruwaily, A.F.; Sanguedolce, F.; Hollingsworth, J.M.; Roberts, W.W.; Faerber, G.J.; Wolf, J.S.; Ghani, K.R. Contemporary Practice Patterns of Flexible Ureteroscopy for Treating Renal Stones: Results of a Worldwide Survey. J. Endourol. 2015, 29, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Song, L.; Xie, D.; Fan, D.; Zhu, L.; Yao, L.; Wang, X.; Liu, S.; Zhang, Y.; Liao, X.; et al. A Novel Flexible Ureteroscopy with Intelligent Control of Renal Pelvic Pressure: An Initial Experience of 93 Cases. J. Endourol. 2016, 30, 1067–1072. [Google Scholar] [CrossRef]
- Thomsen, H.S. Pyelorenal backflow. Clinical and experimental investigations. Radiologic, nuclear, medical and pathoanatomic studies. Dan. Med. Bull. 1984, 31, 438–457. [Google Scholar]
- Jung, H.; Osther, P.J. Intraluminal pressure profiles during flexible ureterorenoscopy. SpringerPlus 2015, 4, 373. [Google Scholar] [CrossRef] [PubMed]
- Corrales, M.; Sierra, A.; Doizi, S.; Traxer, O. Risk of Sepsis in Retrograde Intrarenal Surgery: A Systematic Review of the Literature. Eur. Urol. Open Sci. 2022, 44, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Tokas, T.; Herrmann, T.R.W.; Skolarikos, A.; Nagele, U.; Training and Research in Urological Surgery and Technology (T.R.U.S.T.)-Group. Pressure matters: Intrarenal pressures during normal and pathological conditions, and impact of increased values to renal physiology. World J. Urol. 2019, 37, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Hong, A.; du Plessis, J.; Browne, C.; Jack, G.; Bolton, D. Mechanism of urosepsis: Relationship between intrarenal pressures and pyelovenous backflow. BJU Int. 2023, 132, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Timm, B.; Davis, N.; Wong, L.M.; Bolton, D.M.; Jack, G.S. Pressurized-Bag Irrigation versus Hand-Operated Irrigation Pumps during Ureteroscopic Laser Lithotripsy: Comparison of Infectious Complications. J. Endourol. 2020, 34, 914–918. [Google Scholar] [CrossRef] [PubMed]
- Loftus, C.; Byrne, M.; Monga, M. High pressure endoscopic irrigation: Impact on renal histology. Int. Braz. J. Urol. 2021, 47, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Tokas, T.; Tzanaki, E.; Nagele, U.; Somani, B.K. Role of Intrarenal Pressure in Modern Day Endourology (Mini-PCNL and Flexible URS): A Systematic Review of Literature. Curr. Urol. Rep. 2021, 22, 52. [Google Scholar] [CrossRef]
- Whitaker, R.H. Methods of assessing obstruction in dilated ureters. Br. J. Urol. 1973, 45, 15–22. [Google Scholar] [CrossRef]
- Whitaker, R.H. An evaluation of 170 diagnostic pressure flow studies of the upper urinary tract. J. Urol. 1979, 121, 602–604. [Google Scholar] [CrossRef]
- Lazarus, J.; Wisniewski, P.; Kaestner, L. Beware the bolus size: Understanding intrarenal pressure during ureteroscopic fluid administration. S. Afr. J. Surg. 2020, 58, 220. [Google Scholar]
- Doizi, S.; Uzan, A.; Keller, E.X.; De Coninck, V.; Kamkoum, H.; Barghouthy, Y.; Ventimiglia, E.; Traxer, O. Comparison of intrapelvic pressures during flexible ureteroscopy, mini-percutaneous nephrolithotomy, standard percutaneous nephrolithotomy, and endoscopic combined intrarenal surgery in a kidney model. World J. Urol. 2021, 39, 2709–2717. [Google Scholar] [CrossRef] [PubMed]
- Veenboer, P.W.; de Jong, T.P. Antegrade pressure measurement as a diagnostic tool in modern pediatric urology. World J. Urol. 2011, 29, 737–741. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Walzak, M.P., Jr.; Paquin, A.J., Jr. Renal pelvic pressure levels in management of nephrostomy. J. Urol. 1961, 85, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.; Norby, B.; Frimodt-Moller, P.C.; Osther, P.J. Endoluminal isoproterenol irrigation decreases renal pelvic pressure during flexible ureterorenoscopy: A clinical randomized, controlled study. Eur. Urol. 2008, 54, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Song, L.; Xie, D.; Huang, J.; Zhong, Y.; Tan, W.; Deng, X. Suctioning flexible ureteroscopic lithotripsy in the oblique supine lithotomy position and supine lithotomy position: A comparative retrospective study. Minerva Urol. Nefrol. 2018, 70, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.M.; Jefferson, F.A.; Owyong, M.; Hofmann, M.; Ayad, M.L.; Osann, K.; Okhunov, Z.; Landman, J.; Clayman, R.V. Characterization of intracalyceal pressure during ureteroscopy. World J. Urol. 2021, 39, 883–889. [Google Scholar] [CrossRef]
- Dumbill, R.; Mellati, A.; Yang, B.D.; Ploeg, R.; Turney, B.; Hunter, J. Ureterorenoscopy during normothermic machine perfusion: Effect of varying renal pelvis pressure. BJU Int. 2023, 131, 50–52. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, J.; Kaestner, L. Novel syphon ureteric access sheath has the potential to improve renal pressures and irrigant flow. BJU Int. 2022, 129, 642–647. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Connors, B.A.; Agarwal, D.K.; Assmus, M.A.; Williams, J.C., Jr.; Large, T.; Krambeck, A.E. Determining the threshold of acute renal parenchymal damage for intrarenal pressure during flexible ureteroscopy using an in vivo pig model. World J. Urol. 2022, 40, 2675–2681. [Google Scholar] [CrossRef]
- Doizi, S.; Letendre, J.; Cloutier, J.; Ploumidis, A.; Traxer, O. Continuous monitoring of intrapelvic pressure during flexible ureteroscopy using a sensor wire: A pilot study. World J. Urol. 2021, 39, 555–561. [Google Scholar] [CrossRef]
- Sierra, A.; Corrales, M.; Kolvatzis, M.; Doizi, S.; Traxer, O. Real Time Intrarenal Pressure Control during Flexible Ureterorrenscopy Using a Vascular PressureWire: Pilot Study. J. Clin. Med. 2022, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, F.; Ventimiglia, E.; Corrales, M.; Traxer, O. A Practical Guide for Intra-Renal Temperature and Pressure Management during Rirs: What Is the Evidence Telling Us. J. Clin. Med. 2022, 11, 3429. [Google Scholar] [CrossRef] [PubMed]
- Pauchard, F.; Bhojani, N.; Chew, B.; Ventimiglia, E. How to measure intra-renal pressure during flexible URS: Historical background, technological innovations and future perspectives. Actas Urol. Esp. (Engl. Ed.) 2024, 48, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Croghan, S.M.; Somani, B.K.; Considine, S.W.; Breen, K.J.; McGuire, B.B.; Manecksha, R.P.; Gauhar, V.; Hameed, B.Z.; O’Meara, S.; Emiliani, E.; et al. Perceptions and Practice Patterns of Urologists Relating to Intrarenal Pressure During Ureteroscopy: Findings from a Global Cross-Sectional Analysis. J. Endourol. 2023, 37, 1191–1199. [Google Scholar] [CrossRef] [PubMed]
- Panthier, F.; Pauchard, F.; Traxer, O. Retrograde intra renal surgery and safety: Pressure and temperature. A systematic review. Curr. Opin. Urol. 2023, 33, 308–317. [Google Scholar] [CrossRef]
- Wright, A.; Williams, K.; Somani, B.; Rukin, N. Intrarenal pressure and irrigation flow with commonly used ureteric access sheaths and instruments. Cent. Eur. J. Urol. 2015, 68, 434–438. [Google Scholar]
- Lildal, S.K.; Andreassen, K.H.; Baard, J.; Brehmer, M.; Bultitude, M.; Eriksson, Y.; Ghani, K.R.; Jung, H.; Kamphuis, G.; Kronenberg, P.; et al. Consultation on kidney stones, Copenhagen 2019: Aspects of intracorporeal lithotripsy in flexible ureterorenoscopy. World J. Urol. 2021, 39, 1673–1682. [Google Scholar] [CrossRef]
- Jahrreiss, V.; Nedbal, C.; Castellani, D.; Gauhar, V.; Seitz, C.; Zeng, G.; Juliebø-Jones, P.; Keller, E.; Tzelves, L.; Geraghty, R.; et al. Is suction the future of endourology? Overview from EAU Section of Urolithiasis. Ther. Adv. Urol. 2024, 16, 17562872241232275. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Ma, Q.; Xie, S.; Wang, G.; Li, G.; Chen, G. Comparison of the effectiveness of two adjustable negative pressure ureteral access sheaths combined with flex ureteroscopy for </= 2 cm renal stones. Sci. Rep. 2024, 14, 4745. [Google Scholar]
- Miguel, C.; Sangani, A.; Wiener, S. Exploring ureteroscope design with computational fluid dynamics for improved intra-pelvic pressure. Urolithiasis 2023, 51, 112. [Google Scholar] [CrossRef]
- Zhang, Z.; Leng, S.; Xie, T.; Yuan, Y.; Wang, X. Flexible ureteroscopic lithotripsy with a suctioning ureteral access sheath for removing upper urinary calculi under local anesthesia. Front. Surg. 2023, 10, 1242981. [Google Scholar] [CrossRef] [PubMed]
- Restelli, A.R.; Saeed, W.M.; Fried, N.M. A novel flexible ureteroscope design using a saline light guide channel for combined irrigation and illumination. In Proceedings of the Advanced Photonics in Urology 2023, San Francisco, CA, USA, 28–29 January 2023; Volume 12353, pp. 41–47. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuen, S.K.K.; Traxer, O.; Wroclawski, M.L.; Gadzhiev, N.; Chai, C.A.; Lim, E.J.; Giulioni, C.; De Stefano, V.; Nedbal, C.; Maggi, M.; et al. Scoping Review of Experimental and Clinical Evidence and Its Influence on Development of the Suction Ureteral Access Sheath. Diagnostics 2024, 14, 1034. https://doi.org/10.3390/diagnostics14101034
Yuen SKK, Traxer O, Wroclawski ML, Gadzhiev N, Chai CA, Lim EJ, Giulioni C, De Stefano V, Nedbal C, Maggi M, et al. Scoping Review of Experimental and Clinical Evidence and Its Influence on Development of the Suction Ureteral Access Sheath. Diagnostics. 2024; 14(10):1034. https://doi.org/10.3390/diagnostics14101034
Chicago/Turabian StyleYuen, Steffi Kar Kei, Olivier Traxer, Marcelo Langer Wroclawski, Nariman Gadzhiev, Chu Ann Chai, Ee Jean Lim, Carlo Giulioni, Virgilio De Stefano, Carlotta Nedbal, Martina Maggi, and et al. 2024. "Scoping Review of Experimental and Clinical Evidence and Its Influence on Development of the Suction Ureteral Access Sheath" Diagnostics 14, no. 10: 1034. https://doi.org/10.3390/diagnostics14101034
APA StyleYuen, S. K. K., Traxer, O., Wroclawski, M. L., Gadzhiev, N., Chai, C. A., Lim, E. J., Giulioni, C., De Stefano, V., Nedbal, C., Maggi, M., Sarica, K., Castellani, D., Somani, B., & Gauhar, V. (2024). Scoping Review of Experimental and Clinical Evidence and Its Influence on Development of the Suction Ureteral Access Sheath. Diagnostics, 14(10), 1034. https://doi.org/10.3390/diagnostics14101034










