Comprehensive Genomic Profiling and Therapeutic Implications for Patients with Advanced Cancers: The Experience of an Academic Hospital
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Tumor Samples
2.3. Next Generation Sequencing Panel
2.4. Endpoints and Statistical Analysis
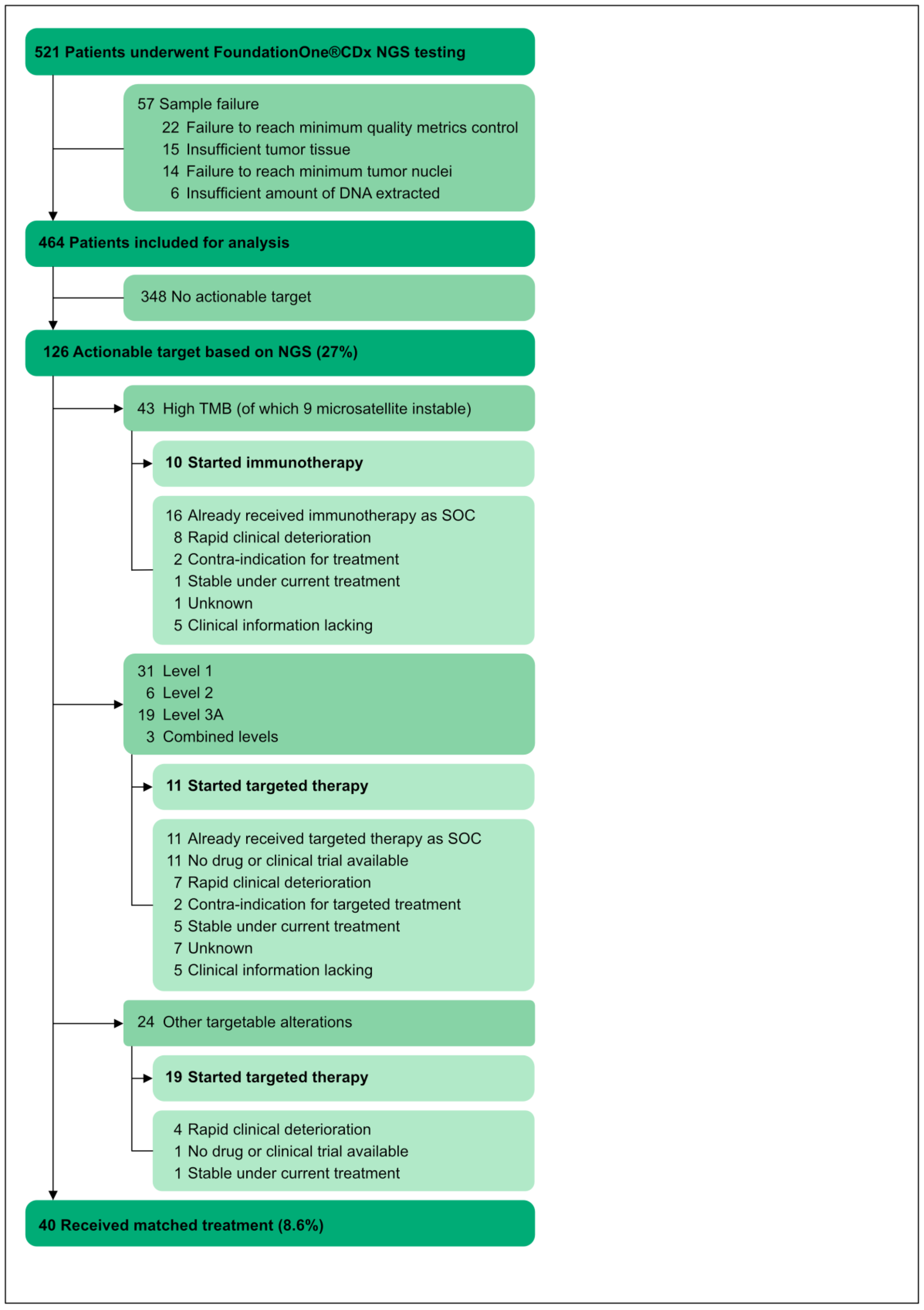
3. Results
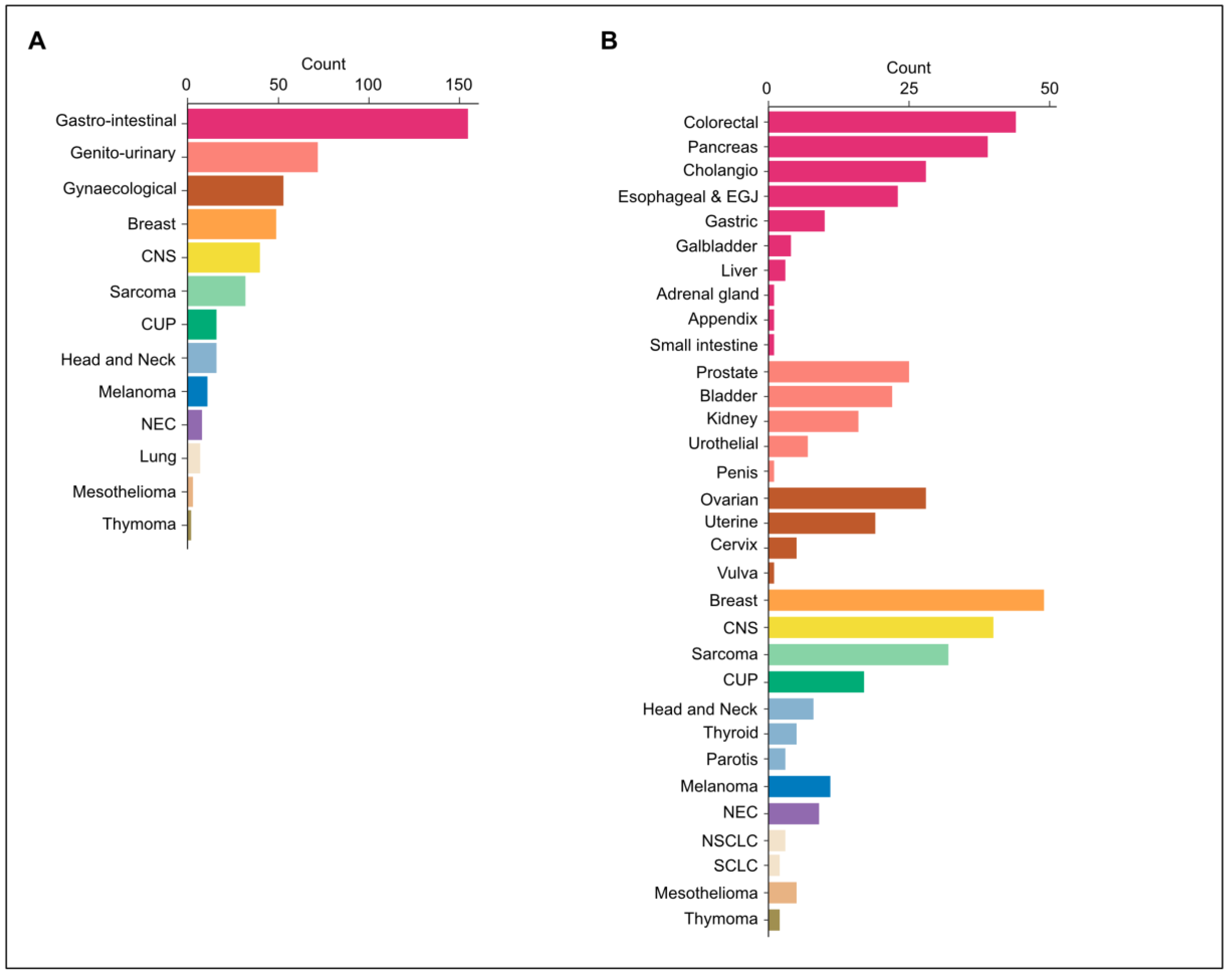
3.1. Demographics
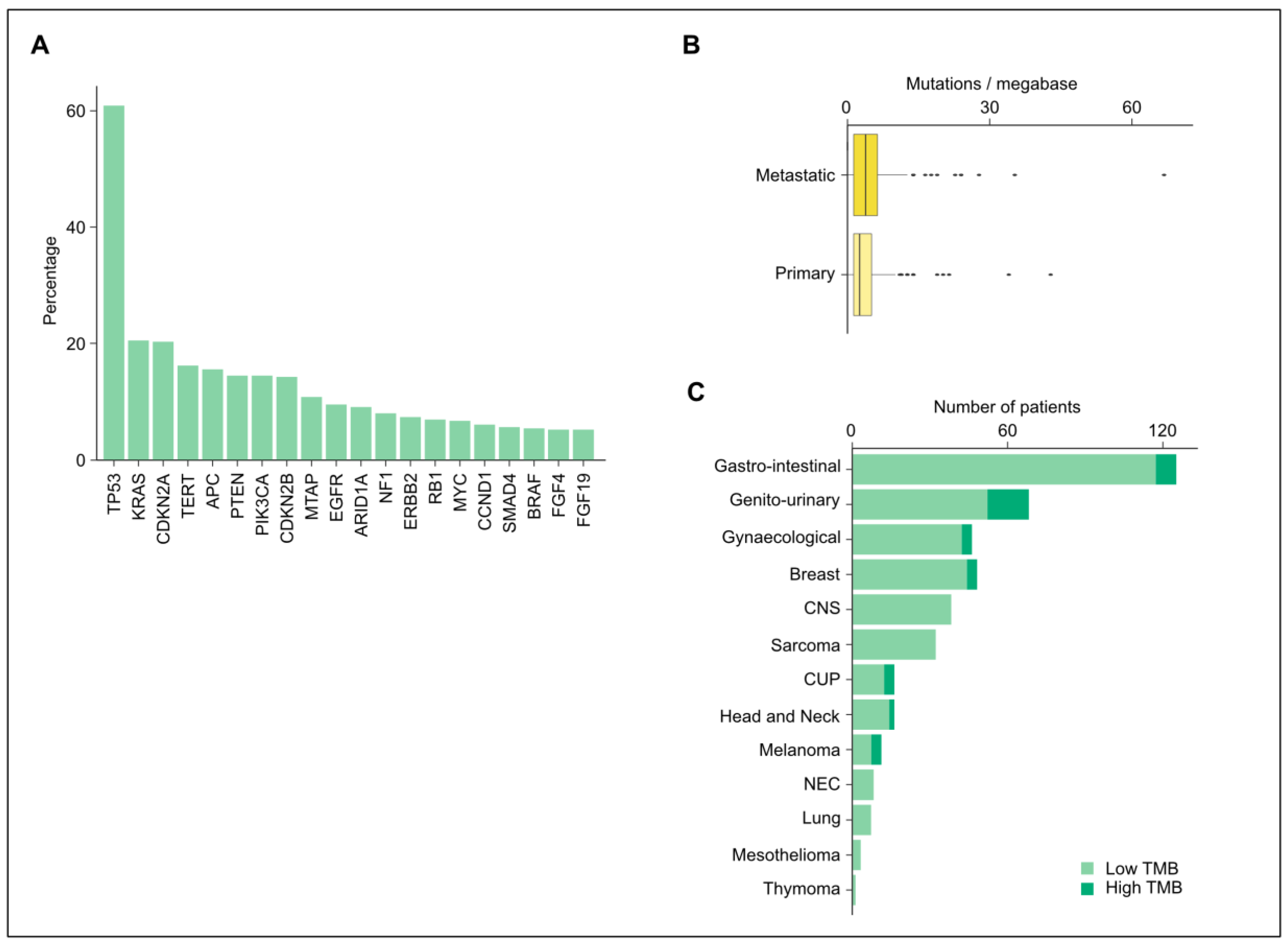
3.2. Altered Genes and Mutations
3.3. Tumor Mutational Burden and Microsatellite Instability
3.4. Targeted Therapy Based on NGS Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slatko, B.E.; Gardner, A.F.; Ausubel, F.M. Overview of Next-Generation Sequencing Technologies. Curr. Protoc. Mol. Biol. 2018, 122, e59. [Google Scholar] [CrossRef] [PubMed]
- Mateo, J.; Steuten, L.; Aftimos, P.; André, F.; Davies, M.; Garralda, E.; Geissler, J.; Husereau, D.; Martinez-Lopez, I.; Normanno, N.; et al. Delivering precision oncology to patients with cancer. Nat. Med. 2022, 28, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Novel Drug Approvals for 2021. Available online: https://www.fda.gov/drugs/new-drugs-fda-cders-new-molecular-entities-and-new-therapeutic-biological-products/novel-drug-approvals-2021 (accessed on 10 August 2022).
- List of EMA-Approved Medicines. Available online: https://www.ema.europa.eu/en/medicines/ema_group_types/ema_medicine/field_ema_web_categories%253Aname_field/Human/field_ema_med_status/authorised-36 (accessed on 10 August 2022).
- Andre, F.; Mardis, E.; Salm, M.; Soria, J.C.; Siu, L.L.; Swanton, C. Prioritizing targets for precision cancer medicine. Ann. Oncol. 2014, 25, 2295–2303. [Google Scholar] [CrossRef] [PubMed]
- Doebele, R.C.; Drilon, A.; Paz-Ares, L.; Siena, S.; Shaw, A.T.; Farago, A.F.; Blakely, C.M.; Seto, T.; Cho, B.C.; Tosi, D.; et al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: Integrated analysis of three phase 1-2 trials. Lancet Oncol. 2020, 21, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y. Tumor agnostic efficacy and safety of erdafitinib in patients (pts) with advanced solid tumors with prespecified fibroblast growth factor receptor alterations (FGFRalt) in RAGNAR: Interim analysis (IA) results. J. Clin. Oncol. 2022, 40, 3007. [Google Scholar] [CrossRef]
- Kato, S.; Kim, K.H.; Lim, H.J.; Boichard, A.; Nikanjam, M.; Weihe, E.; Kuo, D.J.; Eskander, R.N.; Goodman, A.; Galanina, N.; et al. Real-world data from a molecular tumor board demonstrates improved outcomes with a precision N-of-One strategy. Nat. Commun. 2020, 11, 4965. [Google Scholar] [CrossRef]
- Merlin, J.-L.; Gilson, P.; Husson, M.; Harle, A. Targeted PCR vs. NGS for molecular diagnostic in solid tumors and liquid biopsies. How to choose in real-life. J. Clin. Oncol. 2020, 38, e15576. [Google Scholar] [CrossRef]
- Jusakul, A.; Cutcutache, I.; Yong, C.H.; Lim, J.Q.; Huang, M.N.; Padmanabhan, N.; Nellore, V.; Kongpetch, S.; Ng, A.W.T.; Ng, L.M.; et al. Whole-Genome and Epigenomic Landscapes of Etiologically Distinct Subtypes of Cholangiocarcinoma. Cancer Discov. 2017, 7, 1116–1135. [Google Scholar] [CrossRef]
- Milbury, C.A.; Creeden, J.; Yip, W.K.; Smith, D.L.; Pattani, V.; Maxwell, K.; Sawchyn, B.; Gjoerup, O.; Meng, W.; Skoletsky, J.; et al. Clinical and analytical validation of FoundationOne®CDx, a comprehensive genomic profiling assay for solid tumors. PLoS ONE 2022, 17, e0264138. [Google Scholar] [CrossRef]
- Colomer, R.; Mondejar, R.; Romero-Laorden, N.; Alfranca, A.; Sanchez-Madrid, F.; Quintela-Fandino, M. When should we order a next generation sequencing test in a patient with cancer? EClinicalMedicine 2020, 25, 100487. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Kamal, M.; Tsimberidou, A.M.; Bedard, P.; Pierron, G.; Callens, C.; Rouleau, E.; Vincent-Salomon, A.; Servant, N.; Alt, M.; et al. Treatment Algorithms Based on Tumor Molecular Profiling: The Essence of Precision Medicine Trials. J. Natl. Cancer Inst. 2016, 108, djv362. [Google Scholar] [CrossRef]
- Karol, D.; McKinnon, M.; Mukhtar, L.; Awan, A.; Lo, B.; Wheatley-Price, P. The Impact of Foundation Medicine Testing on Cancer Patients: A Single Academic Centre Experience. Front. Oncol. 2021, 11, 687730. [Google Scholar] [CrossRef] [PubMed]
- André, F.; Bachelot, T.; Commo, F.; Campone, M.; Arnedos, M.; Dieras, V.; Lacroix-Triki, M.; Lacroix, L.; Cohen, P.; Gentien, D.; et al. Comparative genomic hybridisation array and DNA sequencing to direct treatment of metastatic breast cancer: A multicentre, prospective trial (SAFIR01/UNICANCER). Lancet Oncol. 2014, 15, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Meric-Bernstam, F.; Brusco, L.; Shaw, K.; Horombe, C.; Kopetz, S.; Davies, M.A.; Routbort, M.; Piha-Paul, S.A.; Janku, F.; Ueno, N.; et al. Feasibility of Large-Scale Genomic Testing to Facilitate Enrollment Onto Genomically Matched Clinical Trials. J. Clin. Oncol. 2015, 33, 2753–2762. [Google Scholar] [CrossRef] [PubMed]
- Sohal, D.P.; Rini, B.I.; Khorana, A.A.; Dreicer, R.; Abraham, J.; Procop, G.W.; Saunthararajah, Y.; Pennell, N.A.; Stevenson, J.P.; Pelley, R.; et al. Prospective Clinical Study of Precision Oncology in Solid Tumors. J. Natl. Cancer Inst. 2015, 108, djv332. [Google Scholar] [CrossRef]
- Nagahashi, M.; Shimada, Y.; Ichikawa, H.; Kameyama, H.; Takabe, K.; Okuda, S.; Wakai, T. Next generation sequencing-based gene panel tests for the management of solid tumors. Cancer Sci. 2019, 110, 6–15. [Google Scholar] [CrossRef] [PubMed]
- FDA. FDA Grants Marketing Approval to FoundationOne CDx In Vitro Diagnostic 2017. 2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-marketing-approval-foundationone-cdx-in-vitro-diagnostic (accessed on 27 April 2023).
- Karlovich, C.A.; Williams, P.M. Clinical Applications of Next-Generation Sequencing in Precision Oncology. Cancer J. 2019, 25, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Medicine, F. Technical Specification. Available online: https://info.foundationmedicine.com/hubfs/FMI%20Labels/FoundationOne_CDx_Label_Technical_Info.pdf (accessed on 27 April 2023).
- Post, T.A. FDA Authorizes MSK-IMPACT Tumor Profiling Assay. 2017. Available online: https://ascopost.com/News/58263 (accessed on 27 April 2023).
- Zehir, A.; Benayed, R.; Shah, R.H.; Syed, A.; Middha, S.; Kim, H.R.; Srinivasan, P.; Gao, J.; Chakravarty, D.; Devlin, S.M.; et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017, 23, 703–713. [Google Scholar] [CrossRef]
- Center, M.S.K.C. MSK-IMPACT: A Targeted Test for Mutations in Both Rare and Common Cancers. Available online: https://www.mskcc.org/msk-impact (accessed on 27 April 2023).
- Jørgensen, J.T. The current landscape of the FDA approved companion diagnostics. Transl. Oncol. 2021, 14, 101063. [Google Scholar] [CrossRef]
- Wakai, T. Precision Cancer Medicine and Super-computing System. Keio J. Med. 2017, 66, 54. [Google Scholar] [CrossRef] [PubMed]
- Conroy, J.M.; Pabla, S.; Glenn, S.T.; Burgher, B.; Nesline, M.; Papanicolau-Sengos, A.; Andreas, J.; Giamo, V.; Lenzo, F.L.; Hyland, F.C.L.; et al. Analytical Validation of a Next-Generation Sequencing Assay to Monitor Immune Responses in Solid Tumors. J. Mol. Diagn. 2018, 20, 95–109. [Google Scholar] [CrossRef]
- Chakravarty, D.; Gao, J.; Phillips, S.M.; Kundra, R.; Zhang, H.; Wang, J.; Rudolph, J.E.; Yaeger, R.; Soumerai, T.; Nissan, M.H.; et al. OncoKB: A Precision Oncology Knowledge Base. JCO Precis Oncol 2017, PO.17.00011. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Takahama, T.; Sakai, K.; Shimizu, S.; Watanabe, S.; Kawakami, H.; Tanaka, K.; Sato, C.; Hayashi, H.; Nonagase, Y.; et al. Clinical Application of the FoundationOne CDx Assay to Therapeutic Decision-Making for Patients with Advanced Solid Tumors. Oncologist 2021, 26, e588–e596. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, V.; Lombardi, P.; Carbonell-Asins, J.A.; Tarazona, N.; Cejalvo, J.M.; González-Barrallo, I.; Martín-Arana, J.; Tébar-Martínez, R.; Viala, A.; Bruixola, G.; et al. Molecular profiling of advanced solid tumours. The impact of experimental molecular-matched therapies on cancer patient outcomes in early-phase trials: The MAST study. Br. J. Cancer 2021, 125, 1261–1269. [Google Scholar] [CrossRef]
- Flaherty, K.T.; Gray, R.; Chen, A.; Li, S.; Patton, D.; Hamilton, S.R.; Williams, P.M.; Mitchell, E.P.; Iafrate, A.J.; Sklar, J.; et al. The Molecular Analysis for Therapy Choice (NCI-MATCH) Trial: Lessons for Genomic Trial Design. J. Natl. Cancer Inst. 2020, 112, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Trédan, O.; Wang, Q.; Pissaloux, D.; Cassier, P.; de la Fouchardière, A.; Fayette, J.; Desseigne, F.; Ray-Coquard, I.; de la Fouchardière, C.; Frappaz, D.; et al. Molecular screening program to select molecular-based recommended therapies for metastatic cancer patients: Analysis from the ProfiLER trial. Ann. Oncol. 2019, 30, 757–765. [Google Scholar] [CrossRef]
- Coquerelle, S.; Darlington, M.; Michel, M.; Durand, M.; Borget, I.; Baffert, S.; Marino, P.; Perrier, L.; Durand-Zaleski, I. Impact of Next Generation Sequencing on Clinical Practice in Oncology in France: Better Genetic Profiles for Patients Improve Access to Experimental Treatments. Value Health 2020, 23, 898–906. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Trédan, O.; Massiani, M.A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef]
- Mateo, J.; Chakravarty, D.; Dienstmann, R.; Jezdic, S.; Gonzalez-Perez, A.; Lopez-Bigas, N.; Ng, C.K.Y.; Bedard, P.L.; Tortora, G.; Douillard, J.Y.; et al. A framework to rank genomic alterations as targets for cancer precision medicine: The ESMO Scale for Clinical Actionability of molecular Targets (ESCAT). Ann. Oncol. 2018, 29, 1895–1902. [Google Scholar] [CrossRef]
- Prasad, V.; Vandross, A. Characteristics of Exceptional or Super Responders to Cancer Drugs. Mayo Clin. Proc. 2015, 90, 1639–1649. [Google Scholar] [CrossRef]
- von Itzstein, M.S.; Smith, M.L.; Railey, E.; White, C.B.; Dieterich, J.S.; Garrett-Mayer, L.; Bruinooge, S.S.; Freedman, A.N.; De Moor, J.; Gray, S.W.; et al. Accessing Targeted Therapies: A Potential Roadblock to Implementing Precision Oncology? JCO Oncol. Pract. 2021, 17, e999–e1011. [Google Scholar] [CrossRef] [PubMed]
- Garralda, E.; Dienstmann, R.; Piris-Giménez, A.; Braña, I.; Rodon, J.; Tabernero, J. New clinical trial designs in the era of precision medicine. Mol. Oncol. 2019, 13, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Reitsma, M.; Fox, J.; Borre, P.V.; Cavanaugh, M.; Chudnovsky, Y.; Erlich, R.L.; Gribbin, T.E.; Anhorn, R. Effect of a Collaboration Between a Health Plan, Oncology Practice, and Comprehensive Genomic Profiling Company from the Payer Perspective. J. Manag. Care Spec. Pharm. 2019, 25, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Mangat, P.K.; Halabi, S.; Bruinooge, S.S.; Garrett-Mayer, E.; Alva, A.; Janeway, K.A.; Stella, P.J.; Voest, E.; Yost, K.J.; Perlmutter, J.; et al. Rationale and Design of the Targeted Agent and Profiling Utilization Registry (TAPUR) Study. JCO Precis. Oncol. 2018, 2018, 1–4. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. A Danish Nationwide Clinical Trial on Targeted Cancer Treatment Based on Genomic Profiling (ProTarget). 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04341181 (accessed on 17 December 2022).
- ClinicalTrials.gov. A MolEcularly Guided Anti-Cancer Drug Off-Label Trial (MEGALiT). Available online: https://clinicaltrials.gov/ct2/show/NCT04185831 (accessed on 17 December 2022).
- Drilon, A.E.; Liu, H.; Wu, F.; Chen, D.; Wilson, T.R.; Simmons, B.P.; Barlesi, F. Tumor-agnostic precision immuno-oncology and somatic targeting rationale for you (TAPISTRY): A novel platform umbrella trial. J. Clin. Oncol. 2021, 39, TPS3154. [Google Scholar] [CrossRef]
- Clinicaltrials.gov. A Study to Examine the Clinical Value of Comprehensive Genomic Profiling Performed by Belgian NGS Laboratories: A Belgian Precision Study of the BSMO in Collaboration With the Cancer Centre (BALLETT). Available online: https://clinicaltrials.gov/ct2/show/NCT05058937 (accessed on 17 December 2022).
- Clinicaltrials.gov. Initiative for Molecular Profiling and Advanced Cancer Therapy (IMPACT II). Available online: https://www.clinicaltrials.gov/ct2/show/NCT02152254 (accessed on 17 December 2022).
- Clinicaltrials.gov. PREcision Medicine in Cancer in Odense, Denmark (PRECODE). Available online: https://clinicaltrials.gov/ct2/show/NCT05385081 (accessed on 17 December 2022).
- Blay, J.Y.; Corset, V.; Mastier, C.; Treilleux, I.; Le Tourneau, C.; Italiano, A.; Delord, J.P.; Attignon, V.; Wang, Q.; Baudet, C.; et al. PROFILER 02—A multicentric, prospective cohort study aiming to evaluate the added value of a large molecular profiling panel (315 cancer-related gene panel [FoundationOne]) versus a limited molecular profiling panel (74 cancer-related gene panel [CONTROL]) in advanced solid tumours. Ann. Oncol. 2017, 28, vii4. [Google Scholar] [CrossRef]
- Skamene, T.; Siu, L.L.; Renouf, D.J.; Laskin, J.J.; Bedard, P.L.; Jones, S.J.M.; Ferrario, C.; Whitlock, J.; Petrie, J.; Sullivan, P.; et al. Canadian profiling and targeted agent utilization trial (CAPTUR/PM.1): A phase II basket precision medicine trial. J. Clin. Oncol. 2018, 36, TPS12127. [Google Scholar] [CrossRef]
- Massard, C.; Michiels, S.; Ferté, C.; Le Deley, M.C.; Lacroix, L.; Hollebecque, A.; Verlingue, L.; Ileana, E.; Rosellini, S.; Ammari, S.; et al. High-Throughput Genomics and Clinical Outcome in Hard-to-Treat Advanced Cancers: Results of the MOSCATO 01 Trial. Cancer Discov. 2017, 7, 586–595. [Google Scholar] [CrossRef]
- Miller, R.W.; Hutchcraft, M.L.; Weiss, H.L.; Wu, J.; Wang, C.; Liu, J.; Jayswal, R.; Buchanan, M.; Anderson, A.; Allison, D.B.; et al. Molecular Tumor Board-Assisted Care in an Advanced Cancer Population: Results of a Phase II Clinical Trial. JCO Precis. Oncol. 2022, 6, e2100524. [Google Scholar] [CrossRef]
- Hoes, L.R.; van Berge Henegouwen, J.M.; van der Wijngaart, H.; Zeverijn, L.J.; van der Velden, D.L.; van de Haar, J.; Roepman, P.; de Leng, W.J.; Jansen, A.M.L.; van Werkhoven, E.; et al. Patients with Rare Cancers in the Drug Rediscovery Protocol (DRUP) Benefit from Genomics-Guided Treatment. Clin. Cancer Res. 2022, 28, 1402–1411. [Google Scholar] [CrossRef] [PubMed]
- Prasad, V.; De Jesús, K.; Mailankody, S. The high price of anticancer drugs: Origins, implications, barriers, solutions. Nat. Rev. Clin. Oncol. 2017, 14, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Remon, J.; Dienstmann, R. Precision oncology: Separating the wheat from the chaff. ESMO Open 2018, 3, e000446. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C.; Soria, J.C.; Bardelli, A.; Biankin, A.; Caldas, C.; Chandarlapaty, S.; de Koning, L.; Dive, C.; Feunteun, J.; Leung, S.Y.; et al. Consensus on precision medicine for metastatic cancers: A report from the MAP conference. Ann. Oncol. 2016, 27, 1443–1448. [Google Scholar] [CrossRef]
- McKenzie, A.J.; Dilks, H.D.; Jones, S.F.; Burris, H., 3rd. Should next-generation sequencing tests be performed on all cancer patients? Expert Rev. Mol. Diagn. 2019, 19, 89–93. [Google Scholar] [CrossRef]
- Schwartzberg, L.; Kim, E.S.; Liu, D.; Schrag, D. Precision Oncology: Who, How, What, When, and When Not? Am. Soc. Clin. Oncol. Educ. Book 2017, 37, 160–169. [Google Scholar] [CrossRef]
- Marabelle, A.; Mouraud, S. Cancer patients can be categorized by a uniform and mutually exclusive pattern of expression of PD-1 or PD-L1 on their tumor infiltrating T lymphocytes. J. Immunother. Cancer 2019, 7, 283. [Google Scholar] [CrossRef]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Christofyllakis, K.; Bittenbring, J.T.; Thurner, L.; Ahlgrimm, M.; Stilgenbauer, S.; Bewarder, M.; Kaddu-Mulindwa, D. Cost-effectiveness of precision cancer medicine-current challenges in the use of next generation sequencing for comprehensive tumour genomic profiling and the role of clinical utility frameworks (Review). Mol. Clin. Oncol. 2022, 16, 21. [Google Scholar] [CrossRef]
| Succesfully Biopsied Patients, n = 464 (%) | |
|---|---|
| Age of tissue (weeks) | |
| Median | 32 |
| Range | 0–876 |
| Tissue of origin | |
| Primary | 271 (58.4%) |
| Metastatic | 190 (40.9%) |
| Unknown | 3 (0.6%) |
| Pretreated | |
| Yes | 155 (33.4%) |
| No | 293 (63.1%) |
| Unknown | 16 (3.4%) |
| Age | |
| Median | 63 |
| Range | 19–88 |
| Gender | |
| Female | 226 (48.7%) |
| Male | 238 (51.3%) |
| Metastasized | |
| Yes | 335 (72.2%) |
| No | 117 (25.2%) |
| Unknown | 12 (2.6%) |
| Tumor Group | |
| Breast | 49 (10.6%) |
| Central nervous system | 40 (8.6%) |
| Carcinoma of unknown primary | 16 (3.4%) |
| Gastro-intestinal | 155 (33.4%) |
| Genito-urinary | 72 (15.5%) |
| Gynaecological | 53 (11.4%) |
| Head and neck | 16 (3.4%) |
| Lung | 7 (1.5%) |
| Melanoma | 11 (2.4%) |
| Mesothelioma | 3 (0.6%) |
| Neuro-endocrine carcinoma | 8 (1.7%) |
| Sarcoma | 32 (6.9%) |
| Thymoma | 2 (0.4%) |
| Tumor Type | |
| Adrenal gland | 1 (0.2%) |
| Appendix | 1 (0.2%) |
| Bladder | 22 (4.7%) |
| Breast | 49 (10.6%) |
| Cervix | 5 (1.1%) |
| Cholangiocarcinoma | 28 (6.0%) |
| Central nervous system | 40 (8.6%) |
| Colorectal | 44 (9.5%) |
| Carcinoma of unknown primary | 17 (3.7%) |
| Esophageal and gastric | 23 (5.0%) |
| Galbladder | 4 (0.9%) |
| Gastric | 10 (2.2%) |
| Head and neck | 8 (1.7%) |
| Kidney | 16 (3.4%) |
| Liver | 3 (0.6%) |
| Melanoma | 11 (2.4%) |
| Mesothelioma | 5 (1.1%) |
| Neuro-endocrine carcinoma | 9 (1.9%) |
| Non-small cell lung cancer | 3 (0.6%) |
| Ovarian | 28 (6.0%) |
| Pancreas | 39 (8.4%) |
| Parotis | 3 (0.6%) |
| Penis | 1 (0.2%) |
| Prostate | 25 (5.4%) |
| Sarcoma | 32 (6.9%) |
| Small cell lung cancer | 2 (0.4%) |
| Small intestine | 1 (0.2%) |
| Thymoma | 2 (0.4%) |
| Thyroid | 5 (1.1%) |
| Urothelial | 7 (1.5%) |
| Uterine | 19 (4.1%) |
| Vulva | 1 (0.2%) |
| Tumor Type | AKT1 | ARID1A | BRAF | CDKN2A | EGFR | ERBB2 | ESR1 | EWSR1 | FGFR2 | FGFR3 | HRAS | IDH1 | KRAS | NRAS | PIK3CA | PTEN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Breast | 3A | 4 | 1 | 3A | 4 | 1, 2 | ||||||||||
| Central nervous system | 4 | 4 | 3A | 4 | ||||||||||||
| Carcinoma of unknown primary | 3A | 4 | ||||||||||||||
| Cholangiocarcinoma | 2 | 1 | 1 | 4 | ||||||||||||
| Colorectal | 1 | 2 | 3A, 4 | |||||||||||||
| Esophageal and gastric | 4 | 1 | 4 | |||||||||||||
| Galbladder | 4 | |||||||||||||||
| Pancreas | 3A, 4 | |||||||||||||||
| Small intestine | 4 | |||||||||||||||
| Bladder | 1 | 4 | ||||||||||||||
| Kidney | 4 | |||||||||||||||
| Penis | 4 | |||||||||||||||
| Prostate | 3A | |||||||||||||||
| Urothelial | 3A | 4 | ||||||||||||||
| Cervix | 4 | |||||||||||||||
| Ovarian | 3A | 4 | 4 | 4 | ||||||||||||
| Uterine | 4 | 4 | 4 | |||||||||||||
| Melanoma | 1, 3A | 3A | ||||||||||||||
| Neuro-endocrine cancer | 4 | |||||||||||||||
| Sarcoma | 4 | 4 | ||||||||||||||
| Thyroid | 1 | 4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teuwen, L.-A.; Roets, E.; D’Hoore, P.; Pauwels, P.; Prenen, H. Comprehensive Genomic Profiling and Therapeutic Implications for Patients with Advanced Cancers: The Experience of an Academic Hospital. Diagnostics 2023, 13, 1619. https://doi.org/10.3390/diagnostics13091619
Teuwen L-A, Roets E, D’Hoore P, Pauwels P, Prenen H. Comprehensive Genomic Profiling and Therapeutic Implications for Patients with Advanced Cancers: The Experience of an Academic Hospital. Diagnostics. 2023; 13(9):1619. https://doi.org/10.3390/diagnostics13091619
Chicago/Turabian StyleTeuwen, Laure-Anne, Evelyne Roets, Pieter D’Hoore, Patrick Pauwels, and Hans Prenen. 2023. "Comprehensive Genomic Profiling and Therapeutic Implications for Patients with Advanced Cancers: The Experience of an Academic Hospital" Diagnostics 13, no. 9: 1619. https://doi.org/10.3390/diagnostics13091619
APA StyleTeuwen, L.-A., Roets, E., D’Hoore, P., Pauwels, P., & Prenen, H. (2023). Comprehensive Genomic Profiling and Therapeutic Implications for Patients with Advanced Cancers: The Experience of an Academic Hospital. Diagnostics, 13(9), 1619. https://doi.org/10.3390/diagnostics13091619







