The Significance of Dual-Energy X-ray Absorptiometry (DXA) Examination in Cushing’s Syndrome—A Systematic Review
Abstract
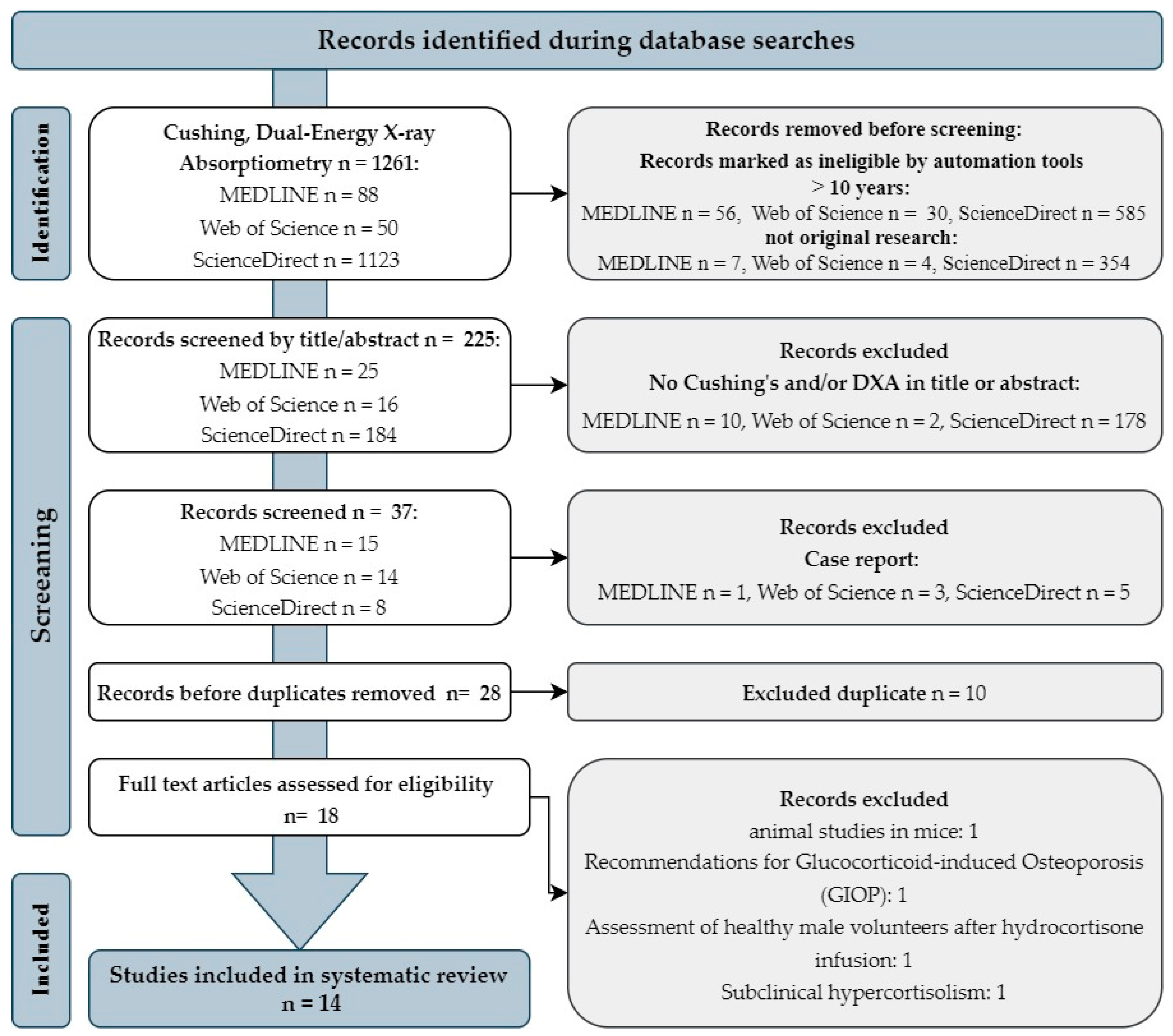
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Search Strategies
2.3. Article Protocol Selection
3. Importance of DXA Analysis in Patients with Cushing’s Syndrome Based on Clinical Studies
3.1. Application of Body Composition Analysis by the DXA Method in Cushing’s Syndrome
3.1.1. Fat Tissue Application
3.1.2. Lean Tissue Application
3.1.3. Bone Tissue Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bazzocchi, A.; Ponti, F.; Albisinni, U.; Battista, G.; Guglielmi, G. DXA: Technical aspects and application. Eur. J. Radiol. 2016, 85, 1481–1492. [Google Scholar] [CrossRef] [PubMed]
- Ward, L.C. Human body composition: Yesterday, today, and tomorrow. Eur. J. Clin. Nutr. 2018, 72, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Gallagher, D. Assessment methods in human body composition. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Lemos, T.; Gallagher, D. Current body composition measurement techniques. Curr. Opin. Endocrinol. Diabetes 2017, 24, 310–314. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J. Med Res. 2018, 148, 648–658. [Google Scholar] [CrossRef]
- Ceniccola, G.D.; Castro, M.G.; Piovacari, S.M.F.; Horie, L.M.; Corrêa, F.G.; Barrere, A.P.N.; Toledo, D.O. Current technologies in body composition assessment: Advantages and disadvantages. Nutrition 2018, 62, 25–31. [Google Scholar] [CrossRef]
- Cheung, Y.; Roff, G.; Grossmann, M. Precision of the Hologic Horizon A dual energy X-ray absorptiometry in the assessment of body composition. Obes. Res. Clin. Pr. 2020, 14, 514–518. [Google Scholar] [CrossRef]
- Achamrah, N.; Colange, G.; DeLay, J.; Rimbert, A.; Folope, V.; Petit, A.; Grigioni, S.; Déchelotte, P.; Coëffier, M. Comparison of body composition assessment by DXA and BIA according to the body mass index: A retrospective study on 3655 measures. PLoS ONE 2018, 13, e0200465. [Google Scholar] [CrossRef]
- Shepherd, J.A.; Ng, B.K.; Sommer, M.J.; Heymsfield, S.B. Body composition by DXA. Bone 2017, 104, 101–105. [Google Scholar] [CrossRef]
- Guglielmi, G.; Ponti, F.; Agostini, M.; Amadori, M.; Battista, G.; Bazzocchi, A. The role of DXA in sarcopenia. Aging Clin. Exp. Res. 2016, 28, 1047–1060. [Google Scholar] [CrossRef]
- Kendler, D.L.; Borges, J.L.; Fielding, R.A.; Itabashi, A.; Krueger, D.; Mulligan, K.; Camargos, B.M.; Sabowitz, B.; Wu, C.-H.; Yu, E.W.; et al. The Official Positions of the International Society for Clinical Densitometry: Indications of Use and Reporting of DXA for Body Composition. J. Clin. Densitom. 2013, 16, 496–507. [Google Scholar] [CrossRef]
- Marra, M.; Sammarco, R.; De Lorenzo, A.; Iellamo, F.; Siervo, M.; Pietrobelli, A.; Donini, L.M.; Santarpia, L.; Cataldi, M.; Pasanisi, F.; et al. Assessment of Body Composition in Health and Disease Using Bioelectrical Impedance Analysis (BIA) and Dual Energy X-ray Absorptiometry (DXA): A Critical Overview. Contrast Media Mol. Imaging 2019, 2019, 1–9. [Google Scholar] [CrossRef]
- Tosato, M.; Marzetti, E.; Cesari, M.; Savera, G.; Miller, R.R.; Bernabei, R.; Landi, F.; Calvani, R. Measurement of muscle mass in sarcopenia: From imaging to biochemical markers. Aging Clin. Exp. Res. 2017, 29, 19–27. [Google Scholar] [CrossRef]
- E Williams, J.; Wells, J.C.; Wilson, C.M.; Haroun, D.; Lucas, A.; Fewtrell, M.S. Evaluation of Lunar Prodigy dual-energy X-ray absorptiometry for assessing body composition in healthy persons and patients by comparison with the criterion 4-component model. Am. J. Clin. Nutr. 2006, 83, 1047–1054. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Toombs, R.J.; Ducher, G.; Shepherd, J.; De Souza, M.J. The Impact of Recent Technological Advances on the Trueness and Precision of DXA to Assess Body Composition. Obesity 2012, 20, 30–39. [Google Scholar] [CrossRef]
- Goulding, A.; Taylor, R.W.; Grant, A.M.; Jones, S.; Taylor, B.J.; Williams, S.M. Relationships of appendicular LMI and total body LMI to bone mass and physical activity levels in a birth cohort of New Zealand five-year olds. Bone 2009, 45, 455–459. [Google Scholar] [CrossRef]
- Shevroja, E.; Cafarelli, F.P.; Guglielmi, G.; Hans, D. DXA parameters, Trabecular Bone Score (TBS) and Bone Mineral Density (BMD), in fracture risk prediction in endocrine-mediated secondary osteoporosis. Endocrine 2021, 74, 20–28. [Google Scholar] [CrossRef]
- Messina, C.; Bignotti, B.; Bazzocchi, A.; Phan, C.M.; Tagliafico, A.; Guglielmi, G.; Sardanelli, F.; Sconfienza, L.M. A critical appraisal of the quality of adult dual-energy X-ray absorptiometry guidelines in osteoporosis using the AGREE II tool: An EuroAIM initiative. Insights Imaging 2017, 8, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Shuhart, C.R.; Yeap, S.S.; Anderson, P.A.; Jankowski, L.G.; Lewiecki, E.M.; Morse, L.R.; Rosen, H.N.; Weber, D.R.; Zemel, B.S.; Shepherd, J.A. Executive Summary of the 2019 ISCD Position Development Conference on Monitoring Treatment, DXA Cross-calibration and Least Significant Change, Spinal Cord Injury, Peri-prosthetic and Orthopedic Bone Health, Transgender Medicine, and Pediatrics. J. Clin. Densitom. 2019, 22, 453–471. [Google Scholar] [CrossRef] [PubMed]
- Cosman, F.; De Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Dimai, H.P. Use of dual-energy X-ray absorptiometry (DXA) for diagnosis and fracture risk assessment; WHO-criteria, T- and Z-score, and reference databases. Bone 2017, 104, 39–43. [Google Scholar] [CrossRef]
- Leib, E.S.; Lewiecki, E.M.; Binkley, N.; Hamdy, R.C. Official Positions of the International Society for Clinical Densitometry. J. Clin. Densitom. 2004, 7, 1–5. [Google Scholar] [CrossRef]
- Trementino, L.; Appolloni, G.; Ceccoli, L.; Marcelli, G.; Concettoni, C.; Boscaro, M.; Arnaldi, G. Bone complications in patients with Cushing’s syndrome: Looking for clinical, biochemical, and genetic determinants. Osteoporos. Int. 2013, 25, 913–921. [Google Scholar] [CrossRef]
- Sawicki, P.; Tałałaj, M.; Życińska, K.; Zgliczyński, W.S.; Wierzba, W. Current Applications and Selected Technical Details of Dual-Energy x-Ray Absorptiometry. Med. Sci. Monitor 2021, 27, e930839. [Google Scholar] [CrossRef]
- Schousboe, J.T.; Vokes, T.; Broy, S.B.; Ferrar, L.; McKiernan, F.; Roux, C.; Binkley, N. Vertebral Fracture Assessment: The 2007 ISCD Official Positions. J. Clin. Densitom. 2008, 11, 92–108. [Google Scholar] [CrossRef]
- Borges, J.L.C.; da Silva, M.S.; Ward, R.J.; Diemer, K.M.; Yeap, S.S.; Lewiecki, E.M. Repeating Vertebral Fracture Assessment: 2019 ISCD Official Position. J. Clin. Densitom. 2019, 22, 484–488. [Google Scholar] [CrossRef]
- Diacinti, D.; Guglielmi, G.; Pisani, D.; Argirò, R.; Serafini, C.; Romagnoli, E.; Minisola, S.; Catalano, C.; David, V. Vertebral morphometry by dual-energy X-ray absorptiometry (DXA) for osteoporotic vertebral fractures assessment (VFA). La Radiol. Med. 2012, 117, 1374–1385. [Google Scholar] [CrossRef]
- Harvey, N.; Glüer, C.; Binkley, N.; McCloskey, E.; Brandi, M.-L.; Cooper, C.; Kendler, D.; Lamy, O.; Laslop, A.; Camargos, B.; et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015, 78, 216–224. [Google Scholar] [CrossRef]
- Roux, J.P.; Wegrzyn, J.; Boutroy, S.; Bouxsein, M.L.; Hans, D.; Chapurlat, R. The predictive value of trabecular bone score (TBS) on whole lumbar vertebrae mechanics: An ex vivo study. Osteoporos. Int. 2013, 24, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- McCloskey, E.V.; Oden, A.; Harvey, N.C.; Leslie, W.D.; Hans, D.; Johansson, H.; Barkmann, R.; Boutroy, S.; Brown, J.; Chapurlat, R.; et al. A meta-analysis oftrabecular bone score in fracture risk prediction and its relationship to FRAX. J. Bone Miner. Res. 2016, 31, 940–948. [Google Scholar] [CrossRef]
- Shevroja, E.; Lamy, O.; Kohlmeier, L.; Koromani, F.; Rivadeneira, F.; Hans, D. Use of Trabecular Bone Score (TBS) as a Complementary Approach to Dual-energy X-ray Absorptiometry (DXA) for Fracture Risk Assessment in Clinical Practice. J. Clin. Densitom. 2017, 20, 334–345. [Google Scholar] [CrossRef]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH Sarcopenia Project: Rationale, Study Description, Conference Recommendations, and Final Estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Messina, C.; Albano, D.; Gitto, S.; Tofanelli, L.; Bazzocchi, A.; Ulivieri, F.M.; Guglielmi, G.; Sconfienza, L.M. Body composition with dual energy X-ray absorptiometry: From basics to new tools. Quant. Imaging Med. Surg. 2020, 10, 1687–1698. [Google Scholar] [CrossRef]
- Dávalos-Yerovi, V.; Marco, E.; Sánchez-Rodríguez, D.; Guillen-Solà, A.; Duran, X.; Pascual, E.M.; Muniesa, J.M.; Escalada, F.; Duarte, E. Sarcopenia According to the Revised European Consensus on Definition and Diagnosis (EWGSOP2) Criteria Predicts Hospitalizations and Long-Term Mortality in Rehabilitation Patients With Stable Chronic Obstructive Pulmonary Disease. J. Am. Med Dir. Assoc. 2019, 20, 1047–1049. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef]
- Duran, I.; Martakis, K.; Rehberg, M.; Stark, C.; Koy, A.; Schoenau, E. The Appendicular Lean Mass Index Is a Suitable Surrogate for Muscle Mass in Children with Cerebral Palsy. J. Nutr. 2019, 149, 1863–1868. [Google Scholar] [CrossRef]
- Santos, D.A.; Dawson, J.A.; Matias, C.N.; Rocha, P.M.; Minderico, C.S.; Allison, D.B.; Sardinha, L.B.; Silva, A.M. Reference Values for Body Composition and Anthropometric Measurements in Athletes. PLoS ONE 2014, 9, e97846. [Google Scholar] [CrossRef]
- Chiu, T.-H.; Chen, S.-C.; Yu, H.-C.; Hsu, J.-S.; Shih, M.-C.; Jiang, H.-J.; Hsu, W.-H.; Lee, M.-Y. Association between Geriatric Nutrition Risk Index and Skeletal Muscle Mass Index with Bone Mineral Density in Post-Menopausal Women Who Have Undergone Total Thyroidectomy. Nutrients 2020, 12, 1683. [Google Scholar] [CrossRef]
- Kim, K.-I.; Kang, M.-G.; Yoon, S.-J.; Choi, J.-Y.; Kim, S.-W.; Kim, C.-H. Relationship between Within-Visit Blood Pressure Variability and Skeletal Muscle Mass. J. Nutr. Heal. Aging 2018, 23, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Teros, M.T.L.; C, F.A.R.; Alemán-Mateo, H. Hyperinsulinemia is associated with the loss of appendicular skeletal muscle mass at 4.6 year follow-up in older men and women. Clin. Nutr. 2015, 34, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Khawaja, A.; Sabbagh, P.; Prioux, J.; Zunquin, G.; Baquet, G.; Maalouf, G.; El Hage, Z.; Antoun, A.; El Hage, R. The Relationships Between Skeletal Muscle Index and Bone Variables in a Group of Young Adults. J. Clin. Densitom. 2021, 24, 78–87. [Google Scholar] [CrossRef]
- Shypailo, R.J.; Wong, W.W. Fat and fat-free mass index references in children and young adults: Assessments along racial and ethnic lines. Am. J. Clin. Nutr. 2020, 112, 566–575. [Google Scholar] [CrossRef]
- Kelly, T.L.; Wilson, K.E.; Heymsfield, S.B. Dual Energy X-Ray Absorptiometry Body Composition Reference Values from NHANES. PLoS ONE 2009, 4, e7038. [Google Scholar] [CrossRef]
- Aucouturier, J.; Meyer, M.; Thivel, D.; Taillardat, M.; Duché, P. Effect of Android to Gynoid Fat Ratio on Insulin Resistance in Obese Youth. Arch. Pediatr. Adolesc. Med. 2009, 163, 826–831. [Google Scholar] [CrossRef]
- Bantle, A.E.; Bosch, T.A.; Dengel, D.R.; Wang, Q.; Mashek, D.G.; Chow, L.S. DXA-Determined Regional Adiposity Relates to Insulin Resistance in a Young Adult Population with Overweight andObesity. J. Clin. Densitom. 2019, 22, 287–292. [Google Scholar] [CrossRef]
- Shetty, S.; Kapoor, N.; Thomas, N.; Paul, T.V. DXA Measured Visceral Adipose Tissue, Total Fat, Anthropometric Indices and its Association With Cardiometabolic Risk Factors in Mother-Daughter Pairs From India. J. Clin. Densitom. 2021, 24, 146–155. [Google Scholar] [CrossRef]
- Bland, V.L.; Kindler, J.M.; Blew, R.M.; Morrill, K.E.; Roe, D.J.; Going, S.B. Visceral adipose tissue and cardiometabolic risk factors in young Hispanic and non-Hispanic girls. Front. Pediatr. 2022, 10, 892206. [Google Scholar] [CrossRef]
- Heshka, S.; Lemos, T.; Astbury, N.M.; Widen, E.; Davidson, L.; Goodpaster, B.H.; DeLany, J.P.; Strain, G.W.; Pomp, A.; Courcoulas, A.P.; et al. Resting Energy Expenditure and Organ-Tissue Body Composition 5 Years After Bariatric Surgery. Obes. Surg. 2019, 30, 587–594. [Google Scholar] [CrossRef]
- Ko, B.-J.; Myung, S.K.; Cho, K.-H.; Park, Y.G.; Kim, S.G.; Kim, D.H.; Kim, S.M. Relationship Between Bariatric Surgery and Bone Mineral Density: A Meta-analysis. Obes. Surg. 2015, 26, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Beraldo, R.A.; Vassimon, H.S.; Navarro, A.M.; Foss-Freitas, M.C. Development of predictive equations for total and segmental body fat in HIV-seropositive patients. Nutrition 2015, 31, 127–131. [Google Scholar] [CrossRef]
- Delpierre, C.; Bonnet, E.; Marion-Latard, F.; Aquilina, C.; Obadia, M.; Marchou, B.; Massip, P.; Perret, B.; Bernard, J. Impact of HIV Infection on Total Body Composition in Treatment—Naive Men Evaluated by Dual-Energy X-ray Absorptiometry Comparison of 90 Untreated HIV-Infected Men to 241 Controls. J. Clin. Densitom. 2007, 10, 376–380. [Google Scholar] [CrossRef]
- Gradidge, P.J.; Norris, S.A.; Crowther, N.J. The Effect of Obesity on the Waist Circumference Cut-Point Used for the Diagnosis of the Metabolic Syndrome in African Women: Results from the SWEET Study. Int. J. Environ. Res. Public Heal. 2022, 19, 10250. [Google Scholar] [CrossRef]
- Papadimas, G.; Terzis, G.; Methenitis, S.; Spengos, K.; Papadopoulos, C.; Vassilopoulou, S.; Kavouras, S.; Michelakakis, H.; Manta, P. Body composition analysis in late-onset Pompe disease. Mol. Genet. Metab. 2011, 102, 41–43. [Google Scholar] [CrossRef]
- Apaydın, T.; Yavuz, D.G. Assessment of non-traumatic vertebral fractures in Cushing’s syndrome patients. J. Endocrinol. Investig. 2021, 44, 1767–1773. [Google Scholar] [CrossRef]
- E Franzoni, E.; Ciccarese, F.; E Di Pietro, E.; Facchini, G.; Moscano, F.; Iero, L.; A Monaldi, A.; Battista, G.; A Bazzocchi, A. Follow-up of bone mineral density and body composition in adolescents with restrictive anorexia nervosa: Role of dual-energy X-ray absorptiometry. Eur. J. Clin. Nutr. 2013, 68, 247–252. [Google Scholar] [CrossRef]
- Karountzos, V.; Lambrinoudaki, I.; Tsitsika, A.; Deligeoroglou, E. The role of total body fat mass and trunk fat mass, combined with other endocrine factors, in menstrual recovery and psychopathology of adolescents with Anorexia Nervosa. Gynecol. Endocrinol. 2017, 33, 757–762. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Chow, S.K.; Hung, V.W.; Wong, C.H.; Wong, R.M.; Tsang, C.S.; Kwok, T.; Cheung, W. Diagnosis of sarcopenia by evaluating skeletal muscle mass by adjusted bioimpedance analysis validated with dual-energy X-ray absorptiometry. J. Cachex- Sarcopenia Muscle 2021, 12, 2163–2173. [Google Scholar] [CrossRef]
- Koo, M.; Wang, Y.-F.; Chuang, T.-L.; Chuang, M.-H.; Lin, C.-H. Association of bone mineral density and trabecular bone score with cardiovascular disease. Tzu Chi Med. J. 2020, 32, 234–239. [Google Scholar] [CrossRef]
- Shin, H.I.; Jung, S.H. Body Fat Distribution and Associated Risk of Cardiovascular Disease in Adults With Cerebral Palsy. Front. Neurol. 2021, 12, 733294. [Google Scholar] [CrossRef] [PubMed]
- Wiklund, P.; Toss, F.; Weinehall, L.; Hallmans, G.; Franks, P.W.; Nordström, A.; Nordström, P. Abdominal and Gynoid Fat Mass Are Associated with Cardiovascular Risk Factors in Men and Women. J. Clin. Endocrinol. Metab. 2008, 93, 4360–4366. [Google Scholar] [CrossRef] [PubMed]
- da Rosa, S.E.; Costa, A.C.; Fortes, M.S.R.; Marson, R.A.; Neves, E.B.; Rodrigues, L.C.; Ferreira, P.F.; Filho, J.F. Cut-Off Points of Visceral Adipose Tissue Associated with Metabolic Syndrome in Military Men. Healthcare 2021, 9, 886. [Google Scholar] [CrossRef]
- Nicklas, B.J.; Penninx, B.W.J.H.; Ryan, A.S.; Berman, D.M.; Lynch, N.A.; Dennis, K.E. Visceral Adipose Tissue Cutoffs Associated With Metabolic Risk Factors for Coronary Heart Disease in Women. Diabetes Care 2003, 26, 1413–1420. [Google Scholar] [CrossRef]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis—2020 Update. Endocr. Pr. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Silva, B.C.; Leslie, W.D.; Resch, H.; Lamy, O.; Lesnyak, O.; Binkley, N.; McCloskey, E.V.; A Kanis, J.; Bilezikian, J.P. Trabecular Bone Score: A Noninvasive Analytical Method Based Upon the DXA Image. J. Bone Miner. Res. 2014, 29, 518–530. [Google Scholar] [CrossRef]
- Lacroix, A.; Feelders, R.A.; Stratakis, C.A.; Nieman, L.K. Cushing’s Syndrome. Lancet 2015, 386, 913–927. [Google Scholar] [CrossRef]
- Raff, H.; Carroll, T. Cushing’s syndrome: From physiological principles to diagnosis and clinical care. J. Physiol. 2015, 593, 493–506. [Google Scholar] [CrossRef]
- Nishioka, H.; Yamada, S. Cushing’s Disease. J. Clin. Med. 2019, 8, 1951. [Google Scholar] [CrossRef]
- Mishra, A.K. Cushing’s Syndrome. In Endocrine Surgery: A South Asian Perspective; MDText.com, Inc.: Abington, UK, 2021; pp. 359–368. ISBN 9780429578885. [Google Scholar]
- Ferraù, F.; Korbonits, M. Metabolic Comorbidities in Cushing’s Syndrome. Eur. J. Endocrinol. 2015, 173, M133–M157. [Google Scholar] [CrossRef]
- Hakami, O.A.; Ahmed, S.; Karavitaki, N. Epidemiology and Mortality of Cushing’s Syndrome. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101521. [Google Scholar] [CrossRef] [PubMed]
- Ceccato, F.; Boccato, M.; Zilio, M.; Barbot, M.; Frigo, A.C.; Luisetto, G.; Boscaro, M.; Scaroni, C.; Camozzi, V. Body Composition is Different After Surgical or Pharmacological Remission of Cushing’s Syndrome: A Prospective DXA Study. Horm. Metab. Res. 2017, 49, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Wagenmakers, M.; Roerink, S.; Gil, L.; Plantinga, T.; Smit, J.; Netea-Maier, R.; Hermus, A. Persistent centripetal fat distribution and metabolic abnormalities in patients in long-term remission of Cushing’s syndrome. Clin. Endocrinol. 2014, 82, 180–187. [Google Scholar] [CrossRef]
- Ragnarsson, O.; Glad, C.A.M.; Bergthorsdottir, R.; Almqvist, E.G.; Ekerstad, E.; Widell, H.; Wängberg, B.; Johannsson, G. Body composition and bone mineral density in women with Cushing’s syndrome in remission and the association with common genetic variants influencing glucocorticoid sensitivity. Eur. J. Endocrinol. 2015, 172, 1–10. [Google Scholar] [CrossRef]
- Stratrova, S.S.; Mishevska, S.J.; Bitoska, I.; Kafedziska, I. Diagnostic Central Obesity Indexes Cut-Off Point Values Determined with Dual-Energy X-Ray Absorptiometry in Cushing’s and Obese Women. Prilozi 2020, 41, 13–21. [Google Scholar] [CrossRef]
- Stratrova, S.S.; Mishevska, S.J.; Efremovska, L.; Bitoska, I.; Spasovski, D. New DXA Diagnostic Indexes of Abdominal Obesity. Prilozi 2021, 42, 37–50. [Google Scholar] [CrossRef]
- Martel-Duguech, L.; Alonso-Jimenez, A.; Bascuñana, H.; Díaz-Manera, J.; Llauger, J.; Nuñez-Peralta, C.; Montesinos, P.; Webb, S.M.; Valassi, E. Prevalence of sarcopenia after remission of hypercortisolism and its impact on HRQoL. Clin. Endocrinol. 2021, 95, 735–743. [Google Scholar] [CrossRef]
- Guo, W.; Li, F.; Zhu, C.; Wang, B.; Wang, K.; Dai, C.; Jia, H.; Wei, H.; He, Q.; Cui, J.; et al. Effect of hypercortisolism on bone mineral density and bone metabolism: A potential protective effect of adrenocorticotropic hormone in patients with Cushing’s disease. J. Int. Med Res. 2017, 46, 492–503. [Google Scholar] [CrossRef]
- Maurice, F.; Dutour, A.; Vincentelli, C.; Abdesselam, I.; Bernard, M.; Dufour, H.; Lefur, Y.; Graillon, T.; Kober, F.; Cristofari, P.; et al. Active Cushing syndrome patients have increased ectopic fat deposition and bone marrow fat content compared to cured patients and healthy subjects: A pilot 1H-MRS study. Eur. J. Endocrinol. 2018, 179, 307–317. [Google Scholar] [CrossRef]
- Belaya, Z.E.; Hans, D.; Rozhinskaya, L.; Dragunova, N.V.; Sasonova, N.I.; Solodovnikov, A.G.; Tsoriev, T.T.; Dzeranova, L.; Mel’Nichenko, G.; Dedov, I.I. The risk factors for fractures and trabecular bone-score value in patients with endogenous Cushing’s syndrome. Arch. Osteoporos. 2015, 10, 1–9. [Google Scholar] [CrossRef]
- Dos Santos, C.V.; Neto, L.V.; Madeira, M.; Coelho, M.C.A.; De Mendonça, L.M.C.; Paranhos-Neto, F.; Lima, I.C.B.; Gadelha, M.R.; Farias, M.L.F. Bone density and microarchitecture in endogenous hypercortisolism. Clin. Endocrinol. 2015, 83, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Vinolas, H.; Grouthier, V.; Mehsen-Cetre, N.; Boisson, A.; Winzenrieth, R.; Schaeverbeke, T.; Mesguich, C.; Bordenave, L.; Tabarin, A. Assessment of vertebral microarchitecture in overt and mild Cushing’s syndrome using trabecular bone score. Clin. Endocrinol. 2018, 89, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Ferraù, F.; Giovinazzo, S.; Messina, E.; Tessitore, A.; Vinci, S.; Mazziotti, G.; Lania, A.; Granata, F.; Cannavò, S. High bone marrow fat in patients with Cushing’s syndrome and vertebral fractures. Endocrine 2019, 67, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.R.; Milner, J.J.; Makowski, L. The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012, 249, 218–238. [Google Scholar] [CrossRef] [PubMed]
- Koenen, M.; Hill, M.A.; Cohen, P.; Sowers, J.R. Obesity, Adipose Tissue and Vascular Dysfunction. Circ. Res. 2021, 128, 951–968. [Google Scholar] [CrossRef]
- Shah, A.; Mehta, N.; Reilly, M.P. Adipose Inflammation, Insulin Resistance, and Cardiovascular Disease. JPEN J. Parenter. Enteral. Nutr. 2008, 32, 638–644. [Google Scholar] [CrossRef]
- Tiemensma, J.; Daskalakis, N.P.; van der Veen, E.M.; Ramondt, S.; Richardson, S.K.; Broadbent, E.; Romijn, J.A.; Pereira, A.M.; Biermasz, N.R.; Kaptein, A.A. Drawings Reflect a New Dimension of the Psychological Impact of Long-Term Remission of Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 2012, 97, 3123–3131. [Google Scholar] [CrossRef]
- Valassi, E.; Crespo, I.; Santos, A.; Webb, S. Clinical consequences of Cushing’s syndrome. Pituitary 2012, 15, 319–329. [Google Scholar] [CrossRef]
- Martel-Duguech, L.; Alonso-Jiménez, A.; Bascuñana, H.; Díaz-Manera, J.; Llauger, J.; Nuñez-Peralta, C.; Biagetti, B.; Montesinos, P.; Webb, S.M.; Valassi, E. Thigh Muscle Fat Infiltration Is Associated With Impaired Physical Performance Despite Remission in Cushing’s Syndrome. J. Clin. Endocrinol. Metab. 2020, 105, e2039–e2049. [Google Scholar] [CrossRef]
- Katsuhara, S.; Yokomoto-Umakoshi, M.; Umakoshi, H.; Matsuda, Y.; Iwahashi, N.; Kaneko, H.; Ogata, M.; Fukumoto, T.; Terada, E.; Sakamoto, R.; et al. Impact of Cortisol on Reduction in Muscle Strength and Mass: A Mendelian Randomization Study. J. Clin. Endocrinol. Metab. 2021, 107, e1477–e1487. [Google Scholar] [CrossRef]
- Fütő, L.; Tőke, J.; Patócs, A.; Szappanos, A.; Varga, I.; Gláz, E.; Tulassay, Z.; Rácz, K.; Tóth, M. Skeletal differences in bone mineral area and content before and after cure of endogenous Cushing’s syndrome. Osteoporos. Int. 2007, 19, 941–949. [Google Scholar] [CrossRef]
- Arnaldi, G.; Mancini, T.; Tirabassi, G.; Trementino, L.; Boscaro, M. Advances in the Epidemiology, Pathogenesis, and Man-agement of Cushing’s Syndrome Complications. J. Endocrinol. Investigat. 2012, 35, 434–448. [Google Scholar] [CrossRef]
- Belaya, Z.E.; Rozhinskaya, L.Y.; Melnichenko, G.A.; Solodovnikov, A.G.; Dragunova, N.V.; Iljin, A.V.; Dzeranova, L.K.; Dedov, I.I. Serum extracellular secreted antagonists of the canonical Wnt/β-catenin signaling pathway in patients with Cushing’s syndrome. Osteoporos. Int. 2013, 24, 2191–2199. [Google Scholar] [CrossRef]
- Garg, M.K.; Kharb, S. Dual energy X-ray absorptiometry: Pitfalls in measurement and interpretation of bone mineral density. Indian J. Endocrinol. Metab. 2013, 17, 203–210. [Google Scholar] [CrossRef]
- Bolotin, H.; Sievänen, H.; Grashuis, J. Patient-Specific DXA Bone Mineral Density Inaccuracies: Quantitative Effects of Nonuniform Extraosseous Fat Distributions. J. Bone Miner. Res. 2003, 18, 1020–1027. [Google Scholar] [CrossRef]
- Yu, E.W.; Thomas, B.J.; Brown, J.K.; Finkelstein, J.S. Simulated increases in body fat and errors in bone mineral density measurements by DXA and QCT. J. Bone Miner. Res. 2011, 27, 119–124. [Google Scholar] [CrossRef]
- Nelson, L.; Gulenchyn, K.Y.; Atthey, M.; Webber, C.E. Is a Fixed Value for the Least Significant Change Appropriate? J. Clin. Densitom. 2010, 13, 18–23. [Google Scholar] [CrossRef]
- Kim, M.-W.; Lee, D.-H.; Huh, J.-W.; Bai, J.-W. The impact of obesity on the accuracy of DXA BMD for DXA-equivalent BMD estimation. BMC Musculoskelet. Disord. 2022, 23, 1–8. [Google Scholar] [CrossRef]
- Evans, E.M.; Mojtahedi, M.C.; Kessinger, R.B.; Misic, M.M. Simulated Change in Body Fatness Affects Hologic QDR 4500A Whole Body and Central DXA Bone Measures. J. Clin. Densitom. 2006, 9, 315–322. [Google Scholar] [CrossRef]
| Tissue | Type of Scan | Indicator | Formula | Unit | Clinical Application | Recommendations | Cut-Off Points | Refs. |
|---|---|---|---|---|---|---|---|---|
| Lean | Whole body | LMI | kg/m2 | Indices for the diagnosis of “low muscle mass” in sarcopenia and as a biomarker in the identification of undernutrition status | ||||
| ALM | ALM = arms LM + legs LM | kg | EWGSOP2 | ♂ < 20 kg ♀ < 15 kg | [16] | |||
| ALMI | kg/m2 | ♂ < 7.0 kg/m2 ♀ < 6.0 kg/m2 | [16] | |||||
| Adipose | Whole body | FMI | kg/ m2 | Proposed for the diagnosis of obesity | NHANES | Normal: ♀ = 5–9, ♂ =3–6 Overweight: ♀ = 9–13, ♂ = 6–9 Obesity class I: ♀ = 13–17, ♂ = 9–12 Obesity class II: ♀ = 17–21, ♂ = 12–15 Obesity class III: ♀ > 21, ♂ > 15 | [45] | |
| AG | n.a | Cardiovascular risk factors: dyslipidemia, insulin resistance, impaired glucose tolerance, hypercholesterolemia, hypertriglyceridemia, hypertension | n.a | AG > 1 | [46,62] | |||
| VAT | n.a | cm2; cm3; g | Metabolic and cardiovascular risk factors | Men ≥1025.0 cm3 (1086.0 g) is associated with the risk factors of MetS | [63] | |||
| Women >100 cm2—metabolic risk >160 cm2—high metabolic risk | [64] | |||||||
| Bone | Whole body | BMD | g/cm2 | Osteoporosis | Diagnosis in post-menopausal women and men aged 65 and older BMD T-score: normal: ≥−1 osteopenia: −1 to −2.5; osteoporosis: ≤−2.5 Diagnosis in premenopausal women, young men, children BMD Z-score * <2.0—low bone mass | |||
| Proximal femur | WHO ISCD * | [23] | ||||||
| Lumbar spine | ||||||||
| Lumbar spine | VFA | The fracture-grading method devised by Genant | n.a | Vertebral fractures | ISCD, AACCE, ACE | n.a | [28,65] | |
| Lumbar spine | TBS | The analysed grey-level variations in the 2D DXA images. | Trabeculogram, TBS values | Vertebral fractures | ISCD | Range 0.5 to 2.0, >1.35—normal 1.35—partial damage <1.20 serious damage | [66] |
| Type of Tissue | Ref. | The Aim of the Study is Directly Connected with DXA | Sample (♀/♂) | Type of Scan/Indices | Densytometr Type |
|---|---|---|---|---|---|
| Fat tissue | [73] | To prospectively evaluate body composition changes with DXA in patients with active hypercortisolism and during the remission phase, considering the different therapeutic plans. | n = 23 (19/4) Active CS: 46.6 ± 12.2 yrs. Complete follow-up from 2009 to 2016. Remission CS: 51 ± 13.4 yrs. Controls n = 25 (21/4) 47 ± 9 yrs. | Whole-body scan: Total body: FM (g), FM (%), Lean (g), Lean + BMC (g) Trunk: FM (g), FM (%), Lean (g), Lean + BMC (g) R1 (the box was manually defined as DXA subregion 4 cm, slice at the top of the iliac crest): FM (g), FM (%), Lean (g), Lean + BMC (g) | Discovery W Hologic QDR 4500 C densitometer (Hologic Inc., Waltham, MA, USA) |
| [74] | To investigate the adipose tissue distribution, adipocytokine profiles, and metabolic risk profiles of patients in long-term remission of CS. | n = 116 (92/24) CS in remission n = 58 (46/12) 50.8 ± 12.3 yrs. HC n = 58 (46/12) 51.2 ± 12.4 yrs. | Whole-body scan: FM (%), LBM (%), Trunk FM (%), Extremity FM (%), Leg FM (%), Trunk: leg, Trunk: extremities | Hologic QDR 4500 densitometer (Hologic, Bedford, Zaventem, Belgium) | |
| [75] | To study body composition and BMD in patients with CS in long-term remission. | n = 100 (100/0) CS in remission n = 50 (50/0) 53 ± 14 yrs. HC n = 50 (50/0) age-matched | Scan of: lumbar spine, femur neck; BMD (g/cm2) Whole-body scan: LBM (kg), LBM (% of body weight) FM (kg), FM (% of body weight) abdominal FM (kg), abdominal FM (% of body weight) ALM (kg), ALM (% of body weight) | Lunar DPX-L, 12 Lunar Corporation, Madison, WI, USA). | |
| [76] | To develop a set of normative standards, reference ranges with the determination of the cut-off points (CP) values of DXA indices of central abdominal obesity (COIs) as a ratio of android (A) to gynoid (G) fat and tissue mass and their percentages that best differentiate CS and obese women (O) and confirm central abdominal obesity and to determine their normal CP values that best differentiate group C from CS, O1, and O2 and exclude abdominal obesity. | n = 72 (72/0) CS n = 18 (18/0) 43.58 ± 13.58 yrs. O1 n = 18 (18/0) 40.4 ± 12.05 yrs. O2 n = 18 (18/0) 45 ± 8 yrs. HW n = 18 (18/0) 40.09 ± 12.72 yrs. | Whole-body scan: COI1 = , COI2 = , COI3 = , COI4 = | System Lunar DPX-NT, enCore | |
| [77] | To develop CP values of new DXA indices of central abdominal obesity as ratios of android and trunk to legs, as well as trunk and legs to total fat and tissue mass and their percentages that best differentiate CS and O1 and confirm central abdominal obesity (m) and to determine their normal (n) CP values that best differentiate group C from CS, O1, and O2 and exclude abdominal obesity. | Whole-body scan: A/L ratios: A/L-t, A/L-f, A/L-t%, A/L-f%, Tr/L ratios: Tr/L-t, Tr/L-f, Tr/L-f%, Tr/L-f% Tr/To: Tr/To-t, Tr/To- f, Tr-f%, Tr-f%, L/To ratios: L/To-t, L/To-f, L/To-t%, L-Tof% | System Lunar DPX-NT, enCore | ||
| Lean tissue | [78] | To assess the prevalence of sarcopenia in CS patients in long-term remission using the EWGSOP2 criteria. | n = 72 (72/0) CS in remission 36 (36/0) median age 51 (45.2–60) yrs. Controls n = 36 (36/0) age-matched | Whole-body scan: ALM (kg) ALMI (kg/m2) | Hologic Discovery W, Software Apex Version 13.4 |
| Bone tissue | [56] | (1) To determine the frequency of non-traumatic vertebral fractures in patients with CS and the factors associated with vertebral fractures. (2) To assess bone mineral density in patients with CS. | n = 242 (188/54) Active CS n = 135 (111/24) HC n = 107 (77/30) age-matched | Scan of: lumbar spine, femoral neck; BMD (g/cm2), T-score, Z-score | Lunar DPX-L |
| [79] | (1) To characterise BMD and bone metabolism in patients with ACTH-dependent or ACTH-independent hypercortisolism versus healthy controls. (2) To analyse the effects of ACTH on BMD in patients with ACS and CD. | n = 73 (57/16) ACS n = 21 (19/2) 35 ± 11.53 yrs. CD n = 34 (25/9) 36.63 ± 11.22 yrs. HC n = 18 (13/5) 38.36 ± 14.19 yrs. | Scan of: lumbar spine, femoral neck, whole body, BMD (g/cm2), T-score, Z-score | Prodigy-GE densitometer (GE Healthcare, Chicago, IL, USA) | |
| [80] | To evaluate bone marrow fat by 1H-MRS in patients in remission (at least two years) vs active ACTH-dependent CS in comparison with age- and sex-matched controls. | n = 51 (38/13) Active CS n = 17 (12/5) 51.6 ± 3.4 yrs. Remission CS n = 17 (13/4) 48.3 ± 3.0 yrs. HC n = 17 (13/4) 50.3 ± 2.1 yrs. | Scan of: lumbar spine, femoral neck; BMD (g/cm2), T-score | GE Lunar iDXA (Healthcare) | |
| [81] | To identify risk factors for fracture in patients with endogenous CS and to evaluate the value of bone microarchitecture as assessed by TBS in this cohort of patients. | CS n = 182 (149/33) Patients with fractures n = 81 (59/22) 39.3 ± 12.9 yrs. Patients without fractures, n = 101 (90/11) 36.6 ± 12.4 yrs. | Scan of: lumbar spine, femoral neck, hip mean spine TBS, mean spine TBS Z- score, mean spine TBS BMD (g/cm2), femoral neck BMD (g/cm2), femoral neck Z-score, total hip BMD (g/cm2), total hip BMD Z-score | Lunar Prodigy (GEHC Lunar, Madison, Madison, WI, USA) | |
| [82] | To investigate alterations in BMD (evaluated by DXA and HR-pQCT) and bone microarchitecture (evaluated by HR-pQCT) in a cohort of patients with active endogenous CS. | n = 81 (68/13) Active CS (24/6) 38.1 ± 14 yrs. HC (44/7) 36.1 ± 8.3 yrs. | Scan of: lumbar spine, femoral neck, total femur, radius; BMD (g/cm2), T-score, Z-score | GE Lunar Prodigy Advance; (GE Healthcare Madison, Madison, WI, USA) | |
| [83] | To compare lumbar spine BMD and TBS in a large cohort of cases of overt CS and MACE of various etiologies and to assess the evolution of BMD and TBS in the subset of patients after remission of overt CS. | n = 110 (89/21) CS n = 53 (42/11) 49.9 ± 12.8 yrs. MACE n = 39 (34/5) 57.8 ± 9.3 yrs. patients with non-secreting adrenal incidentalomas n = 18 (13/5) 59.2 ± 9.1 yrs. | Lumbar spine scan BMD (g/cm2), T-scores; TBS | Lunar iDXA, GE, Madison, USA; TBS insight v2.1 software (Medimaps, Mérignac, France). | |
| [84] | To evaluate the association between bone marrow fat (BNF), as assessed by magnetic resonance spectroscopy (MRS), and morphometric vertebral fractures (VFs) in patients with active endogenous CS. | n = 35 (26/9) Active endogenous CS n = 20 (15/5) 44 ± 13 yrs. HC n= 15 (11/4) age 43 ± 12 yrs. | Lumbar spine scan BMD (g/cm2) A quantitative morphometric; six points were marked on each vertebral body to describe: anterior (Ha), middle (Hm), and posterior (Hp) vertebral heights; Height ratios (Ha/Hp, Hm/Hp, Hp/Hp of the above vertebrae, Hp/Hp of the below vertebrae) were calculated for each vertebra from L1 to L4; | Hologic Discovery W | |
| [25] | (1) To evaluate the prevalence of bone complications (bone demineralisation, vertebral and peripheral fractures) in patients with CS. (2) To investigate the role of gender, disease aetiology, duration, and degree of hypercortisolism, as well as the impact of GR gene polymorphisms, on the development of these complications. | n = 52 (43/9) CD n = 38 (32/6) 43.58 ± 13.23 yrs. ACS n = 14 (11/3) 48.50 ± 1 5.05 yrs. | DXA morphometry; Scan of: lumbar spine, femoral neck, total femur; BMD (g/cm2), T-score, Z-score | Lunar Prodigy® DXA (GE Healthcare, Madison, WI, USA). enCore 2007 version 11.4. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radecka, A.; Lubkowska, A. The Significance of Dual-Energy X-ray Absorptiometry (DXA) Examination in Cushing’s Syndrome—A Systematic Review. Diagnostics 2023, 13, 1576. https://doi.org/10.3390/diagnostics13091576
Radecka A, Lubkowska A. The Significance of Dual-Energy X-ray Absorptiometry (DXA) Examination in Cushing’s Syndrome—A Systematic Review. Diagnostics. 2023; 13(9):1576. https://doi.org/10.3390/diagnostics13091576
Chicago/Turabian StyleRadecka, Aleksandra, and Anna Lubkowska. 2023. "The Significance of Dual-Energy X-ray Absorptiometry (DXA) Examination in Cushing’s Syndrome—A Systematic Review" Diagnostics 13, no. 9: 1576. https://doi.org/10.3390/diagnostics13091576
APA StyleRadecka, A., & Lubkowska, A. (2023). The Significance of Dual-Energy X-ray Absorptiometry (DXA) Examination in Cushing’s Syndrome—A Systematic Review. Diagnostics, 13(9), 1576. https://doi.org/10.3390/diagnostics13091576







