Abstract
We field-assessed the accuracy, acceptability, and feasibility of the SD BIOLINE HIV/Syphilis Duo rapid diagnostic test in three groups: pregnant women, female sex workers (FSW), and men who have sex with men (MSM). Venous blood samples collected in the field were compared with the respective gold standard methods: SD BIOLINE HIV/Syphilis Duo Treponemal Test versus FTA-abs (Wama brand) treponemal laboratory test for syphilis, and SD BIOLINE HIV/Syphilis Duo Test versus the fourth generation Genscreen Ultra HIV Ag-Ag (Bio-Rad brand) laboratory test for HIV. From a total of 529 participants, 397 (75.1%) were pregnant women, 76 (14.3%) FSW and 56 (10.6%) MSM. Sensitivity and specificity parameters of HIV were 100.0% (95% CI: 82.35–100.0%) and 100.0% (95% CI: 99.28–100.0%), respectively. Sensitivity and specificity parameters found for TP antibody detection were 95.00% (95% CI: 87.69–98.62%) and 100.0% (95% CI: 98.18–100.0%), respectively. The SD BIOLINE HIV/Syphilis Duo Test showed high acceptability among participants (85.87%) and health professionals (85.51%), as well as easy usability by professionals (91.06%). The usability of the SD BIOLINE HIV/Syphilis Duo Test kit would not be a barrier to accessing rapid testing, if the product were incorporated into the list of health service supplies.
Keywords:
HIV; Syphilis; screening; diagnosis; duo test; SD bioline duo assay; sensitivity; specificity; kappa coefficient; Amazon 1. Introduction
The global pharmaceutical industry has produced several point-of-care health technologies aimed to improve communicable disease diagnosis, with emphasis on rapid solid-phase immunochromatographic assays for qualitative detection of antibodies and antigens. Since 2012, the Brazilian Ministry of Health has invested in rapid testing strategies, offering individual rapid tests for HIV and syphilis, combined with a structured process of acquisition, distribution, online technical training, and quality assessment by referral laboratories before implementation and availability in the healthcare system [1,2].
From the beginning of the epidemic in 1980s to June 2021, a total of 1,045,355 AIDS cases were reported in Brazil, including 381,793 new cases of HIV registered in 2021, with a detection rate of 14.1/100,000 inhabitants [3]. In 2020, 115,371 cases of acquired syphilis (detection rate of 54.5 cases/100,000 inhabitants), 61,441 cases of syphilis in pregnant women (detection rate of 21.6/1000 live births), 22,065 cases of congenital syphilis (with an incidence rate of 7.7/1000 live births), and 186 deaths from congenital syphilis (mortality rate of 6.5/1000 live births), were reported [4]. Aiming to enhance diagnosis and detection rates, the World Health Organization (WHO) prequalified the SD Bioline HIV/Syphilis Duo Test, making it the first simultaneous diagnostic point of care available for purchase by the public and private sectors, and recommended the application of the Duo rapid test in antenatal services and other testing sites, citing the simplification of acquisition process and advantages such as: reduction of storage space; simplification of training for healthcare professionals; use of a single finger prick; receiving test and treatment results in a shorter period; and trying to reduced unit cost for reagents compared to two single rapid tests for HIV and syphilis [5,6].
For HIV and syphilis, only rapid tests demonstrating sensitivity performance of ≥99.5% and ≥94.5%, and specificity performance of ≥99.0% and ≥93.0%, respectively, are acquired [3,4]. Further, operational performance criteria are also considered, which require the use of rapid tests presenting only a single reagent, storage at room temperature, execution in a maximum of four steps, reading in up to 30 min, and no laboratory experience requirement to perform the rapid test [7]. The SD Bioline HIV/Syphilis Duo Test results from laboratory prequalification revealed performance parameters of 100% (95%CI 98.2–100%) and 99.5% (95%CI 97.2–100%) on sensitivity and specificity for HIV, respectively, while for Treponema pallidum antibodies detection, the final sensitivity was 87% (95%CI 81.5–91.3%), with specificity of 99.5% (95%CI 97.2–100%) compared to reference (gold standard) essays [5].
Despite the aforementioned advantages, there is a lack of data on the duo test acceptance by service users in Brazil, especially in specific population segments where cases of HIV infection and syphilis has high incidence, such as men who have sex with men (MSM), female sex workers (FSW), and pregnant women in imminent risk for vertical transmission. Another relevant aspect, but less investigated, is the usability perception by healthcare professionals. The present work report results on the first survey on accuracy, acceptability and usability of the SD BIOLINE HIV/Syphilis Duo Test conducted in a real-life scenario in Brazil, allowing understanding the advantages and disadvantages of incorporating this health technology in the healthcare service and guarantee access to safe and quality diagnosis.
2. Materials and Methods
2.1. Study Design and Settings
This an epidemiological field-based, cross-sectional study, one which evaluated the accuracy, acceptability and usability performance of the SD BIOLINE HIV/Syphilis Duo Test for HIV and syphilis screening in the three different key populations—pregnant woman, MSM and FSW—in the city of Belém, capital of Pará State, Northern Brazil, from April to July 2021. The study design was based on a previously described protocol by the ProSPeRo Network [8].
Convenience sampling was employed considering the availability of individuals willing to participate in the study, supplies, and personal staff. Eligible participants were recruited at distinct sites: pregnant women attending to antenatal care routine services were recruited at three clinical sites, including Icoaraci, Bengui II and Paraiso dos Pássaros healthcare centers; MSM were recruited at Belém’s Testing and Counseling Center for STI’s, and female sex workers were recruited during a targeted action for testing and counseling at their working places (three nightclubs).
2.2. Participant Enrollment and Testing Procedures
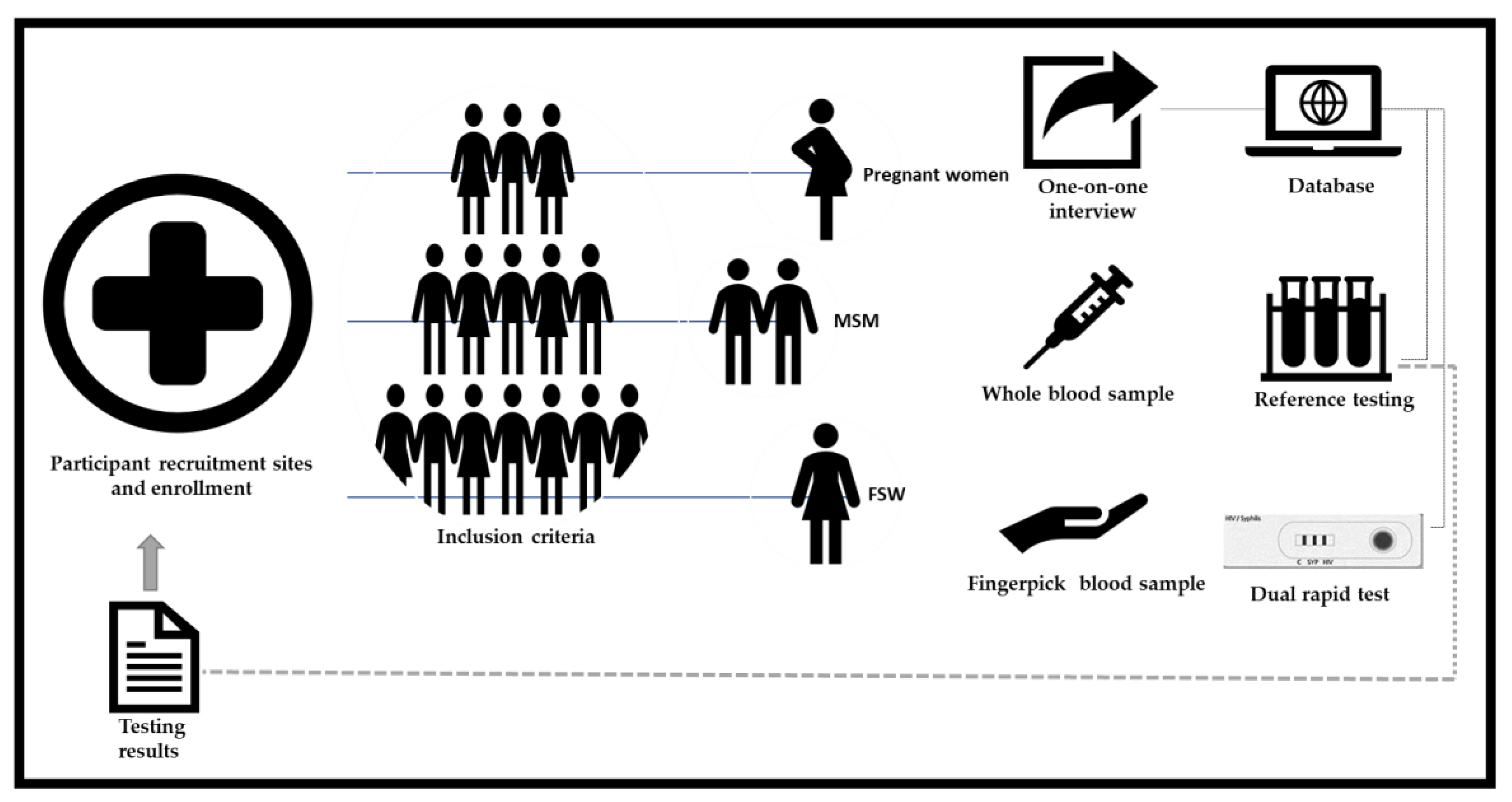
Participants were invited and enrolled by a trained field researcher at each recruitment site, who explained the study purpose, testing procedures and applied an informed consent form (ICF) (Figure 1). General inclusion criteria consisted of participants 18 years and older, who provided informed written consent and were willing to be interviewed and tested. In particular for MSM, included individuals had to report engaging in sexual activity with another male in the last 12 months, and for FSW, engaging in sexual activity in exchange of payment or some other benefit in the last 12 months. Restriction regarding period of pregnancy or prenatal care were not applied for inclusion of pregnant women. Participants who refused to provide consent, who were not willing to be interviewed and tested, or who were under the influence of drugs or alcohol at the time of their participation in the study were excluded. The use of antimicrobials by participants who received prescribed treatment for syphilis or other infections in any period prior to study enrollment was recorded, but not used as exclusion criteria.
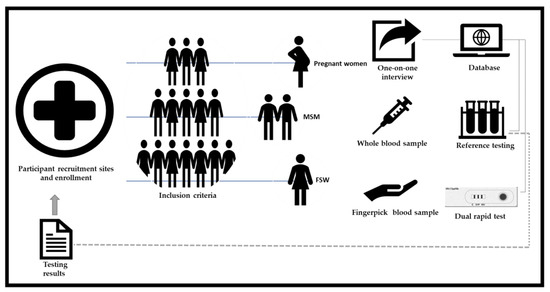
Figure 1.
Key populations enrollment and testing procedures.
The evaluated kit consisted of the SD BIOLINE HIV/Syphilis Duo Test for HIV (Abbot, South Korea) is a solid phase immunochromatographic assay for the qualitative, simultaneous detection of antibodies to all isotypes (IgG, IgM, IgA) specific to HIV-1/2 and Treponema pallidum in human serum, plasma or whole blood. The manufacturer reports sensitivity and specificity values of 99.91% and 99.67% for HIV, and 99.67% and 99.72% for syphilis, respectively [5]. Each kit includes 25 essays, containing a vial of diluent solution, 25 pipettes, 70% alcohol wipes and an instruction form. The evaluated lancet was a 23 g safety lancet type, a different model from the one provided in the kit, because the only lancet accepted for use by the Unified Health System of Brazil (SUS) is of this type.
A fingerpick/whole blood sample was used to perform the SD BIOLINE HIV/Syphilis Duo Test according to the manufacturer’s instructions. Testing results were recorded in worksheets, counter-checked by a technician and a trained field researcher, reviewed online after photographic registration and sending via message app, and finally sealed in envelopes. In case of an invalid result, another test was performed. The test was reported as “Invalid” if the result of the repeated test was still invalid. In a case of discordant reading between the two independent evaluators, a third reader performed a final reading.
Results of this duo rapid antibody test under evaluation were not reported to participants. In addition, 5 mL of venipuncture whole blood was collected into an EDTA collection tube which was stored and then transported to the reference laboratory, in a temperature-controlled container within six hours of specimen collection.
Reference testing considered the national HIV and syphilis testing algorithm, which was performed at reference laboratories (STI’s laboratory, Bacteriology and Mycology section; and Retrovirus laboratory, Virology Section—Evandro Chagas Institute, Ministry of Health) by a laboratory staff, who were blinded to prior test results and clinic information of the participants. Standard reference test results were reported to participants, who received an issued report with HIV and syphilis tests results. All the documents were filed and delivered sealed in the health units where the participants were recruited, except for the FSW. For FSW, the tests results were filed at the fixed healthcare unit belonging to the “Consultório na Rua/Street Clinic strategy” and at the nightclubs in which were recruited. Pregnant women and MSM participants received a text message via messaging application and/or an email with the conclusion and research achievements, except for the FSW, as they did not provide any phone number or e-mail address. Finally, a seminar aiming to present the project results was held on 17 September 2021 at the Evandro Chaga Institute (IEC/PA). The event was directed to SESMA (Municipal Secretary of Health), basic healthcare units and CTA managers and staff, as well as to GEMPAC (Group of Female Prostitutes From the State of Pará) members, a non-governmental association representing the FSW.
2.3. Acceptability and Usability Survey
To determine the acceptability of the investigated SD BIOLINE HIV/Syphilis Duo Test, a trained research assistant conducted a one-on-one interview for completing a socio-demographic questionnaire with each participant at field study sites. Acceptability among participants was determined by evaluating preference for rapid POC testing or laboratory testing; preference for rapid duo test or single test for each disease; willingness to wait for results and how much time they would be willing to wait for these results, and finally which of the tests would stimulate testing for HIV/Syphilis by the participants.
The usability of the SD BIOLINE HIV/Syphilis Duo Test was evaluated by health professionals from nine different services linked to the Primary Health Care strategy in the city of Belém. Health professionals contacted in advance by research staff were enrolled after an explanation of the study’s purpose, and gave consent by signing the specific ICF. The research staff provided the kit and supplies for evaluation, together with a video with guidelines for test execution. Then, the healthcare professionals organized themselves into pairs, executed the test in each other, and finally, filled out the survey usability form.
2.4. Data Analysis
A data entry form was created (Google Form) for each key population investigated, where questionnaires data and test results (laboratory and rapid test) were uploaded. All collected data was counter-checked and submitted to quality control daily. From this database, proportions were calculated for categorical variables by using excel.
Contingency tables were constructed in order to calculate sensitivity, specificity, positive predictive value, negative predictive value and accuracy performance. The rapid test results were compared with the respective reference (gold standard) methods: SD BIOLINE HIV/Syphilis Duo Treponemal Test versus FTA-abs (Wama brand) treponemal laboratory test for syphilis, and SD BIOLINE HIV/Syphilis Duo Test versus the 4th generation Genscreen Ultra HIV Ag-Ag (Bio-Rad brand) laboratory test for HIV. Confidence intervals (CIs) of 95% were considered for each estimate. The calculations were performed using the software MedCalc (https://www.medcalc.org/, accessed on 19 July 2022). The kappa index was calculated, to compare concordance between the reference tests results and the SD BIOLINE HIV/Syphilis Duo Test, using the software VassarStats (http://vassarstats.net/, accessed on 20 July 2022). The results were compared to performance values described by the manufacturer and prequalification values by the World Health Organization and the Brazilian Ministry of Health manuals for diagnosis.
2.5. Ethical Considerations
The present study had approval granted by the Research and Ethics Committee of the Evandro Chagas Institute (No. 19146919.3.0000.0019, approval date: 27 August 2019) and the Pan American Health Organization (No. PAHOERC-2019-08-0059, approval date: 27 August 2019). Consent was also provided by the Permanent Education Center of the Health Department of Belém (Date: 25 July 2019). Each participant was invited and voluntarily enrolled by providing informed consent. Study data and blood samples were identified by unique identification codes, aiming to protect data and maintain confidentiality.
3. Results
3.1. SD Bioline HIV/Syphilis Duo Test Kit Performance
From a total of 529 participants, 397 (75.1%) were pregnant women, 76 (14.3%) FSW and 56 (10.6%) MSM. For the syphilis, rapid Duo testing revealed a total of 76 (14.3%) reactive participants, including 29 (13.7%) pregnant women, 18 (32.1%) MSM and 29 (38.1%) FSW; while on the reference testing by FTA-Abs, 80 (15.1%) of participants were reactive, including 31 (7.8%) of pregnant women, 18 (32.1%) MSM and 31 (40.7%) FSW. For HIV, the rapid Duo testing and reference standard essay presented concordant results with 19 (3.6%) reactive participants, including four (1.0%) of pregnant women, 11 (19.6%) MSM and four (5.2%) FSW.
The concordance performance of the rapid Duo test with the reference test demonstrated sensitivity and specificity parameters of 100.0% (95% CI: 82.35–100.0%) and 100.0% (95% CI: 99.28–100.0%), respectively, for HIV (kappa coefficient: 1.00 [95% CI, 1.00–1.00]). As for the differences, sensitivity and specificity parameters found for TP antibody detection were of 95.00% (95% CI: 87.69–98.62%) and 100.0% (95% CI: 98.18–100.0%), respectively (kappa coefficient: 0.9705 [95% CI: 0.9417–0.9993]) (Table 1 and Table 2).

Table 1.
Concordance performance of the rapid Duo test with the reference test for Syphilis.

Table 2.
Concordance performance of the rapid Duo test with the reference test for HIV.
3.2. Pregnant Women, MSM and FSW Acceptability
Among the studied groups, over 50% of both pregnant women and MSM would choose the rapid test over the laboratory test. Regarding the use of the rapid test to detect one or two simultaneous infections (duo), most participants would prefer the duo test. As for the waiting time, despite not exceeding 50% of the audience in each group, most participants would like to receive the test results swiftly (30 min to 1 h) (Table 3).

Table 3.
Acceptability of the HIV-syphilis rapid diagnostic test among pregnant women, MSM and FSW.
3.3. Healthcare Professional’s Acceptability
Among the interviewed healthcare professionals, the rapid Duo test was accepted by 85.51%, with 5.80% responding not preferring the simple or Duo test, as they have the ability to handle both tests. In relation to healthcare professionals’ opinion on the test acceptance by the target public, the majority responded that all groups would agree to be tested using the rapid Duo test (89.86%). According to the professionals, the group with the greatest refusal could be the FSW, with a percentage of 4.35% (Table 4).

Table 4.
Acceptability of the HIV-syphilis rapid diagnostic test among healthcare professionals.
3.4. Feasibility of the Duo Rapid Test among Healthcare Professionals
A total of 138 healthcare professionals answered the questionnaire regarding professional training, operational characteristics of the Duo test kit, and usability suggestion by the target public. Among the healthcare professionals, the majority have graduated from high school (57.24%) and were professionally trained as a nursing technician (51.44%). Also, most of the professionals classified the test as easy (91.06%) to operate and apply, and specifically reported difficult in the use of the provided pipette for blood collection; “interpretation of results” was the characteristic with the best acceptability among healthcare professionals (95.65%) (Table 5).

Table 5.
Operational feasibility of the HIV-Syphilis rapid diagnostic test among healthcare professionals.
4. Discussion
The present study reports one of the first investigations conducted in Brazil on the accuracy, acceptability and usability of a rapid test for simultaneous diagnosis of HIV and syphilis. The SD BIOLINE kit, the only kit available in the country, during the pandemic period, was evaluated, given the limitations imposed by the shortages during the pandemic. However, there are products with the same purpose registered in Brazil whose manufacturers are MedLevensohn® (Rio de Janeiro, Brazil), Eco Diagnóstica (Minas Gerais, Brazil), Lumiradx (São Paulo, Brazil) and Bio Manguinhos (Rio de Janeiro, Brazil).
Understanding the epidemiological scenario is highly important for guiding the prioritization of prevention measures, such as rapid testing. It is also known that, despite the availability of diagnostic tools for HIV and syphilis in the market and in the healthcare system, other factors are pointed out as barriers to access to testing. Regarding STIs, and from the perspective of users, especially key populations such as MSM and FSW, prejudice and stigma, acceptance of sexuality, fear of the result, and lack of information are highlighted as the main barriers to accessing testing [9]. The present study revealed that offering a simultaneous rapid test for HIV and syphilis diagnosis would not drive users away from health services given that most prefer rapid testing over laboratory testing (Table 3). Among the pregnant women, MSM, and FSW groups, wide acceptance of the rapid Duo test was observed (Table 3). In the USA, 28% of patients screened for HIV in an emergency medical service believed that rapid testing was less or much less accurate than conventional testing, reinforcing the need for acceptability studies prior to the decision to implement new diagnostic devices in healthcare services [10]. A study conducted in Peru concluded high acceptability of rapid testing among transgender women (98.8%), however, in Argentina, only 60.7% of this public preferred simultaneous HIV and syphilis diagnostic testing [11]. Regarding the waiting time, we observed that most of participants prefer to receive the results as quickly as possible and in less than an hour. Flores [12], demonstrated that the implementation of simultaneous screening for HIV/syphilis testing in a health service in Peru resulted in 52% completely satisfied patients and 48% satisfied with the improvements in care processes.
The acceptability of rapid tests was not a consensus among healthcare professionals, as in the past there has been enormous resistance during their application in healthcare services in Brazil. Many professionals did not trust the results expressed on the test strip, and preferred to wait for results issued by the reference laboratories, and by means of referenced methods, for the purposes of clinical management. However, scientific evidence generated on the accuracy of rapid tests in comparison to gold standard tests, and the adoption of minimum parameters of sensitivity and specificity, mitigated this resistance. Such scenario was also observed in the present study (Table 4).
In addition, practitioners in service demonstrated a preference to use the duo test over the single test, mainly due to its ease of application and reduction supplies (data not shown). The 138 volunteer healthcare professionals enrolled in this study reported that the SD BIOLINE was easy to perform (Table 5). The use of the collection pipette was the most difficult feature described, but classified as such only by a small proportion of participants (Table 5). Although most healthcare professionals are qualified to apply the rapid test, some reported not using it frequently or had started working with this function only recently, which may explain their lower skill with the collection pipette (data not shown). Other participants also stated that users of health services would accept the simultaneous rapid test for diagnosis of HIV and syphilis, which was ratified by the users’ responses (Table 3).
Despite specific strategies for healthcare professionals’ qualification on rapid tests’ application in the context of STIs, Brazil adopts such technologies for several other infections. At the time of this study, many professionals had also been trained to perform rapid tests for COVID-19 detection, which may contribute to the high rate of ease performing the SD BIOLINE test. Even though, the reading of results being one of the features rated as easiest by the professionals, such finding is limited as the ProSPeRo Network protocol for usability investigations recommend that each professional perform at least 50 rapid tests before answering the questionnaire [8]. During the field application of the duo test, the project staff reported that variable color intensity in the syphilis band resulted, a fact also observed by Olugbenga [13], Bristow [14,15] and Heuvel [16]. This shows the need for training of positive test readings by professionals despite the easy classification indicated by them in this study, which may aid to avoid interpretation of erroneous results.
The observation of rapid test performance in a real-life scenario is another extremely relevant criteria for its incorporation in healthcare systems. The rapid tests for syphilis presented a sensitivity of 95%, a specificity of 100% and an accuracy of 99.25% (Table 1). For HIV, all measures were 100% in all groups (Table 2), which are above the sensitivity and specificity criteria recommended by the Brazilian Ministry of Health and WHO pre-qualifications [17]. Although the kappa value indicated almost-perfect agreement with the reference test, the results of the group of pregnant women for syphilis showed a sensitivity of 93.55%, as well for the FSW group, which is slightly below the established criteria of 94.5% [17]. These values are higher than showed by WHO in prequalification report for the case of antibodies to Treponema pallidum, with 87.0% (95% CI: 81.5–91.3%) for final sensitivity and the final specificity of 99.5% (95% CI: 97.2–100%) compared to the reference assays. Until now, the minimal sensitivity as specificity accepted by the Ministry of Health of Brazil was the same for single and for duo tests, and for all types of specimens (e.g., serum, plasma, or whole blood). It would be important to review these parameters considering the methodology differences, different specimens’ type and other aspects, such as the increase of access and acceptability.
Studies conducted by Black et al. [18], in South Africa, also showed low sensitivity of the SD BIOLINE for the diagnosis of syphilis in FSW from the finger stick whole blood sample. Studies with pregnant women in Zambia and Vietnam also revealed low sensitivity for syphilis diagnosis from SD BIOLINE finger stick whole blood sample [19,20]. Assays of the SD BIOLINE with serum and plasma samples also showed low sensitivity for syphilis diagnosis performed in pregnant women and MSM in the US, South Sudan and Zimbabwe [21,22,23]. SD BIOLINE was 100% sensitive from plasma of pregnant women in Uganda [24]. Despite the low sensitivity shown for syphilis diagnosis, such studies provided a satisfactory specificity. The specificity values for pregnant women and FSW groups are within the limit recommended by the Brazilian Ministry of Health (93% specificity). In areas of high prevalence rates of a disease, the higher the test specificity, the higher the positive predictive value are found, therefore, higher the probability of disease in cases of positive result. It is worth mentioning that some factors can interfere with the accuracy of a diagnostic test, such as execution failures, the use of the incorrect volume of buffer or sample, reading the test result at the incorrect time, incorrect interpretation of the result, difficulty in interpreting weakly reagent bands, etc.
In this study, the field phase included double-checking of rapid test results. The survey also asked the volunteers about some factors already reported as possible causes of false-reactive results in rapid tests, such as autoimmune diseases, liver disease, pregnancy, recent H1N1 vaccination, hemodialysis patients, and patients on interferon therapy, among others. However, none of these factors seems to be related to the four false-negative cases for syphilis (Table 1). These were serological scars. In relation to HIV, all indices of all specimens tested were 100% compared to the gold standard, meeting the criteria defined by the ministry and PAHO [17], which is 99.5% for sensitivity and 99.0% for specificity. In addition, the syphilis results for the MSM group showed all rates at 100%, meeting the recommended criteria [7].
5. Conclusions
This study concludes that the accuracy, acceptability and usability of the SD BIOLINE HIV/Syphilis Duo Test kit would not be barriers to access to rapid testing, if the product were incorporated into the list of inputs of the health services.
Author Contributions
Conceptualization, D.C.S., G.N.D., P.C.G., N.M.C.V., A.S.B. and M.L.B.; methodology, D.C.S., G.N.D., P.C.G., N.M.C.V., A.S.B. and M.L.B.; validation, D.C.S., L.C.F.F., H.S.d.R., Y.C.R., F.B.F., C.d.O.S., O.M., G.N.D. and J.d.F.R.F.; formal analysis, L.C.F.F. and H.S.d.R.; investigation, D.C.S., L.C.F.F., H.S.d.R., Y.C.R., F.B.F. and C.d.O.S.; resources, J.d.F.R.F. and O.M.; data curation, L.C.F.F., H.S.d.R. and G.N.D.; writing—original draft preparation, D.C.S., L.C.F.F. and H.S.d.R.; writing—review and editing, D.C.S., H.S.d.R., Y.C.R., P.C.G., A.S.B., M.L.B. and C.d.O.S.; visualization, Y.C.R. and H.S.d.R.; supervision, D.C.S. and M.L.B.; project administration, D.C.S. and M.L.B.; funding acquisition, D.C.S., G.N.D., P.C.G., N.M.C.V., A.S.B. and M.L.B. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded through Letter Agreement TC 66–SCON2019-00400 between the Pan American Health Organization (PAHO/WHO) and the Department of Chronic Diseases and Sexually Transmitted Infections—DCCI/SVS/MS, with the aim of support the national response to HIV/AIDS, Syphilis and other STIs and Viral Hepatitis (Available online: https://www.paho.org/pt/documentos/relatorio-tecnico-do-termo-cooperacao-no-66-controle-dsthivaids—e-hepatitis-virals-2, accessed on 30 November 2022).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Research and Ethics Committee of the Evandro Chagas Institute (Nº 19146919.3.0000.0019, approval date: 27 August 2019) and the Pan American Health Organization (Nº PAHOERC-2019-08-0059, approval date: 7 October 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All relevant data is presented within the manuscript.
Acknowledgments
To the volunteers, the team technicians, the team from Evandro Chagas Institute, the Municipal Health Department of Belém, GEMPAC, the team from the Federal University of Santa Catarina and FAPEU, Pan American Health Organization (PAHO/WHO) and the Department of Diseases, Chronic Conditions and Sexually Transmitted Infections (DCCI/SVS/MS).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ministério da Saúde. Protocolo Clínico e Diretrizes Terapêuticas para Prevenção da Transmissão Vertical de HIV, Sífilis e Hepatites Virais; Ministério da Saúde: Brasília, Brazil, 2020.
- Gaspar, P.C.; Bigolin, Á.; Alonso Neto, J.B.; Pereira, E.D.D.S.; Bazzo, M.L. Brazilian Protocol for sexually transmitted infections 2020: Syphilis diagnostic tests. Rev. Soc. Bras. Med. Trop. 2021, 54, e2020630. [Google Scholar] [CrossRef]
- Ministério da Saúde. Boletim Epidemiológico do HIV/Aids; Ministério da Saúde: Brasília, Brazil, 2021.
- Ministério da Saúde. Boletim Epidemiológico de Sífilis; Ministério da Saúde: Brasília, Brazil, 2021.
- World Health Organization. WHO Information Note on the Use of Dual HIV/Syphilis Rapid Diagnostic Tests (RDT). 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/252849/WHO-RHR-17.01-eng.pdf?sequence=1 (accessed on 10 November 2022).
- World Health Organization. Product: SD Bioline HIV/Syphilis Duo. Number PQDx 0179-012-00. 28 October 2015; WHO Prequalification of In Vitro Diagnostics Programmes Public Report; World Health Organization: Geneva, Switzerland, 2022. [Google Scholar]
- Ministério da Saúde; Secretaria de Vigilância em Saúde; Departamento de Vigilância, Prevenção e Controle das Doenças Sexualmente Transmissíveis, Aids e Hepatites Virais. Manual Técnico para Diagnóstico da Sífilis; Ministério da Saúde: Brasília, Brazil, 2016. Available online: http://www.aids.gov.br/pt-br/pub/2016/manual-tecnico-para-diagnostico-da-sifilis (accessed on 17 November 2022).
- ProSPeRo Network. Standardised protocol for a prospective cross-sectional multicentre clinic-based evaluation of two dual point-of-care tests for the screening of HIV and syphilis in men who have sex with men, sex workers and pregnant women. BMJ Open 2020, 10, e044479. [Google Scholar]
- Cota, V.L.; Cruz, M.M.D. Barreiras de acesso para Homens que fazem Sexo com Homens à testagem e tratamento do HIV no município de Curitiba (PR). Saúde Debate 2021, 45, 393–405. [Google Scholar] [CrossRef]
- Merchant, R.C.; Clark, M.A.; Seage, G.R., III; Mayer, K.H.; Degruttola, V.G.; Becker, B.M. Emergency department patient perceptions and preferences on opt-in rapid HIV screening program components. AIDS Care 2009, 21, 490–500. [Google Scholar] [CrossRef]
- Zalazar, V.; Frola, C.E.; Gun, A.; Radusky, P.D.; Panis, N.K.; Cardozo, N.F.; Fabian, S.; Duarte, M.I.; Aristegui, I.; Cahn, P.; et al. Acceptability of dual HIV/syphilis rapid test in community-and home-based testing strategy among transgender women in Buenos Aires, Argentina. Int. J. STD AIDS 2021, 32, 501–509. [Google Scholar] [CrossRef]
- Flores, E.C.; Lluque, M.E.; Chiappe, M.; Lino, R.; Bayer, A.M. Operations research study to implement HIV and syphilis point-of-care tests and assess client perceptions in a marginalised area of Lima, Peru. Int. J. STD AIDS 2015, 26, 723–728. [Google Scholar] [CrossRef]
- Olugbenga, I.; Taiwo, O.; Laverty, M.; Ngige, E.; Anyaike, C.; Bakare, R.; Ogunleye, V.; Maddox, B.L.P.; Newman, D.R.; Gliddon, H.D.; et al. Clinic-based evaluation study of the diagnostic accuracy of a dual rapid test for the screening of HIV and syphilis in pregnant women in Nigeria. PLoS ONE 2018, 13, e0198698. [Google Scholar] [CrossRef]
- Bristow, C.C.; Leon, S.R.; Huang, E.; Brown, B.J.; Ramos, L.B.; Vargas, S.K.; Flores, J.A.; Caceres, C.F.; Klausner, J.D. Field evaluation of a dual rapid diagnostic test for HIV infection and syphilis in Lima, Peru. Sex. Transm. Infect. 2016, 92, 182–185. [Google Scholar] [CrossRef]
- Bristow, C.C.; Severe, L.; Pape, J.W.; Javanbakht, M.; Lee, S.-J.; Comulada, W.S.; Klausner, J.D. Dual rapid lateral flow immunoassay fingerstick wholeblood testing for syphilis and HIV infections is acceptable and accurate, Port-au-Prince, Haiti. BMC Infect. Dis. 2016, 16, 302. [Google Scholar] [CrossRef]
- Van Den Heuvel, A.; Smet, H.; Prat, I.; Sands, A.; Urassa, W.; Fransen, K.; Crucitti, T. Laboratory evaluation of four HIV/syphilis rapid diagnostic tests. BMC Infect. Dis. 2019, 19, 1. [Google Scholar] [CrossRef]
- Ministério da Saúde; Secretaria de Vigilância em Saúde; Prevenção e Controle das Infecções Sexualmente Transmissíveis, do HIV/Aids e das Hepatites Virais. Manual Técnico para o Diagnóstico da Infecção pelo HIV em Adultos e Crianças, 4th ed.; Ministério da Saúde: Brasília, Brazil, 2017; 85p.
- Black, V.; Williams, B.G.; Maseko, V.; Radebe, F.; Rees, H.V.; Lewis, D.A. Field evaluation of Standard Diagnostics’ Bioline HIV/Syphilis Duo test among female sex workers in Johannesburg, South Africa. Sex. Transm. Infect. 2016, 92, 495–498. [Google Scholar] [CrossRef]
- Kasaro, M.P.; Bosomprah, S.; Taylor, M.M.; Sindano, N.; Phiri, C.; Tambatamba, B.; Malumo, S.; Freeman, B.; Chibwe, B.; Laverty, M.; et al. Field performance evaluation of dual rapid HIV and syphilis tests in three antenatal care clinics in Zambia. Int. J. STD AIDS 2019, 30, 323–328. [Google Scholar] [CrossRef]
- Withers, K.; Bristow, C.; Nguyen, M.; Stafylis, C.; Giang, L.M.; Klausner, J.D. A field evaluation of a rapid dual immunoassay for human immunodeficiency virus and syphilis antibodies, Hanoi, Vietnam. Int. J. STD AIDS 2019, 30, 173–180. [Google Scholar] [CrossRef]
- Lodiongo, D.K.; Bior, B.K.; Dumo, G.W.; Katoro, J.S.; Mogga, J.J.H.; Lokore, M.L.; Abias, A.G.; Carter, J.Y.; Deng, L.L. Field evaluation of SD BIOLINE HIV/Syphilis Duo assay among pregnant women attending routine antenatal care in Juba, South Sudan. PLoS ONE 2018, 13, e0205383. [Google Scholar] [CrossRef]
- Holden, J.; Goheen, J.; Jett-Goheen, M.; Barnes, M.; Hsieh, Y.H.; Gaydos, C.A. An evaluation of the SD Bioline HIV/syphilis duo test. Int. J. STD AIDS 2018, 29, 57–62. [Google Scholar] [CrossRef]
- Rietmeijer, C.A.; Mungati, M.; Kilmarx, P.H.; Barr, B.T.; Gonese, E.; Kularatne, R.S.; Lewis, D.A.; Klausner, J.D.; Rodgers, L.; Handsfield, H.H. Performance of a Dual Human Immunodeficiency Virus/Syphilis Rapid Test Compared with Conventional Serological Testing for Syphilis and Human Immunodeficiency Virus in a Laboratory Setting: Results From the Zimbabwe STI Etiology Study. Sex. Transm. Dis. 2019, 46, 584–587. [Google Scholar] [CrossRef]
- Taremwa, I.M.; Twelwanike, A.; Mwambi, B.; Atuhairwe, C. Laboratory assessment of SD Bioline HIV/Syphilis Duo Kit among pregnant women attending antenatal clinic Mayuge Health Center III, East central Uganda. BMC Res. Notes 2019, 12, 238. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).