Diffuse Pulmonary Meningotheliomatosis: Clinic-Pathologic Entity or Indolent Metastasis from Meningioma (or Both)?
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Korn, D.; Bensch, K.; Liebow, A.A.; Castleman, B. Multiple minute pulmonary tumors resembling chemodectomas. Am. J. Pathol. 1960, 37, 641–672. [Google Scholar] [PubMed]
- Zak, F.G.; Chabes, A. Pulmonary chemodectomatosis. JAMA 1963, 183, 887–889. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, M.J.; Mills, S.E.; Askin, F.B. Minute pulmonary meningothelial-like nodules. A clinicopathologic study of so-called minute pulmonary chemodectoma. Am. J. Surg. Pathol. 1988, 12, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Colby, T.V.; Yousem, S.A. Pulmonary histology for the surgical pathologist. Am. J. Surg. Pathol. 1988, 12, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; El-Zammar, O.A.; Katzenstein, A.L. Pulmonary meningothelial-like nodules: New insights into a common but poorly understood entity. Am. J. Surg. Pathol. 2009, 33, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, E.; Tsuta, K.; Maeshima, A.M.; Asamura, H.; Matsuno, Y. Minute pulmonary meningothelial-like nodules: Clinicopathologic analysis of 121 patients. Hum. Pathol. 2009, 40, 678–682. [Google Scholar] [CrossRef] [PubMed]
- Tamburini, N.; Morandi, L.; Rinaldi, R.; Maniscalco, P.; Quarantotto, F.; Montinari, E.; Papi, A.; Anania, G.; Cavallesco, G. Diffuse pulmonary meningothelial like nodules simulating metastatic thymoma. J. Thorac. Dis. 2018, 10, E442–E446. [Google Scholar] [CrossRef]
- Kfoury, H.; Arafah, M.A.; Arafah, M.M.; Alnassar, S.; Hajjar, W. Mimicry of Minute Pulmonary Meningothelial-like Nodules to Metastatic Deposits in a Patient with Infiltrating Lobular Carcinoma: A Case Report and Review of the Literature. Korean J. Pathol. 2012, 46, 87–91. [Google Scholar] [CrossRef]
- Suster, S.; Moran, C.A. Diffuse pulmonary meningotheliomatosis. Am. J. Surg. Pathol. 2007, 31, 624–631. [Google Scholar] [CrossRef]
- Kuroki, M.; Nakata, H.; Masuda, T.; Hashiguchi, N.; Tamura, S.; Nabeshima, K.; Matsuzaki, Y.; Onitsuka, T. Minute pulmonary meningothelial-like nodules: High-resolution computed tomography and pathologic correlations. J. Thorac. Imaging 2002, 17, 227–229. [Google Scholar] [CrossRef]
- Jayaschandran, V.; Gjorgova-Gjeorgjievski, S.; Siddique, H. An uncommon cause of miliary pattern of pulmonary nodules—Diffuse pulmonary meningotheliomatosis. Respir. Case Rep. 2017, 5, e00238. [Google Scholar] [CrossRef] [PubMed]
- Sellami, D.; Gotway, M.B.; Hanks, D.K.; Webb, W.R. Minute pulmonary meningothelial-like nodules: Thin-section CT appearance. J. Comput. Assist. Tomogr. 2001, 25, 311–313. [Google Scholar] [CrossRef] [PubMed]
- Kraushaar, G.; Ajlan, A.M.; English, J.C.; Muller, N.L. Minute pulmonary meningothelial-like nodules: A case of incidentally detected diffuse cystic micronodules on thin-section computed tomography. J. Comput. Assist. Tomogr. 2012, 34, 780–782. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kim, G.J.; Kim, Y.J.; Leem, A.Y.; Hwang, E.D.; Kim, S.K.; Chang, J.; Kang, Y.A.; Kim, S.Y. Minute pulmonary meningothelial-like nodules simulating hematogenous lung metastasis: A case report. Tuberc. Respir. Dis. 2013, 75, 67–70. [Google Scholar] [CrossRef]
- Sen, N.; Canpolat, E.T.; Koc, Z. A rarely seen diffuse parenchymal lung disease: Diffuse pulmonary meningotheliomatosis. Tuberk. Toraks Tuberc. Thorax 2015, 63, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Harada, M.; Aono, Y.; Tasui, H.; Uto, T.; Sato, J.; Imokawa, S.; Suzuki, S.; Tanioka, F.; Suda, T. Minute pulmonary meningothelial-like nodules showing multiple ring-shaped opacities. Intern. Med. 2019, 58, 3149–3152. [Google Scholar] [CrossRef]
- Maasdorp, S.D.; Nel, J.M.; Prins, M. Diffuse pulmonary meningotheliomatosis. A case report. Afr. J. Thorac. Crit. Care Med. 2019, 26, 18–19. [Google Scholar] [CrossRef]
- Alkurashi, A.K.; Almodallal, Y.; Albitar, H.A.H.; Cheville, J.C.; Iyer, V.N. Diffuse pulmonary meningotheliomatosis: A rare lung disease presenting with diffuse ground-glass opacities and cavitation. Am. J. Case Rep. 2020, 21, e926172. [Google Scholar] [CrossRef]
- Dzian, A.; Malik, M.; Hamada, L.; Micak, J.; Gregorova, I.; Kosturiakova, G. A rare case diagnosed by videothoracoscopic lung biopsy: Diffuse pulmonary meningotheliomatosis. Case Rep. Pulmonol. 2021, 2021, 1990433. [Google Scholar] [CrossRef]
- Yun, G.; Huang, T.; O’Dwyer, D.; Chugtai, A.; Agarwal, P. Diffuse pulmonary meningotheliomatosis. Clin. Imaging 2021, 70, 111–113. [Google Scholar] [CrossRef]
- Fernandez-Sarabia, M.T.; Cardenal Escarcena, A.; Furones Diez, M.; Rodriguez Garcia, J.M. Meningoteliomatosis pulmonar difusa: Causa infrecuente de patrón micronodularDiffuse pulmonary meningotheliomatosis: An uncommon cause of the micronodular pattern. Radiologia 2010, 52, 357–360. [Google Scholar] [CrossRef] [PubMed]
- Shimaoka, Y.; Ichikawa, H.; Ohara, T.; Asakawa, K.; Terada, M.; Toyama, J. A case of diffuse pulmonary meningotheliomatosis. Ann. Jpn. Respir. Soc. 2014, 3, 695–699. [Google Scholar]
- Noguchi-Konaka, M.; Endoh, M.; Sasage, T.; Nakahashi, K.; Suzuki, H.; Ogata, S.; Shiono, S. Diffuse Pulmonary Meningotheliomatosis Needed to be Differentiate from Metastatic Lung Tumor: Report of a Case. Kyobu Geka Jpn. J. Thorac. Surg. 2022, 75, 232–235. [Google Scholar]
- Ding, Y.L.; Zhu, H.; Yang, W.; Liu, B.B.; Zhu, X.; Li, M.J.; He, B. Diffuse pulmonary meningotheliomatosis: A case report and literature review. Chin. J. Tuberc. Respir. Dis. 2019, 42, 24–29. [Google Scholar]
- Mizutani, E.; Morita, R.; Emoto, N.; Moda, M.; Kasai, S.; Okochi, Y.; Kodama, M.; Abe, K. Multiple Minute Pulmonary Meningothelial-like Nodules;Report of a Case. Kyobu Geka Jpn. J. Thorac. Surg. 2020, 73, 968–971. [Google Scholar]
- Park, H.Y.; Nishi, Y.; Tabe, K.; Yamamoto, H.; Shibasaki, M.; Sakata, K.; Nagata, M.; Kuramitu, K.; Nakamura, S.; Morita, R.; et al. Minute pulmonary meningothelial-like nodules: A case report. J. Jpn. Respir. Soc. 2002, 40, 499–502. [Google Scholar]
- Bernabeu Mora, A.; Sánchez Nieto, J.M.; Hu, C.; Alcaraz Mateos, E.; Gimenez Bascunana, A.; Rodriguez-Rodrigues, M. Diffuse pulmonary meningotheliomatosis diagnosed by transbronchial lung biopsy. Respiration 2013, 86, 145–148. [Google Scholar] [CrossRef]
- Gleason, J.B.; Schroeder, J.; Ramirez, J. Meningotheliomatosis: A rare cause of diffuse miliary pattern pulmonary opacities. J. Clin. Diagn. Res. 2016, 10, OJ05. [Google Scholar] [CrossRef]
- Morresi-Hauf, A.T.; Reu, S.; Heiss-Neumann, M.; Gesierich, W.; Richter, T.; Wagner, P.K. Diffuse pulmonary meningotheliomatosis. Pneumologe 2015, 69, 553–559. [Google Scholar]
- Kumar, A.; Cherian, S.V.; Farver, C.; Mehta, A.C. Pulmonary meningotheliomatosis. Arch. Bronconeumol. 2018, 54, 104–105. [Google Scholar] [CrossRef]
- Huang, E.C.; Zhang, Y.; Bishop, J.W.; Gandour-Edwards, R.F.; Afify, A.M. Diffuse pulmonary meningotheliomatosis: A diagnostically challenging entity on fine-needle aspiration cytology. Diagn. Cytopathol. 2015, 43, 727–730. [Google Scholar] [CrossRef] [PubMed]
- Tzilas, V.; Bouros, D. A 58-year-old woman with lung nodules and chronic cough. Chest 2021, 160, e285–e288. [Google Scholar] [CrossRef] [PubMed]
- Swenson, K.E.; VanderLaan, P.; Parikh, M. Diffuse Pulmonary Meningotheliomatosis Diagnosed Via Transbronchial Cryobiopsy. J. Bronchol. Interv. Pulmonol. 2022, 29, e63–e65. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Cooke, D.T.; Kamangar, E.; Inaty, H. Diffuse Pulmonary Meningotheliomatosis in a Patient with Neurodermatitis with Prurigo Nodularis. J. Bronchol. Interv. Pulmonol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, J.; Zhao, Y.; Zhang, T.; Xu, H.; Chen, Y.C. Minute pulmonary meningothelial-like nodules: Associations between computed tomography and pathology features. Quant. Imaging Med. Surg. 2023, 13, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Murata, D.; Zaizen, Y.; Tokisawa, S.; Matama, G.; Chikasue, T.; Nishii, Y.; Ohno, S.; Tsumura, K.; Tominaga, M.; Fukuoka, J.; et al. A Rare Case of Diffuse Bilateral Minute Pulmonary Meningothelial-like Nodules Increasing Over the Short Term and Resembling Metastatic Lung Cancer. Intern. Med. 2022. [Google Scholar] [CrossRef]
- Wang, Y.X.; Lei, Z.; Yang, M.; Wang, Z.Y.; Zhang, X.; Pan, G.Q. Case Report: Clinicopathological Analysis of Minute Pulmonary Meningothelial-Like Nodules: Report of 7 Cases. Front. Oncol. 2022, 12, 942517. [Google Scholar] [CrossRef]
- Huang, X.; Mou, Y.F.; Ren, F.Q.; Wang, Y.; Yang, Y. Multiple primary pulmonary meningioma: A case report and literature review. Thorac. Cancer 2022, 13, 2257–2259. [Google Scholar] [CrossRef]
- Colby, T.V. The pathologist’s approach to bronchoscopic biopsies. Pathologica 2010, 102, 432–442. [Google Scholar]
- Agozzino, M.; Inzani, F.; Cavallero, A.; Arbustini, E.; Meloni, F.; Oggionni, T.; Dore, R.; D’Armini, A.; Viganò, M. Minute pulmonary meningothelial-like nodules in the transbronchial biopsy of a lung transplant recipient. J. Heart Lung Transplant. 2016, 25, 148–150. [Google Scholar] [CrossRef]
- Ionescu, D.N.; Sasatomi, E.; Aldeeb, D.; Omalu, B.I.; Finkelstein, S.D.; Swalsky, P.A.; Yousem, S.A. Pulmonary meningothelial-like nodules: A genotypic comparison with meningiomas. Am. J. Surg. Pathol. 2004, 28, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Niho, S.; Yokose, T.; Nishiwaki, Y.; Mukai, K. Immunohistochemical and clonal analysis of minute pulmonary meningothelial-like nodules. Hum. Pathol. 1999, 30, 425–429. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Watanabe, M.; Inoue, T.; Yamaura, T.; Suzuki, T.; Saito, M.; Niitsuma, K.; Endo, K.; Oshibe, I.; Soeta, N.; et al. Brief report on similar mutational changes in neurofibromatosis type 2 gene in minute pulmonary meningothelial-like nodule and meningioma of the central nervous system. Oncotarget 2018, 89, 36012–36016. [Google Scholar] [CrossRef] [PubMed]
- Weissferdt, A.; Tang, X.; Suster, S.; Wistuba, I.I.; Moran, C.A. Pleuropulmonary Meningothelial Proliferations: Evidence for a Common Histogenesis. Am. J. Surg. Pathol. 2015, 39, 1673–1678. [Google Scholar] [CrossRef]
- Adlakha, A.; Rao, K.; Adlakha, H.; Perry, A.; Crotty, T.B.; Scheithauer, B.W.; Ryu, J.H. Meningioma metastatic to the lung. Mayo Clin. Proc. 1999, 74, 1129–1133. [Google Scholar] [CrossRef]
- Stefani, A.; Rossi, G.; Pecchi, A.; Bertolini, F.; Falasca, A.; Aramini, B.; Morandi, U. An unusual case of cystic interstitial lung disease. Lancet 2013, 381, 1246. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.X.; Yan, L.X.; Liu, C.; Wang, S.Y.; Li, W.F.; Gao, X.; Wei, X.W.; Zhou, Q. Benign disease prone to be misdiagnosed as malignant pulmonary nodules: Minute meningothelioid nodules. Thorac. Cancer 2019, 10, 1182–1187. [Google Scholar] [CrossRef]
- Asakawa, A.; Horio, H.; Hishima, T.; Yamamich, T.; Okui, M.; Harada, M. Clinicopathologic features of minute pulmonary meningothelial-like nodules. Asian Cardiovasc. Thorac. Ann. 2017, 25, 509–512. [Google Scholar] [CrossRef]
- Rossi, G.; Cavazza, A.; Spagnolo, P.; Sverzellati, N.; Longo, L.; Jukna, A.; Montanari, G.; Carbonelli, C.; Vincenzi, G.; Bogina, G.; et al. Diffuse idiopathic pulmonary neuroendocrine cell hyperplasia syndrome. Eur. Respir. J. 2016, 47, 1829–1841. [Google Scholar] [CrossRef]
- Masago, K.; Hosada, W.; Sasaki, E.; Murakami, Y.; Sugano, M.; Nagasaka, T.; Yamada, M.; Yatabe, Y. Is primary pulmonary meningioma a giant form of a meningothelial-like nodule? A case report and review of the literature. Case Rep. Oncol. 2012, 5, 471–478. [Google Scholar] [CrossRef]
- Simonetti, G.; Terreni, M.R.; DiMeco, F.; Fariselli, L.; Gaviani, P. Letter to the editor: Lung metastasis in WHO grade I meningioma. Neurol. Sci. 2018, 39, 1781–1783. [Google Scholar] [CrossRef] [PubMed]
- Sathirareuangchai, S.; Kakazu, K.; Tauchi-Nishi, P.; Morris, P.; Sae-Ow, W. Low grade intracranial meningioma presenting with pulmonary metastasis: Case report and literature review. Pathol. Res. Pract. 2019, 215, 152390. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.C.; Myers, J.L.; Colby, T.V.; Leslie, K.O.; Tazelaar, H.D. Endometrial stromal sarcoma metastatic to the lung: A detailed analysis of 16 patients. Am. J. Surg. Pathol. 2002, 26, 440–449. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, C.; Gupta, N.; Johnson, S.R.; Yu, J.J.; McCormack, F.X. Lymphangioleiomyomatosis: Pathogenesis, clinical features, diagnosis, and management. Lancet Respir. Med. 2021, 9, 1313–1327. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Chen, Y.; Huang, Q.; Yong, J.; Yan, S.; Huang, Y. Constant expression of somatostatin receptor 2a in minute pulmonary meningothelial-like nodules. J. Clin. Pathol. 2019, 72, 525–528. [Google Scholar] [CrossRef]

| Case (Year at Diagnosis of DPM) | Gender | Age | Smoke | Symptoms | CT Findings | Sampling Procedure | Original Clinic–Radiologic Diagnosis | Brain Imaging | Specific Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| #1 (2018) | M | 55 | N | No; thorax CT performed after car accident | Diffuse bilateral micronodules randomly distributed; some with cavitation | TBB | Sarcoid | Yes; negative | No | Alive and well (38 months) |
| #2 (2016) | F | 56 | Y | No; CT performed during a medical check for abdominal discomfort | Diffuse bilateral micronodules randomly distributed | TBB | Sarcoid | Yes; presence of a right frontal lesion (8 mm in maximum diameter) consistent with meningioma (no surgery was performed) | No | Alive and well (63 months) |
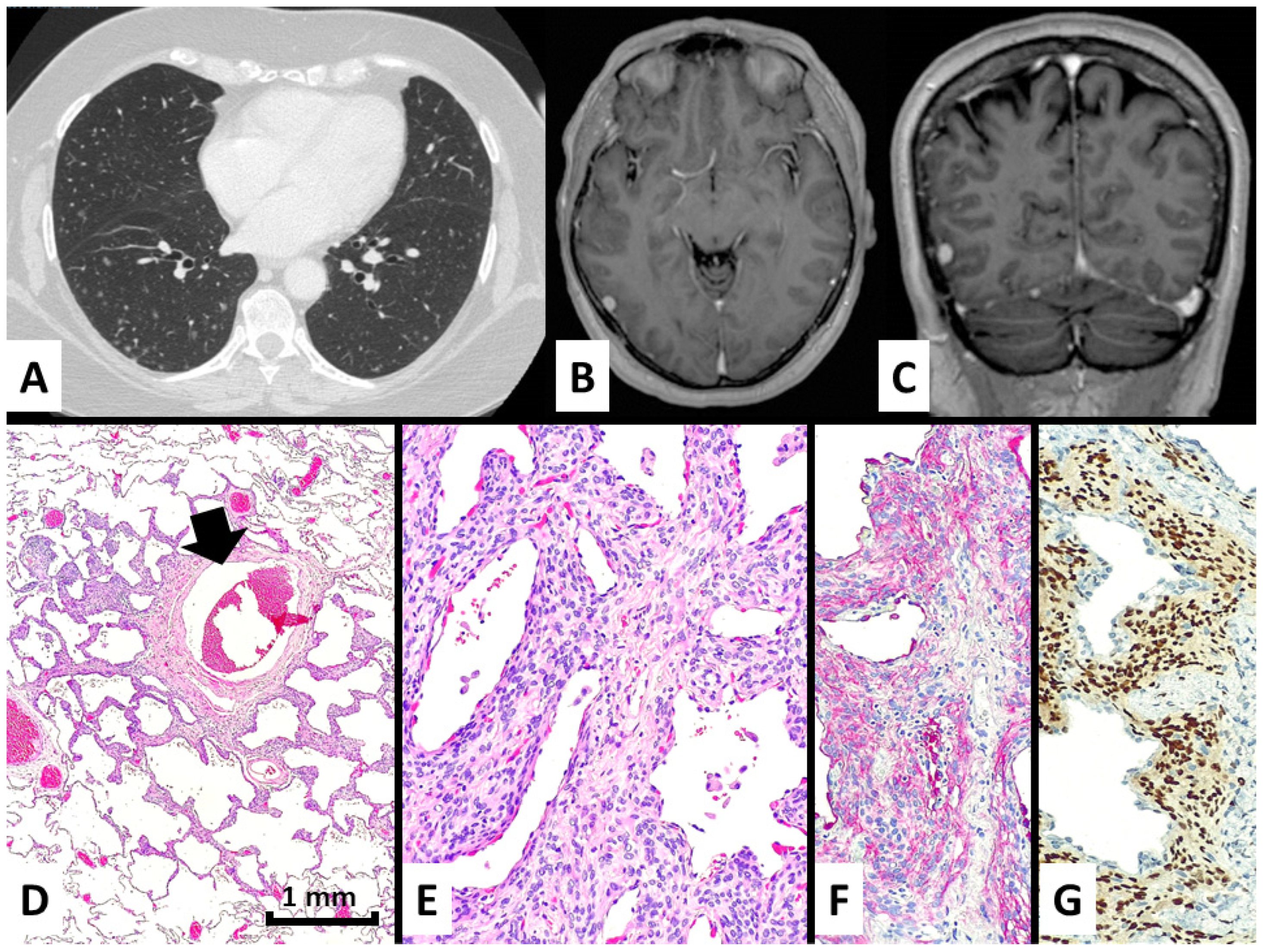
| #3 (2013) | F | 68 | N | No; incidentally discovered together with a minimally-invasive adenocarcinoma) | Diffuse bilateral micronodules randomly distributed | SLB | Pulmonary metastasis | Yes; previous unknown history of left frontal meningioma (grade 1; 10 mm in maximum diameter) | No | Alive and well (99 months) |
| #4 (2015) | F | 51 | N | No; CT scan performed for acute cholecystitis | Diffuse bilateral micronodules with GGO | SLB | DIPNECH | Yes; previous unknown history of right temporal meningioma (4 mm in maximum diameter) without surgery | No | Alive and well (78 months) |
| Reference | Gender | Age | Smoke | Symptoms | Past Medical History | CT Findings | Sampling Procedure | Brain Imaging | Specific Therapy | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Suster/Moran [9] | F | 75 | NA | Dyspnea, shortness of breath, fatigue | Ischemic heart disease; hypertension; colon cancer | Diffuse bilateral reticulonodular infiltrates | SLB | No | No | Alive |
| Suster/Moran [9] | F | 54 | NA | Spontaneous pnx, dyspnea | Uterine leiomyosarcoma | Diffuse bilateral reticulonodular infiltrates | SLB | No | No | Alive |
| Suster/Moran [9] | M | 51 | NA | Cough, shortness of breath | Diffuse bilateral reticulonodular infiltrates | SLB | No | No | Alive | |
| Suster/Moran [9] | F | 63 | NA | Cough, shortness of breath | Coronary artery disease; hypertension | Diffuse bilateral reticulonodular infiltrates | SLB | No | No | Alive |
| Suster/Moran [9] | F | 71 | NA | Dyspnea, shortness of breath, fatigue | Papillary thyroid carcinoma; invasive breast cancer; lung adenocarcinoma | Diffuse bilateral reticulonodular infiltrates | SLB | No | No | Alive |
| Jayaschandran et al. [11] | F | 74 | N | No | Hypertension; dyslipidemia; prediabetes; asthma | Diffuse bilateral micronodules up to 5 mm with GGO and cavitation | TBB | No | No | Alive |
| Sellami et al. [12] | F | 64 | Y | Shortness of breath | GERD; hysterectomy; lung adenocarcinoma | Diffuse bilateral micronodules randomly distributed | SLB | No | No | Alive |
| Kraushaar et al. [13] | F | 54 | N | No | Hypertension; GERD | Diffuse bilateral micronodules up to 4 mm randomly distributed with thin-walled cavitation | SLB | No | No | Alive |
| Lee et al. [14] | M | 52 | Y | Persistent cough and mild dyspnea | Erythrocytosis | Diffuse bilateral micronodules up to 11 mm | CT-guided PNA with atypical cells SLB | No | No | Alive |
| Sen et al. [15] | F | 66 | Y | Dry cough | Diabetes mellitus; hypertension; chronic renal disease | Diffuse bilateral micronodules up to 4 mm randomly distributed | SLB | No | No | Alive |
| Harada et al. [16] | F | 56 | N | No | Turner’s syndrome; iron deficiency anemia | Diffuse bilateral micronodules up to 5 mm with GGO and central lucency ring-like | SLB | Yes | No | Alive |
| Maasdorp et al. [17] | F | 58 | N | Exertional dyspnea, dry cough | Frontal lobe syndrome due to trauma | Diffuse bilateral micronodules with miliary pattern and GGO | SLB | Yes | No | Alive |
| Alkurashi et al. [18] | F | 55 | N | Non-exertional dyspnea, dry cough | Prediabetes; hypertension; hypercholesterolemia; hypothyroidism; GERD | Diffuse bilateral micronodules with GGO and cavitation | SLB | No | No | Alive |
| Dzian et al. [19] | M | 60 | Y | No | Carotid artery disease with stroke; hypertension; nephro/urolithiasis; | Diffuse bilateral micronodules up to 5 mm | TBB with neuroendocrine cell hyperplasia; SLB | No | No | Alive |
| Yun et al. [20] | F | 80 | N | Dry cough, progressive dyspnea | Hypertension; type 2 diabetes mellitus; GERD; chronic kidney disease; colon polyps | Diffuse bilateral micronodules with GGO up to 4 mm | SLB | No | No | Alive |
| Fernandez Sarabia et al. [21] | F | 66 | NA | No | Invasive breast cancer; colon adenocarcinoma; | Diffuse bilateral micronodules | SLB | No | No | Alive |
| Shimaoka et al. [22] | F | 54 | N | No | Turner’s syndrome | Diffuse bilateral micronodules | SLB | No | No | Alive |
| Noguchi-Konaka et al. [23] | F | 77 | NA | No | Breast cancer; colorectal cancer | Diffuse bilateral micronodules | TBB non diagnostic SLB | No | No | Alive |
| Ding et al. [24] | F | 68 | NA | Intermittent cough, expectoration | Diffuse bilateral micronodules | SLB | No | No | Alive | |
| Mizutani et al. [25] | F | 57 | NA | No | Diffuse bilateral micronodules | SLB | No | No | Alive | |
| Park et al. [26] | F | 56 | NA | No | Breast cancer | Diffuse bilateral micronodules | SLB | No | No | Alive |
| Bernabeu Mora et al. [27] | F | 59 | Y | No | Uterine carcinoma; anal abscesses | Diffuse bilateral, randomly-distributed micronodules, most poorly-defined with a cotton wool appearance and cavitation | TBB | No | No | Alive |
| Gleason et al. [28] | F | 63 | Y | No | Diffuse bilateral micronodules up to 4 mm with miliary pattern | TBB | No | No | Alive | |
| Morresi-Hauf et al. [29] | F | 73 | N | No | Goiter | Diffuse bilateral micronodules randomly distributed | SLB | No | No | Alive |
| Morresi-Hauf et al. [29] | F | 60 | Y | No | Diabetes mellitus type 2; hypertension; GERD; prurigo nodularis | Diffuse bilateral micronodules randomly distributed | Cryo-TBB | No | No | Alive |
| Kumar et al. [30] | F | 58 | N | Intermitted dry cough | Diffuse bilateral micronodules with GGO | TBB not diagnostic SLB | No | No | Alive | |
| Huang et al. [31] | F | 57 | N | No | Hypercholesterolemia; obesity; hypertension; asthma | Diffuse bilateral micronodules up to 9 mm with GGO | CT-FNA (inflammatory histiocytic process/LCH) SLB | No | No | Alive |
| Tzilas and Bouros [32] | F | 58 | N | Chronic cough | Diffuse bilateral micronodules with a peripheral zone predilection and central cavitation (“cheerio sign”) | SLB | No | No | Alive | |
| Swenson et al. [33] | F | 61 | Y | Dry cough and non-exertional dyspnea | Diffuse bilateral micronodules with an upper lobe predilection | Cryo-TBB | No | No | Alive | |
| Kim et al. [34] | F | 55 | N | Shortness of breath and exertional dyspnea | Neurodermatitis with prurigo nodularis Obesity and hysterectomy | Innumerable small ground glass nodules, some with cavitation | SLB | No | No | Alive and well |
| Murata et al. [36] | F | 54 | Y | No | Cholelithiasis; hypercholesterolemia | Diffuse bilateral micronodules with a peripheral zone predilection. Some nodules with central lucency and ring-shaped appearance (“cheerio sign”) | TBB non diagnostic SLB | No | No | Alive and well |
| Wang et al. [37] | F | 30 | NA | No | Microinvasive lung adenocarcinoma | Multiple GGO nodules | SLB | No | No | Alive and well |
| Wang et al. [37] | F | 40 | NA | No | Multiple invasive lung adenocarcinoma | Multiple microscopic nodules | SLB | No | No | Alive and well |
| Wang et al. [37] | F | 50 | NA | No | Multiple GGO nodules | SLB | No | No | Alive and well | |
| Wang et al. [37] | F | 50 | NA | No | Multiple GGO nodules | SLB | No | No | Alive and well | |
| Wang et al. [37] | F | 50 | NA | Chest pain radiating to the right shoulder | Multiple microscopic nodules | SLB | No | No | Alive and well | |
| Wang et al. [37] | F | 56 | NA | Cough with yellow sputum | Organizing pneumonia; atypical adenomatous nodules | Multiple microscopic nodules | SLB | No | No | |
| Wang et al. [37] | M | 66 | NA | Recurrent cough, shortness of breath, hemoptysis | Nonkeratinizing squamous cell carcinoma; organizing pneumonia | Multiple microscopic nodules | SLB | No | No | NA |
| Huang et al. [38] | F | 64 | N | No | Hypertension | Multiple GGO nodules | SLB | Yes (ischemic foci in the left centrum semiovale and mild demyelination of white matter; no intracranial meningioma) | No | NA |
| Peng et al. [47] | F | 63 | NA | No | Squamous cell carcinoma of the esophagus | Diffuse bilateral micronodules | SLB | No | No | Alive |
| Peng et al. [47] | F | 68 | NA | No | Diffuse bilateral micronodules with GGO | SLB | No | No | Alive | |
| Peng et al. [47] | F | 54 | NA | No | Diffuse bilateral micronodules with GGO | SLB | No | No | Alive | |
| Peng et al. [47] | F | 59 | NA | No | Diffuse bilateral micronodules | SLB | No | No | Alive | |
| Asakawa et al. [48] | F | 76 | NA | NA | Basedow goiter; hypertension | Diffuse bilateral micronodules up to 6 mm | SLB | Yes | No | Alive |
| Clinical features |
| Female > Male (up to 15:1) |
| Sixth decade |
| Asymptomatic/incidental presentation/mild nonspecific symptoms |
| Stable disease and good prognosis |
| Radiologic features |
| Diffuse, bilateral, and randomly-distributed micronodules up to 6 mm of maximum diameter with/without ground-glass opacities and with/without central “cavitation” (ring-like lucency) |
| Pathologic features |
| Histologic evidence of MMNs; absence of alternative histologic cause of radiologic nodules |
| Lack of intra/extracranial meningioma * |
| Definition | Incidence | Histology | Radiology | Other |
|---|---|---|---|---|
| Diffuse pulmonary meningotheliomatosis (DPM) | Rare; female prevalence | Multiple meningothelial-like nodules with perivenular and interstitial distribution | Diffuse bilateral micronodules (up to 5–6 mm) with a peripheral zone predilection; some nodules show central lucency (“cheerio sign”) | Brain imaging should be performed to exclude intracranial meningioma |
| Metastatic meningioma with DPM features | Rare; no gender prevalence | Multiple meningothelial-like nodules with perivenular and interstitial distribution | Diffuse bilateral micronodules (up to 5–6 mm) with a peripheral zone predilection; some nodules show central lucency (“cheerio sign”) | Positive past medical history of meningioma or concomitant meningioma |
| Multiple meningothelial-like nodules | Not infrequent; no gender prevalence | Multiple meningothelial-like nodules with perivenular and interstitial distribution | No changes | Incidental findings in several conditions (infections; primary/metastatic lung cancer; pulmonary infarcts; hypoxemic conditions) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melocchi, L.; Rossi, G.; Valli, M.; Mengoli, M.C.; Mondoni, M.; Lazzari-Agli, L.; Santandrea, G.; Davoli, F.; Baldovini, C.; Cavazza, A.; et al. Diffuse Pulmonary Meningotheliomatosis: Clinic-Pathologic Entity or Indolent Metastasis from Meningioma (or Both)? Diagnostics 2023, 13, 802. https://doi.org/10.3390/diagnostics13040802
Melocchi L, Rossi G, Valli M, Mengoli MC, Mondoni M, Lazzari-Agli L, Santandrea G, Davoli F, Baldovini C, Cavazza A, et al. Diffuse Pulmonary Meningotheliomatosis: Clinic-Pathologic Entity or Indolent Metastasis from Meningioma (or Both)? Diagnostics. 2023; 13(4):802. https://doi.org/10.3390/diagnostics13040802
Chicago/Turabian StyleMelocchi, Laura, Giulio Rossi, Mirca Valli, Maria Cecilia Mengoli, Michele Mondoni, Luigi Lazzari-Agli, Giacomo Santandrea, Fabio Davoli, Chiara Baldovini, Alberto Cavazza, and et al. 2023. "Diffuse Pulmonary Meningotheliomatosis: Clinic-Pathologic Entity or Indolent Metastasis from Meningioma (or Both)?" Diagnostics 13, no. 4: 802. https://doi.org/10.3390/diagnostics13040802
APA StyleMelocchi, L., Rossi, G., Valli, M., Mengoli, M. C., Mondoni, M., Lazzari-Agli, L., Santandrea, G., Davoli, F., Baldovini, C., Cavazza, A., & Colby, T. V. (2023). Diffuse Pulmonary Meningotheliomatosis: Clinic-Pathologic Entity or Indolent Metastasis from Meningioma (or Both)? Diagnostics, 13(4), 802. https://doi.org/10.3390/diagnostics13040802






