Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review
Abstract
:1. Introduction
2. Indications to Perform EUS in the Evaluation of PCLs
3. EUS Imaging of PCLs
3.1. EUS Imaging and Morphology in the Differentiation of PCLs
3.1.1. Serous Cystadenomas (SCN)
3.1.2. Intraductal Papillary Mucinous Neoplasm
3.1.3. Mucinous Cystic Neoplasms (MCNs)
3.1.4. Pancreatic Neuroendocrine Tumor (pNET) and Solid Pseudopapillary Tumor (SPT)
3.1.5. Pancreatic Pseudocysts
3.1.6. Differentiation of PCLs Based on EUS Morphology
3.2. EUS Imaging and Detection of Malignancy in Mucinous PCLs
3.2.1. Advances in Endosonographic Imaging for Defining Cyst Morphology (Diagnosis and Risk Stratification)
Contrast-Harmonic Mode Endoscopic Ultrasound
EUS-Guided Needle-Based Confocal Laser Endomicroscopy (EUS-nCLE)
4. EUS-Fluid Acquisition
5. EUS-Tissue Acquisition
5.1. EUS-Fine Needle Aspiration (FNA)
5.2. Other EUS-TA Modalities
EUS and Artificial Intelligence
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of Incidental Pancreatic Cysts in the Adult Population on MR Imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef]
- Litchinko, A.; Kobayashi, K.; Halkic, N. A Retrospective Study of Histological Outcome for IPMN after Surgery in Lausanne, Switzerland: A Case Series. Ann. Med. Surg. 2020, 60, 110–114. [Google Scholar] [CrossRef]
- Springer, S.; Masica, D.L.; Dal Molin, M.; Douville, C.; Thoburn, C.J.; Afsari, B.; Li, L.; Cohen, J.D.; Thompson, E.; Allen, P.J.; et al. A Multimodality Test to Guide the Management of Patients with a Pancreatic Cyst. Sci. Transl. Med. 2019, 11, eaav4772. [Google Scholar] [CrossRef]
- Vege, S.S.; Ziring, B.; Jain, R.; Moayyedi, P.; Adams, M.A.; Dorn, S.D.; Dudley-Brown, S.L.; Flamm, S.L.; Gellad, Z.F.; Gruss, C.B.; et al. American Gastroenterological Association Institute Guideline on the Diagnosis and Management of Asymptomatic Neoplastic Pancreatic Cysts. Gastroenterology 2015, 148, 819–822. [Google Scholar] [CrossRef]
- European Evidence-Based Guidelines on Pancreatic Cystic Neoplasms. Gut 2018, 67, 789–804. [CrossRef] [PubMed]
- Tanaka, M.; Fernández-del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of International Consensus Fukuoka Guidelines for the Management of IPMN of the Pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Jais, B.; Rebours, V.; Malleo, G.; Salvia, R.; Fontana, M.; Maggino, L.; Bassi, C.; Manfredi, R.; Moran, R.; Lennon, A.M.; et al. Serous Cystic Neoplasm of the Pancreas: A Multinational Study of 2622 Patients under the Auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016, 65, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Kimura, W.; Moriya, T.; Hanada, K.; Abe, H.; Yanagisawa, A.; Fukushima, N.; Ohike, N.; Shimizu, M.; Hatori, T.; Fujita, N.; et al. Multicenter Study of Serous Cystic Neoplasm of the Japan Pancreas Society. Pancreas 2012, 41, 380–387. [Google Scholar] [CrossRef]
- O’Toole, D.; Palazzo, L.; Hammel, P.; Yaghlene, L.B.; Couvelard, A.; Felce-Dachez, M.; Fabre, M.; Dancour, A.; Aubert, A.; Sauvanet, A.; et al. Macrocystic Pancreatic Cystadenoma: The Role of EUS and Cyst Fluid Analysis in Distinguishing Mucinous and Serous Lesions. Gastrointest. Endosc. 2004, 59, 823–829. [Google Scholar] [CrossRef]
- Goh, B.K.P.; Tan, Y.-M.; Yap, W.-M.; Cheow, P.-C.; Chow, P.K.H.; Chung, Y.-F.A.; Wong, W.-K.; Ooi, L.L.P.J. Pancreatic Serous Oligocystic Adenomas: Clinicopathologic Features and a Comparison with Serous Microcystic Adenomas and Mucinous Cystic Neoplasms. World J. Surg. 2006, 30, 1553–1559. [Google Scholar] [CrossRef] [PubMed]
- Leite, I.; Palmeiro, M.; Farchione, A.; Matos, C.; Bali, M.A.; Demetter, P.; Delhaye, M. Unilocular Macrocystic Serous Cystadenoma of the Pancreas—Atypical Features: A Case Report. Clin. Imaging 2014, 38, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Stark, A.; Donahue, T.R.; Reber, H.A.; Hines, O.J. Pancreatic Cyst Disease. J. Am. Med. Assoc. 2016, 315, 1882. [Google Scholar] [CrossRef] [PubMed]
- Oyama, H.; Tada, M.; Takagi, K.; Tateishi, K.; Hamada, T.; Nakai, Y.; Hakuta, R.; Ijichi, H.; Ishigaki, K.; Kanai, S.; et al. Long-Term Risk of Malignancy in Branch-Duct Intraductal Papillary Mucinous Neoplasms. Gastroenterology 2020, 158, 226–237.e5. [Google Scholar] [CrossRef] [PubMed]
- Zerboni, G.; Signoretti, M.; Crippa, S.; Falconi, M.; Arcidiacono, P.G.; Capurso, G. Systematic Review and Meta-Analysis: Prevalence of Incidentally Detected Pancreatic Cystic Lesions in Asymptomatic Individuals. Pancreatology 2019, 19, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Marchegiani, G.; Pollini, T.; Andrianello, S.; Tomasoni, G.; Biancotto, M.; Javed, A.A.; Kinny-Köster, B.; Amini, N.; Han, Y.; Kim, H.; et al. Progression vs. Cyst Stability of Branch-Duct Intraductal Papillary Mucinous Neoplasms After Observation and Surgery. JAMA Surg. 2021, 156, 654. [Google Scholar] [CrossRef]
- Sugiyama, M.; Atomi, Y. Intraductal Papillary Mucinous Tumors of the Pancreas. Ann. Surg. 1998, 228, 685–691. [Google Scholar] [CrossRef]
- Zamboni, G.; Scarpa, A.; Bogina, G.; Iacono, C.; Bassi, C.; Talamini, G.; Sessa, F.; Capella, C.; Solcia, E.; Rickaert, F.; et al. Mucinous Cystic Tumors of the Pancreas. Am. J. Surg. Pathol. 1999, 23, 410–422. [Google Scholar] [CrossRef]
- Postlewait, L.M.; Ethun, C.G.; McInnis, M.R.; Merchant, N.; Parikh, A.; Idrees, K.; Isom, C.A.; Hawkins, W.; Fields, R.C.; Strand, M.; et al. Association of Preoperative Risk Factors With Malignancy in Pancreatic Mucinous Cystic Neoplasms. JAMA Surg. 2017, 152, 19. [Google Scholar] [CrossRef]
- Keane, M.G.; Shamali, A.; Nilsson, L.N.; Antila, A.; Millastre Bocos, J.; Marijinissen Van Zanten, M.; Verdejo Gil, C.; Maisonneuve, P.; Vaalavuo, Y.; Hoskins, T.; et al. Risk of Malignancy in Resected Pancreatic Mucinous Cystic Neoplasms. Br. J. Surg. 2018, 105, 439–446. [Google Scholar] [CrossRef]
- Park, J.W.; Jang, J.-Y.; Kang, M.J.; Kwon, W.; Chang, Y.R.; Kim, S.-W. Mucinous Cystic Neoplasm of the Pancreas: Is Surgical Resection Recommended for All Surgically Fit Patients? Pancreatology 2014, 14, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shi, S.; Hua, J.; Xu, J.; Zhang, B.; Liu, J.; Yang, X.-J.; Yu, X.-J. Differentiation of Solid-Pseudopapillary Tumors of the Pancreas from Pancreatic Neuroendocrine Tumors by Using Endoscopic Ultrasound. Clin. Res. Hepatol. Gastroenterol. 2020, 44, 947–953. [Google Scholar] [CrossRef] [PubMed]
- Law, J.K.; Ahmed, A.; Singh, V.K.; Akshintala, V.S.; Olson, M.T.; Raman, S.P.; Ali, S.Z.; Fishman, E.K.; Kamel, I.; Canto, M.I.; et al. A Systematic Review of Solid-Pseudopapillary Neoplasms. Pancreas 2014, 43, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Ro, C.; Chai, W.; Yu, V.E.; Yu, R. Pancreatic Neuroendocrine Tumors: Biology, Diagnosis, and Treatment. Chin. J. Cancer 2013, 32, 312–324. [Google Scholar] [CrossRef]
- Misra, D.; Sood, T. Pancreatic Pseudocyst; StatPearls: Tampa, FL, USA, 2022; Volume 1. [Google Scholar]
- Breslin, N.; Wallace, M.B. Diagnosis and Fine Needle Aspiration of Pancreatic Pseudocysts: The Role of Endoscopic Ultrasound. Gastrointest. Endosc. Clin. N. Am. 2002, 12, 781–790. [Google Scholar] [CrossRef]
- Koito, K.; Namieno, T.; Nagakawa, T.; Shyonai, T.; Hirokawa, N.; Morita, K. Solitary Cystic Tumor of the Pancreas: EUS-Pathologic Correlation. Gastrointest. Endosc. 1997, 45, 268–276. [Google Scholar] [CrossRef]
- Brugge, W.R.; Lewandrowski, K.; Lee-Lewandrowski, E.; Centeno, B.A.; Szydlo, T.; Regan, S.; del Castillo, C.F.; Warshaw, A.L. Diagnosis of Pancreatic Cystic Neoplasms: A Report of the Cooperative Pancreatic Cyst Study. Gastroenterology 2004, 126, 1330–1336. [Google Scholar] [CrossRef] [PubMed]
- Gerke, H.; Jaffe, T.; Mitchell, R.; Byrne, M.; Stiffler, H.; Branch, M.; Baillie, J.; Jowell, P. Endoscopic Ultrasound and Computer Tomography Are Inaccurate Methods of Classifying Cystic Pancreatic Lesions. Dig. Liver Dis. 2006, 38, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.A.; Kochman, M.L.; Brensinger, C.; Brugge, W.R.; Faigel, D.O.; Gress, F.G.; Kimmey, M.B.; Nickl, N.J.; Savides, T.J.; Wallace, M.B.; et al. Interobserver Agreement among Endosonographers for the Diagnosis of Neoplastic versus Non-Neoplastic Pancreatic Cystic Lesions. Gastrointest. Endosc. 2003, 58, 59–64. [Google Scholar] [CrossRef]
- Anand, N.; Sampath, K.; Wu, B.U. Cyst Features and Risk of Malignancy in Intraductal Papillary Mucinous Neoplasms of the Pancreas: A Meta-Analysis. Clin. Gastroenterol. Hepatol. 2013, 11, 913–921. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Borin, A.; Dal Borgo, C.; Perri, G.; Pollini, T.; Romanò, G.; D’Onofrio, M.; Gabbrielli, A.; Scarpa, A.; et al. Systematic Review, Meta-Analysis, and a High-Volume Center Experience Supporting the New Role of Mural Nodules Proposed by the Updated 2017 International Guidelines on IPMN of the Pancreas. Surgery 2018, 163, 1272–1279. [Google Scholar] [CrossRef]
- Hirono, S.; Tani, M.; Kawai, M.; Okada, K.; Miyazawa, M.; Shimizu, A.; Kitahata, Y.; Yamaue, H. The Carcinoembryonic Antigen Level in Pancreatic Juice and Mural Nodule Size Are Predictors of Malignancy for Branch Duct Type Intraductal Papillary Mucinous Neoplasms of the Pancreas. Ann. Surg. 2012, 255, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Okabayashi, T.; Kobayashi, M.; Nishimori, I.; Sugimoto, T.; Namikawa, T.; Okamoto, K.; Okamoto, N.; Kosaki, T.; Onishi, S.; Araki, K. Clinicopathological Features and Medical Management of Intraductal Papillary Mucinous Neoplasms. J. Gastroenterol. Hepatol. 2006, 21, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Yamaue, H.; Maguchi, H.; Yamao, K.; Hirono, S.; Osanai, M.; Hijioka, S.; Hosoda, W.; Nakamura, Y.; Shinohara, T.; et al. Predictors of Malignancy in Intraductal Papillary Mucinous Neoplasm of the Pancreas. Pancreas 2013, 42, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Uehara, H.; Ishikawa, O.; Katayama, K.; Kawada, N.; Ikezawa, K.; Fukutake, N.; Takakura, R.; Takano, Y.; Tanaka, S.; Takenaka, A. Size of Mural Nodule as an Indicator of Surgery for Branch Duct Intraductal Papillary Mucinous Neoplasm of the Pancreas during Follow-Up. J. Gastroenterol. 2011, 46, 657–663. [Google Scholar] [CrossRef]
- Iwaya, H.; Hijioka, S.; Mizuno, N.; Kuwahara, T.; Okuno, N.; Tajika, M.; Tanaka, T.; Ishihara, M.; Hirayama, Y.; Onishi, S.; et al. Usefulness of Septal Thickness Measurement on Endoscopic Ultrasound as a Predictor of Malignancy of Branched-duct and Mixed-type Intraductal Papillary Mucinous Neoplasm of the Pancreas. Dig. Endosc. 2019, 31, 672–681. [Google Scholar] [CrossRef]
- Olar, M.P.; Bolboacă, S.D.; Pojoga, C.; Moșteanu, O.; Gheorghiu, M.; Seicean, R.; Rusu, I.; Sparchez, Z.; al Hajjar, N.; Seicean, A. Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst. Diagnostics 2022, 12, 2209. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of Contrast-Enhanced Endoscopic Sonography for Discriminating Mural Nodules From Mucous Clots in Intraductal Papillary Mucinous Neoplasms. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef]
- Lisotti, A.; Napoleon, B.; Facciorusso, A.; Cominardi, A.; Crinò, S.F.; Brighi, N.; Gincul, R.; Kitano, M.; Yamashita, Y.; Marchegiani, G.; et al. Contrast-Enhanced EUS for the Characterization of Mural Nodules within Pancreatic Cystic Neoplasms: Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021, 94, 881–889.e5. [Google Scholar] [CrossRef]
- Krishna, S.G.; Hart, P.A.; Malli, A.; Kruger, A.J.; McCarthy, S.T.; El-Dika, S.; Walker, J.P.; Dillhoff, M.E.; Manilchuk, A.; Schmidt, C.R.; et al. Endoscopic Ultrasound-Guided Confocal Laser Endomicroscopy Increases Accuracy of Differentiation of Pancreatic Cystic Lesions. Clin. Gastroenterol. Hepatol. 2020, 18, 432–440.e6. [Google Scholar] [CrossRef]
- Kovacevic, B.; Antonelli, G.; Klausen, P.; Hassan, C.; Larghi, A.; Vilmann, P.; Karstensen, J. EUS-Guided Biopsy versus Confocal Laser Endomicroscopy in Patients with Pancreatic Cystic Lesions: A Systematic Review and Meta-Analysis. Endosc. Ultrasound 2021, 10, 270. [Google Scholar] [CrossRef]
- Lee, L.S. Updates in Diagnosis and Management of Pancreatic Cysts. World J. Gastroenterol. 2021, 27, 5700–5714. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.; Ge, P.S.; Keach, J.W.; Mullady, D.; Fukami, N.; Edmundowicz, S.A.; Azar, R.R.; Shah, R.J.; Murad, F.M.; Kushnir, V.M.; et al. Suboptimal Accuracy of Carcinoembryonic Antigen in Differentiation of Mucinous and Nonmucinous Pancreatic Cysts: Results of a Large Multicenter Study. Gastrointest. Endosc. 2015, 82, 1060–1069. [Google Scholar] [CrossRef]
- Wang, J.; Paris, P.L.; Chen, J.; Ngo, V.; Yao, H.; Frazier, M.L.; Killary, A.M.; Liu, C.-G.; Liang, H.; Mathy, C.; et al. Next Generation Sequencing of Pancreatic Cyst Fluid MicroRNAs from Low Grade-Benign and High Grade-Invasive Lesions. Cancer Lett. 2015, 356, 404–409. [Google Scholar] [CrossRef]
- Lopes, C.V. Cyst Fluid Glucose: An Alternative to Carcinoembryonic Antigen for Pancreatic Mucinous Cysts. World J. Gastroenterol. 2019, 25, 2271–2278. [Google Scholar] [CrossRef]
- Van der Waaij, L.A.; van Dullemen, H.M.; Porte, R.J. Cyst Fluid Analysis in the Differential Diagnosis of Pancreatic Cystic Lesions: A Pooled Analysis. Gastrointest. Endosc. 2005, 62, 383–389. [Google Scholar] [CrossRef]
- Faias, S.; Cravo, M.; Chaves, P.; Pereira, L. WITHDRAWN: A Comparative Analysis of Glucose and Carcinoembryonic Antigen in Diagnosis of Pancreatic Mucinous Cysts: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Paniccia, A.; Polanco, P.M.; Boone, B.A.; Wald, A.I.; McGrath, K.; Brand, R.E.; Khalid, A.; Kubiliun, N.; O’Broin-Lennon, A.M.; Park, W.G.; et al. Prospective, Multi-Institutional, Real-Time Next-Generation Sequencing of Pancreatic Cyst Fluid Reveals Diverse Genomic Alterations That Improve the Clinical Management of Pancreatic Cysts. Gastroenterology 2023, 164, 117–133.e7. [Google Scholar] [CrossRef] [PubMed]
- Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; Khashab, M.A.; et al. The Role of Endoscopy in the Diagnosis and Treatment of Cystic Pancreatic Neoplasms. Gastrointest. Endosc. 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Thornton, G.D.; McPhail, M.J.W.; Nayagam, S.; Hewitt, M.J.; Vlavianos, P.; Monahan, K.J. Endoscopic Ultrasound Guided Fine Needle Aspiration for the Diagnosis of Pancreatic Cystic Neoplasms: A Meta-Analysis. Pancreatology 2013, 13, 48–57. [Google Scholar] [CrossRef]
- Rogart, J.N.; Loren, D.E.; Singu, B.S.; Kowalski, T.E. Cyst Wall Puncture and Aspiration During EUS-Guided Fine Needle Aspiration May Increase the Diagnostic Yield of Mucinous Cysts of the Pancreas. J. Clin. Gastroenterol. 2011, 45, 164–169. [Google Scholar] [CrossRef]
- Lim, L.G.; Lakhtakia, S.; Ang, T.L.; Vu, C.K.F.; Dy, F.; Chong, V.H.; Khor, C.J.L.; Lim, W.C.; Doshi, B.K.; Varadarajulu, S.; et al. Factors Determining Diagnostic Yield of Endoscopic Ultrasound Guided Fine-Needle Aspiration for Pancreatic Cystic Lesions: A Multicentre Asian Study. Dig. Dis. Sci. 2013, 58, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Jiang, F.; Zhu, J.; Du, Y.; Jin, Z.; Li, Z. Assessment of Morbidity and Mortality Associated with Endoscopic Ultrasound-guided Fine-needle Aspiration for Pancreatic Cystic Lesions: A Systematic Review and Meta-analysis. Dig. Endosc. 2017, 29, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Al-Haddad, M.; Raimondo, M.; Woodward, T.; Krishna, M.; Pungpapong, S.; Noh, K.; Wallace, M.B. Safety and Efficacy of Cytology Brushings versus Standard FNA in Evaluating Cystic Lesions of the Pancreas: A Pilot Study. Gastrointest. Endosc. 2007, 65, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.D.; Subtil, J.C.; Miravalles, T.L.; Echeveste, J.I.; Prieto, C.; Betes, M.; Alvarez Cienfuegos, F.J.; Idoate, M.A. EchoBrush May Be Superior to Standard EUS-Guided FNA in the Evaluation of Cystic Lesions of the Pancreas. Cancer Cytopathol. 2011, 119, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Garcia, J.; Larino-Noia, J.; MacíAs, M.; Martin, A.; Legaz, M.; Rendon, P.; Vila, J.; Reyes, A.; Abdulkader, I.; Dominguez-Munoz, E. Sa1499 A Multicenter, Prospective, Comparative, Randomized Open-Trial of Endoscopic Ultrasound Cytologic Brushing vs. Fine-Needle Aspiration (FNA) for the Pathological Diagnosis of Cystic Pancreatic Lesions. Gastrointest. Endosc. 2012, 75, AB181. [Google Scholar] [CrossRef]
- Balaban, V.; Cazacu, I.; Pinte, L.; Jinga, M.; Bhutani, M.; Saftoiu, A. EUS-through-the-Needle Microbiopsy Forceps in Pancreatic Cystic Lesions: A Systematic Review. Endosc. Ultrasound 2021, 10, 19. [Google Scholar] [CrossRef]
- Kovacevic, B.; Klausen, P.; Rift, C.V.; Toxværd, A.; Grossjohann, H.; Karstensen, J.G.; Brink, L.; Hassan, H.; Kalaitzakis, E.; Storkholm, J.; et al. Clinical impact of endoscopic ultrasound-guided through-the-needle microbiopsy in patients with pancreatic cysts. Endoscopy 2021, 53, 44–52. [Google Scholar] [CrossRef]
- Vilas-Boas, F.; Ribeiro, T.; Afonso, J.; Cardoso, H.; Lopes, S.; Moutinho-Ribeiro, P.; Ferreira, J.; Mascarenhas-Saraiva, M.; Macedo, G. Deep Learning for Automatic Differentiation of Mucinous versus Non-Mucinous Pancreatic Cystic Lesions: A Pilot Study. Diagnostics 2022, 12, 2041. [Google Scholar] [CrossRef]
- Schulz, D.A.H.O.; Heilmaier, M.; Phillip, V.; Treiber, M.; Mayr, U.; Lahmer, T.; Mueller, J.; Demir, I.E.; Friess, H.; Reichert, M.; et al. Accurate Prediction of Histological Grading of Intraductal Papillary Mucinous Neoplasia Using Deep Learning. Endoscopy 2022. [Google Scholar] [CrossRef]
- Rangwani, S.; Ardeshna, D.R.; Rodgers, B.; Melnychuk, J.; Turner, R.; Culp, S.; Chao, W.-L.; Krishna, S.G. Application of Artificial Intelligence in the Management of Pancreatic Cystic Lesions. Biomimetics 2022, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Machicado, J.D.; Chao, W.-L.; Carlyn, D.E.; Pan, T.-Y.; Poland, S.; Alexander, V.L.; Maloof, T.G.; Dubay, K.; Ueltschi, O.; Middendorf, D.M.; et al. High Performance in Risk Stratification of Intraductal Papillary Mucinous Neoplasms by Confocal Laser Endomicroscopy Image Analysis with Convolutional Neural Networks (with Video). Gastrointest. Endosc. 2021, 94, 78–87.e2. [Google Scholar] [CrossRef] [PubMed]
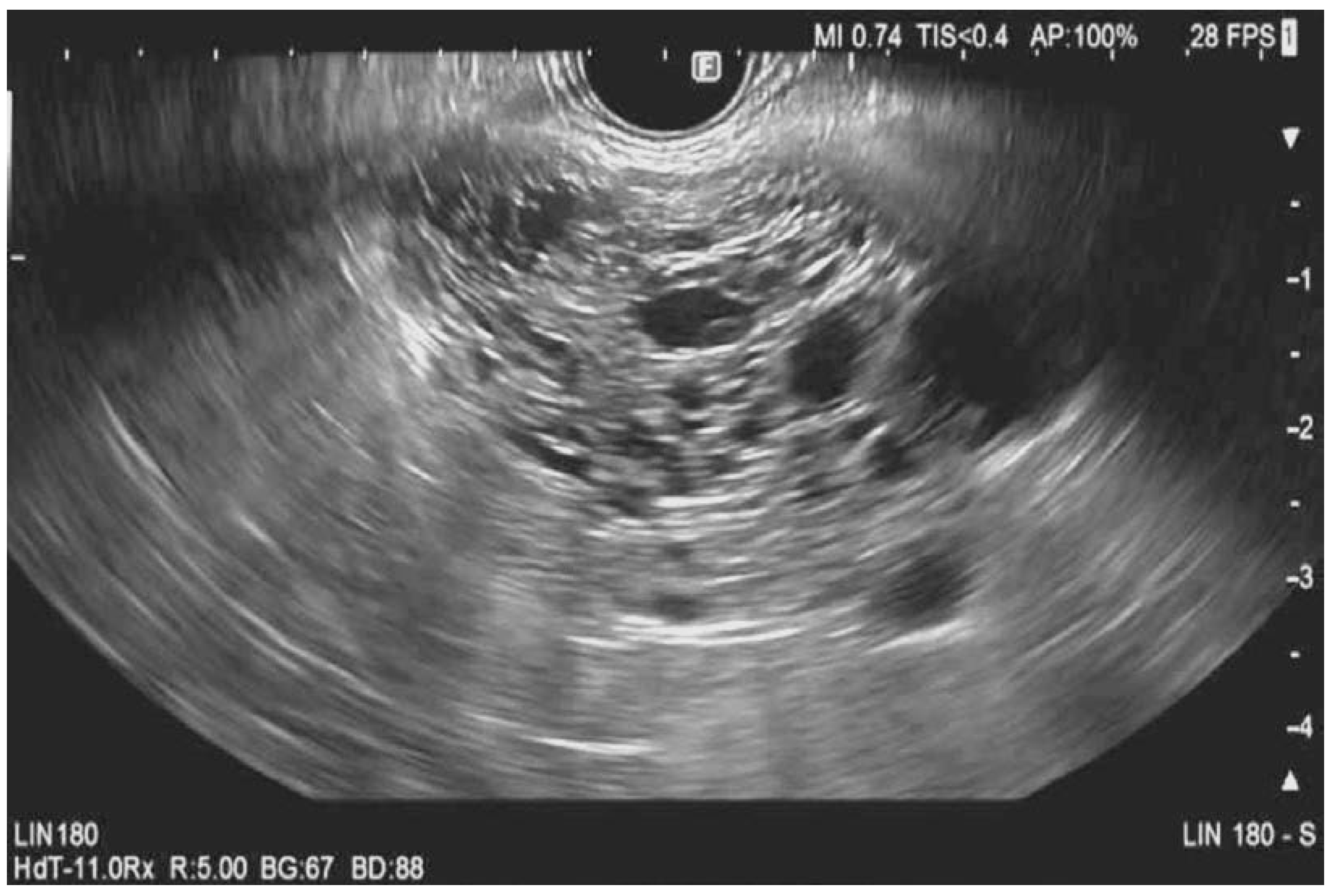

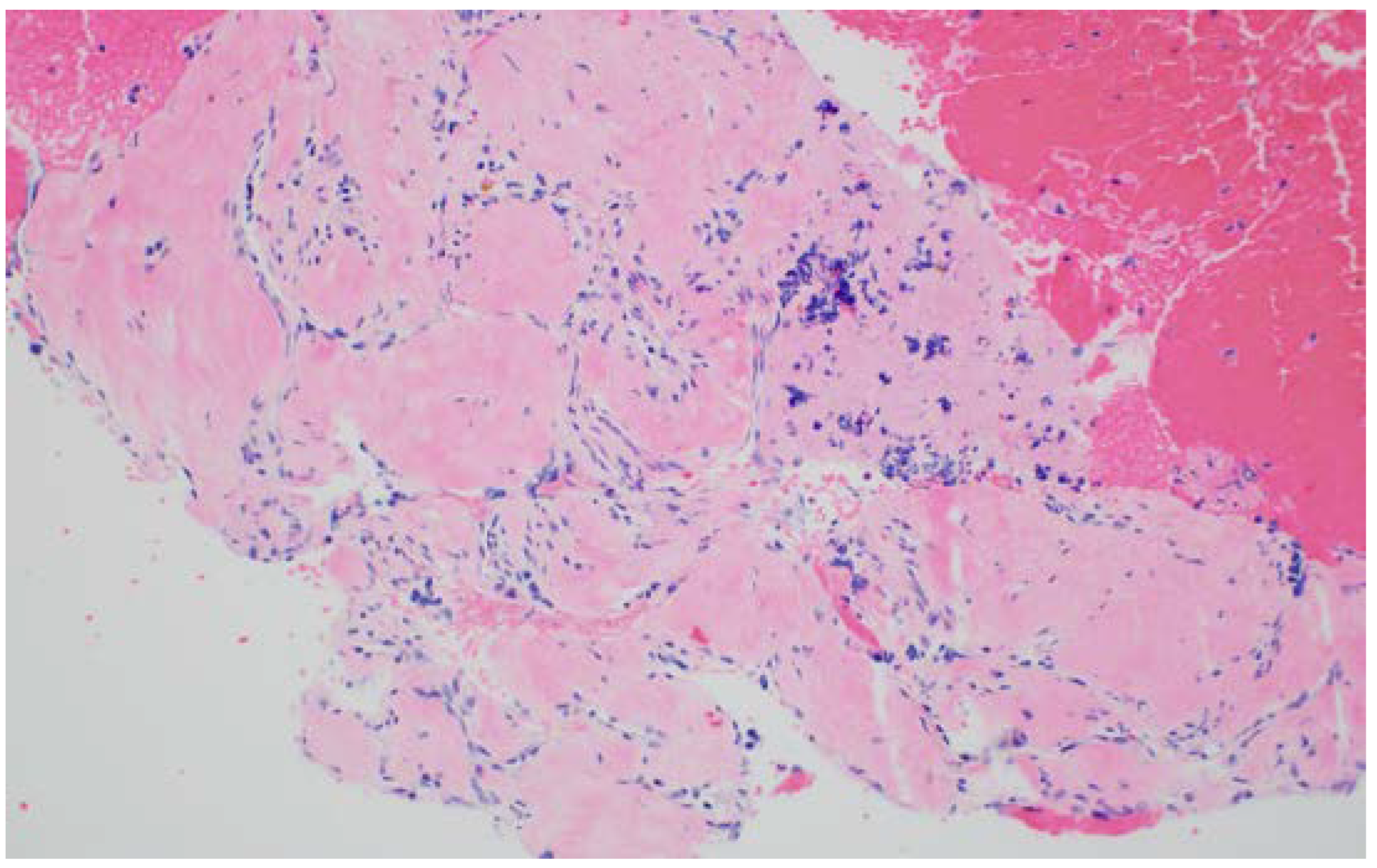

| Guideline | Indications |
|---|---|
| American Gastroenterological Association (AGA) (2015) | >2 high risk features: • PCL Size > 3 cm • Dilated main pancreatic duct • Presence of a solid component |
| International Consensus Guidelines (2017) | With any of the below features: • PCL size > 3 cm • Thickened/enhanced PCL wall • MPD 5–9 mm • Abrupt change in MPD with distal pancreatic atrophy • Lymphadenopathy • Elevated CA 19-9 • Rapid growth (>5 mm/2 years) |
| American College of Gastroenterology (2018) | With any of the below features: • MPD > 5 mm • IPMN or MCN > 3 cm • Change in MPD caliber with upstream atrophy • Size increase > 3 mm/year • Jaundice secondary to PCL • Pancreatitis secondary to PCL • Presence of mural nodule or solid component |
| European (2018) | Radiologic or clinical features of concern for malignancy: Radiologic: • MPD ≥ 5mm • Size increase ≥ 5 mm/year • Presence of mural nodule or solid component Clinical: • Jaundice secondary to PCL • New onset diabetes • Increased CA 19-9 |
| Assay | Pancreatic Lesion Association |
| Carcinoembryonic Antigen (CEA) | Used to differentiate between mucinous and non-mucinous pancreatic cysts with varying accuracy depending on cutoff values. Generally agreed upon that CEA < 5 ng/mL is highly specific for non-mucinous PCL, with a specificity reaching 95%, and CEA > 800 ng/mL is highly specific for mucinous PCL, with a specificity of 98%. |
| Cyst Fluid Glucose | Newer PCL fluid marker–cyst fluid glucose of <50 mg/dL indicates a mucinous cyst with a sensitivity of 91% and specificity of 75% vs. 67% and 80%, respectively, for CEA in the same study population. |
| DNA Markers | Pancreatic Lesion Association |
| VHL Gene Mutations | von Hippel–Lindau (VHL) mutations are correlated with serous cystadenomas. This loss of function mutation has been shown to have a sensitivity of 71% and specificity of 100% in determining serous cystadenomas when evaluating pancreatic cystic lesions. |
| MEN1, LOH Gene Mutations | Multiple endocrine neoplasia 1 (MEN1) and loss of heterozygosity (LOH) genes are correlated with pancreatic neuroendocrine tumors. When evaluated together, these mutations have a sensitivity and specificity of 68% and 98%, respectively, in identifying a lesion as a pancreatic neuroendocrine tumor. |
| MAPK, GNAS Mutations | MAPK and GNAS mutations are used to identify a pancreatic cystic lesion as mucinous with a sensitivity of 90% and specificity of 100%. |
| TP53, SMAD4, CTNNB1, mTOR | When combined with MAPK/GNAS mutation in a sequencing panel, the addition of TP53/SMAD4/CTNNB1/mTOR was able to identify advanced neoplasia with a sensitivity and specificity of 88% and 98%, respectively. |
| Guideline | Indications |
|---|---|
| American Gastroenterological Association (AGA) (2015) | EUS-FNA if >2 high risk features: • Cyst size > 3 cm • Dilated main pancreatic duct • Presence of a solid component |
| International Consensus Guidelines (2017) | EUS-FNA if PCL with any of the below worrisome features: • Cyst size > 3 cm • Thickened/enhanced cyst walls • MPD 5–9 mm • Abrupt change in MPD with distal pancreatic atrophy • Lymphadenopathy • Elevated CA 19-9 • Rapid growth (>5 mm/2 years) |
| American College of Gastroenterology (2018) | EUS-FNA if PCL with any of the below features: • MPD > 5 mm • IPMN or MCN > 3 cm • Change in MPD caliber with upstream atrophy • Size increase > 3 mm/year • Jaundice secondary to cyst • Pancreatitis secondary to cyst • Presence of mural nodule or solid component |
| European (2018) | For cysts when the diagnosis is unclear and the results are expected to change clinical management. EUS-FNA is not to be performed if the diagnosis is available or if there is a clear indication for surgery. |
| American Society for Gastrointestinal Endoscopy | EUS-FNA recommended for PCLs > 3 cm, presence of an epithelial nodule, dilated MPD, or suspicious mass lesion. In the absence of these features, EUS-FNA is considered optional. |
| Technique | Description |
|---|---|
| Endoscopic Ultrasound- Fine Needle Aspiration (EUS-FNA) | Low sensitivity of 54%, but when positive, has a specificity of 93%. Targeted cyst wall puncture after aspiration of cyst fluid was shown to improve the yield and provide an adequate specimen in 77% of cases. |
| Endoscopic Ultrasound Throught-the-Needle Biopsy (EUS-TTNB) | Introduction of microforceps through a 19-gauge needle, allowing for maintainence of cellular structure and potentially a higher yield. Pooled analysis showed a diagnostic yield of 74% and a diagnostic performance of 80%. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rangwani, S.; Juakiem, W.; Krishna, S.G.; El-Dika, S. Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review. Diagnostics 2023, 13, 705. https://doi.org/10.3390/diagnostics13040705
Rangwani S, Juakiem W, Krishna SG, El-Dika S. Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review. Diagnostics. 2023; 13(4):705. https://doi.org/10.3390/diagnostics13040705
Chicago/Turabian StyleRangwani, Shiva, Wasseem Juakiem, Somashekar G. Krishna, and Samer El-Dika. 2023. "Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review" Diagnostics 13, no. 4: 705. https://doi.org/10.3390/diagnostics13040705
APA StyleRangwani, S., Juakiem, W., Krishna, S. G., & El-Dika, S. (2023). Role of Endoscopic Ultrasound in the Evaluation of Pancreatic Cystic Neoplasms: A Concise Review. Diagnostics, 13(4), 705. https://doi.org/10.3390/diagnostics13040705







