Osteomyelitis of the Lower Limb: Diagnostic Accuracy of Dual-Energy CT versus MRI
Abstract
:1. Introduction
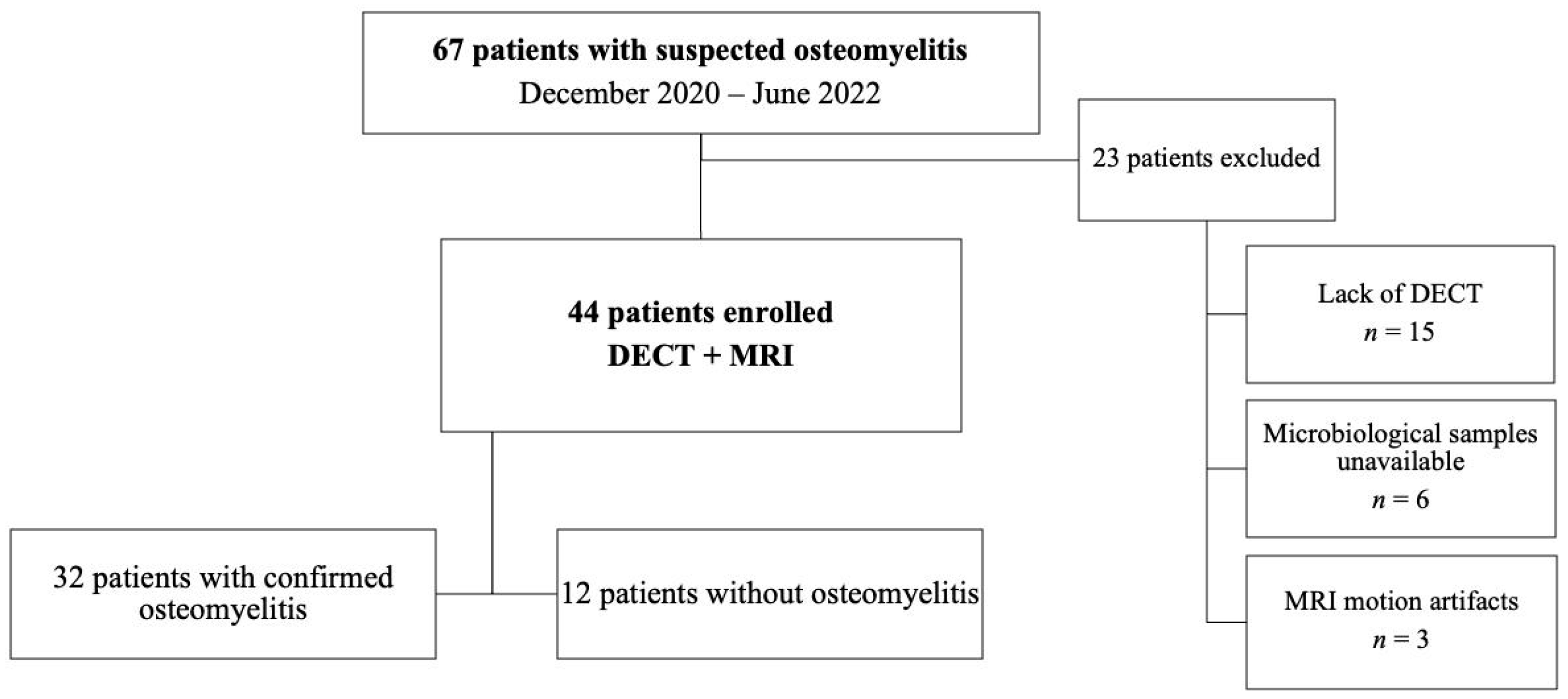
2. Material and Methods
2.1. Participants
2.2. Magnetic Resonance Imaging
2.3. DECT Protocol
2.4. DECT Post-Processing
2.5. Image Analysis
2.6. Clinical Findings and Microbiological Analysis
2.7. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Clinical and Microbiological Results
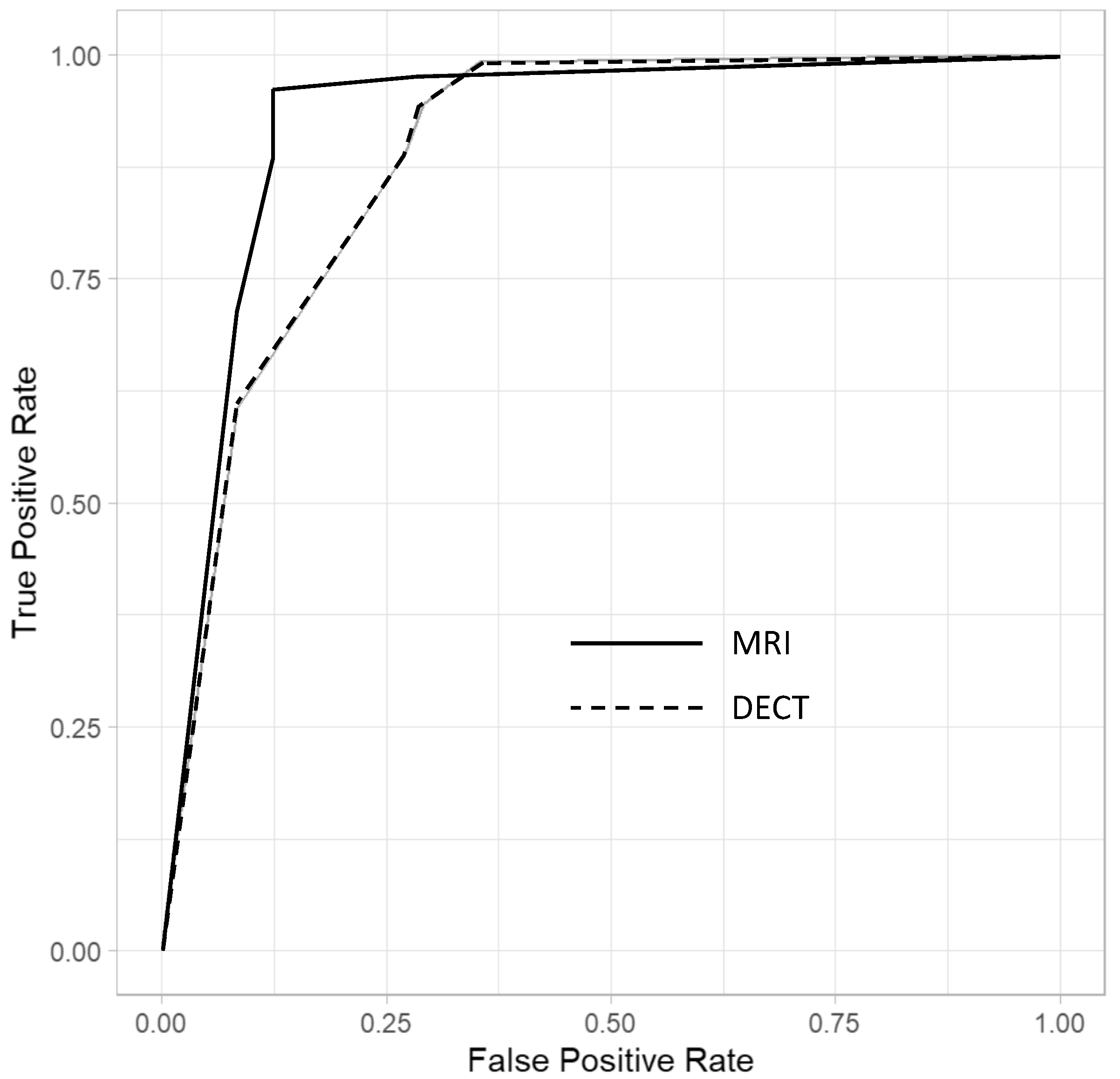
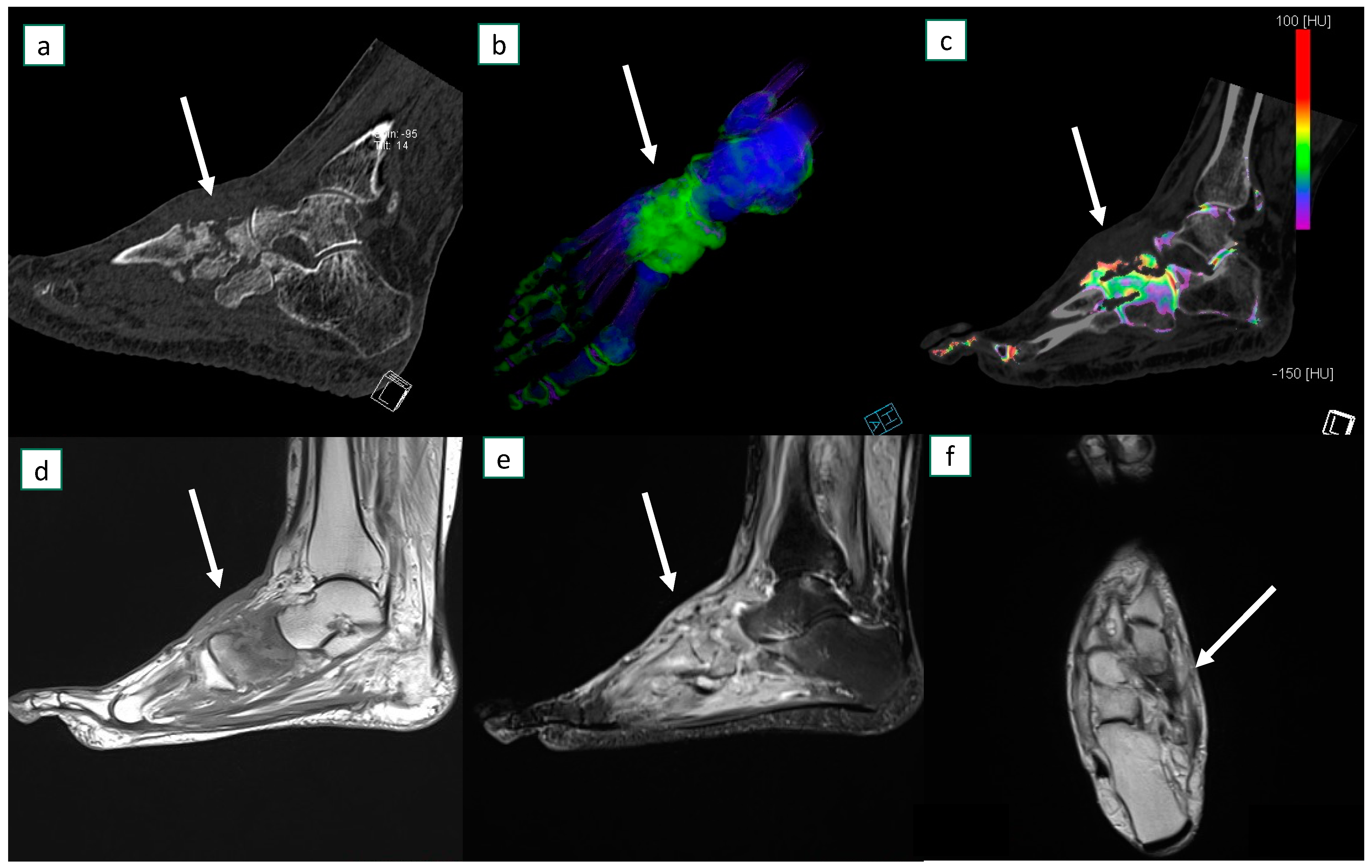
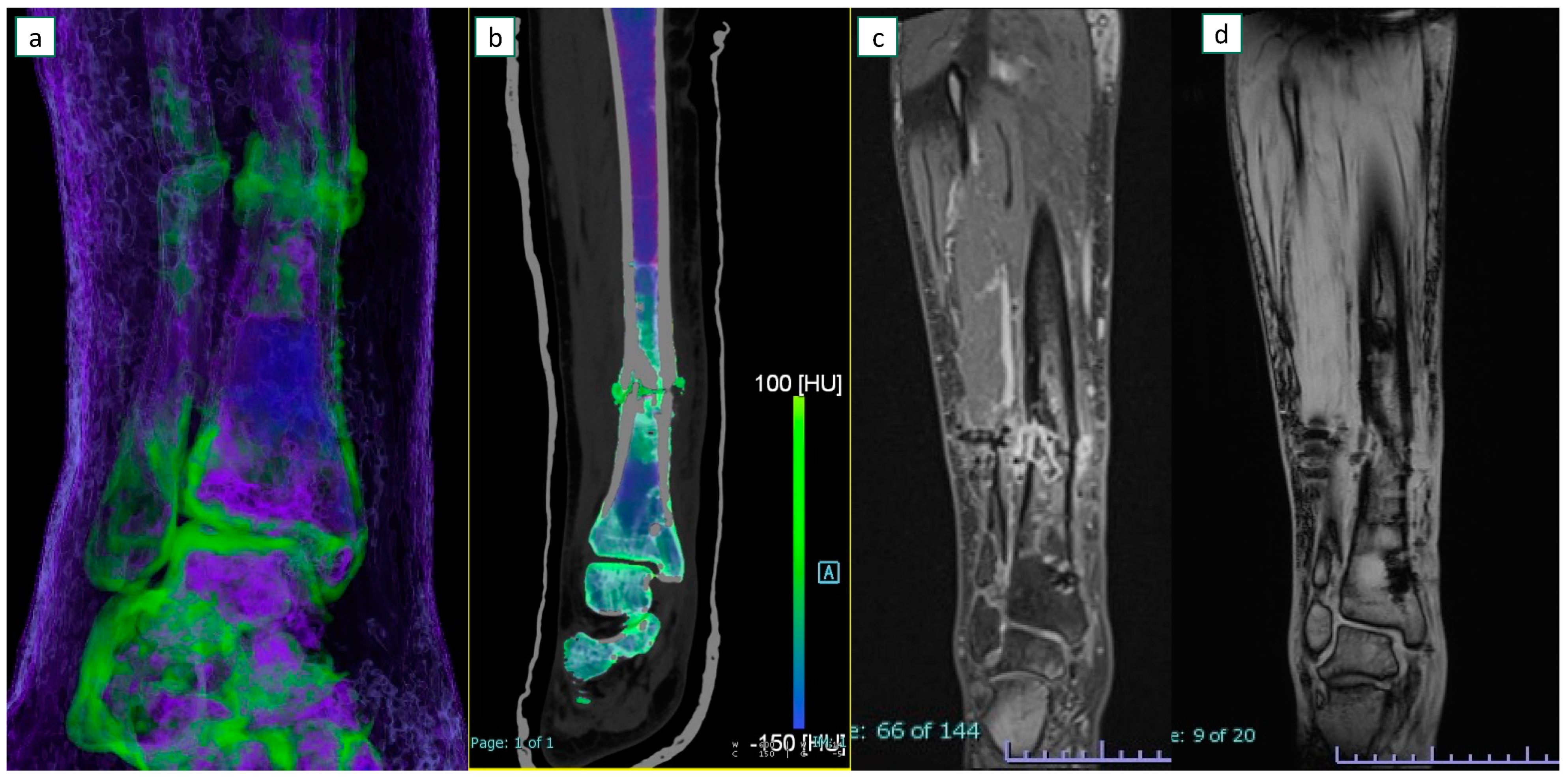
3.3. Imaging Results
3.4. Inter-Observer Agreement
3.5. Multivariable Logistic Analysis
4. Discussion
4.1. Discussion of Background
4.2. Role of DECT and Comparison with Previous Studies
4.3. Discussion of Specific Imaging Parameters
4.4. Strenghts and Drawbacks of DECT
4.5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alaia, E.F.; Chhabra, A.; Simpfendorfer, C.S.; Cohen, M.; Mintz, D.N.; Vossen, J.A.; Zoga, A.C.; Fritz, J.; Spritzer, C.E.; Armstrong, D.G.; et al. MRI nomenclature for musculoskeletal infection. Skelet. Radiol. 2021, 50, 2319–2347. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, S.K. Osteomyelitis. Infect. Dis. Clin. N. Am. 2017, 31, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, H.P.; Morrison, W.B.; Schweitzer, M.E. MR image analysis of pedal osteomyelitis: Distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology 2002, 223, 747–755. [Google Scholar] [CrossRef]
- Resnick, D. Diagnosis of Bone and Joint Disorders, 4th ed.; W.B. Saunders Company: Philadephia, PA, USA, 2002. [Google Scholar]
- Glaudemans, A.W.; Jutte, P.C.; Cataldo, M.A.; Cassar-Pullicino, V.; Gheysens, O.; Borens, O.; Trampuz, A.; Wörtler, K.; Petrosillo, N.; Winkler, H.; et al. Consensus document for the diagnosis of peripheral bone infection in adults: A joint paper by the EANM, EBJIS, and ESR (with ESCMID endorsement). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America clinical practice guideline for the diagnosis and treatment of diabetic foot infections. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Hirschfeld, C.B.; Kapadia, S.N.; Bryan, J.; Jannat-Khah, D.P.; May, B.; Vielemeyer, O.; Esquivel, E.L. Impact of diagnostic bone biopsies on the management of non-vertebral osteomyelitis: A retrospective cohort study. Medicine 2019, 98, e16954. [Google Scholar] [CrossRef]
- Schweitzer, M.E.; Daffner, R.H.; Weissman, B.N.; Bennett, D.L.; Blebea, J.S.; Jacobson, J.A.; Morrison, W.B.; Resnik, C.S.; Roberts, C.C.; Rubin, D.A.; et al. ACR Appropriateness Criteria on suspected osteomyelitis in patients with diabetes mellitus. J. Am. Coll. Radiol. 2008, 5, 881–886. [Google Scholar] [CrossRef]
- Dinh, M.T.; Abad, C.L.; Safdar, N. Diagnostic accuracy of the physical examination and imaging tests for osteomyelitis underlying diabetic foot ulcers: Meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2008, 47, 519–527. [Google Scholar] [CrossRef]
- Donovan, A.; Schweitzer, M.E. Use of MR imaging in diagnosing diabetes-related pedal osteomyelitis. Radiographics 2010, 30, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Kotecha, H.M.; Lo, H.S.; Vedantham, S.; Shin, H.; Cerniglia, C.A. Abbreviated MRI of the foot in patients with suspected osteomyelitis. Emerg. Radiol. 2020, 27, 9–16. [Google Scholar] [CrossRef]
- McCarthy, E.; Morrison, W.B.; Zoga, A.C. MR Imaging of the Diabetic Foot. Magn. Reson. Imaging Clin. N. Am. 2017, 25, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Page, S.; Lavalley, M.; Gale, D.R.; Felson, D.T. Magnetic resonance imaging for diagnosing foot osteomyelitis: A meta-analysis. Arch. Intern. Med. 2007, 167, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Beltran, J.; Noto, A.M.; McGhee, R.B.; Freedy, R.M.; McCalla, M.S. Infections of the musculoskeletal system: High-field-strength MR imaging. Radiology 1987, 164, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Chandnani, V.P.; Beltran, J.; Morris, C.S.; Khalil, S.N.; Mueller, C.F.; Burk, J.M.; Bennett, W.F.; Shaffer, P.B.; Vasila, M.S.; Reese, J. Acute experimental osteomyelitis and abscesses: Detection with MR imaging versus CT. Radiology 1990, 174, 233–236. [Google Scholar] [CrossRef]
- Baffour, F.I.; Glazebrook, K.N.; Morris, J.M.; Michalak, G.J.; Fletcher, J.G.; Leng, S.; McCollough, C.H. Clinical utility of virtual noncalcium dual-energy CT in imaging of the pelvis and hip. Skelet. Radiol. 2019, 48, 1833–1842. [Google Scholar] [CrossRef]
- Jang, S.W.; Chung, B.M.; Kim, W.T.; Gil, J.R. Nondisplaced fractures on hip CT: Added value of dual-energy CT virtual non-calcium imaging for detection of bone marrow edema using visual and quantitative analyses. Acta Radiol. 2019, 60, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Kellock, T.T.; Nicolaou, S.; Kim, S.S.; Al-Busaidi, S.; Louis, L.J.; O’Connell, T.W.; Ouellette, H.A.; McLaughlin, P.D. Detection of Bone Marrow Edema in Nondisplaced Hip Fractures: Utility of a Virtual Noncalcium Dual-Energy CT Application. Radiology 2017, 284, 798–805. [Google Scholar] [CrossRef]
- Foti, G.; Faccioli, N.; Silva, R.; Oliboni, E.; Zorzi, C.; Carbognin, G. Bone marrow edema around the hip in non-traumatic pain: Dual-energy CT vs MRI. Eur. Radiol. 2020, 30, 4098–4106. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Zhang, H.; Jia, X.; Zheng, X.; Zuo, T. Diagnostic accuracy of dual-energy computed tomography (DECT) to detect non-traumatic bone marrow edema: A systematic review and meta-analysis. Eur. J. Radiol. 2022, 153, 110359. [Google Scholar] [CrossRef]
- Ghazi Sherbaf, F.; Sair, H.I.; Shakoor, D.; Fritz, J.; Schwaiger, B.J.; Johnson, M.H.; Demehri, S. DECT in Detection of Vertebral Fracture–associated Bone Marrow Edema: A Systematic Review and Meta-Analysis with Emphasis on Technical and Imaging Interpretation Parameters. Radiology 2021, 300, 110–119. [Google Scholar] [CrossRef]
- Foti, G.; Guerriero, M.; Faccioli, N.; Fighera, A.; Romano, L.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema around the ankle joint in non-traumatic patients: Diagnostic accuracy of dual-energy computed tomography. Clin. Imaging 2021, 69, 341–348. [Google Scholar] [CrossRef]
- Yan, Y.Y.; Ouellette, H.A.; Saththianathan, M.; Munk, P.L.; Mallinson, P.I.; Sheikh, A. The Role of a Virtual Noncalcium Dual-Energy CT Application in the Detection of Bone Marrow Edema in Peripheral Osteomyelitis. Can. Assoc. Radiol. J. J. Assoc. Can. Radiol. 2022, 73, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Beltramello, A.; Catania, M.; Rigotti, S.; Serra, G.; Carbognin, G. Diagnostic accuracy of dual-energy CT and virtual non-calcium techniques to evaluate bone marrow edema in vertebral compression fractures. Radiol. Med. 2019, 124, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Serra, G.; Iacono, V.; Zorzi, C. Identification of Traumatic Bone Marrow Oedema: The Pearls and Pitfalls of Dual-Energy CT (DECT). Tomography 2021, 7, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Erdman, W.A.; Tamburro, F.; Jayson, H.T.; Weatherall, P.T.; Ferry, K.B.; Peshock, R.M. Osteomyelitis: Characteristics and pitfalls of diagnosis with MR imaging. Radiology 1991, 180, 533–539. [Google Scholar] [CrossRef]
- Morrison, W.B.; Schweitzer, M.E.; Batte, W.G.; Radack, D.P.; Russel, K.M. Osteomyelitis of the foot: Relative importance of primary and secondary MR imaging signs. Radiology 1998, 207, 625–632. [Google Scholar] [CrossRef]
- Lee, Y.J.; Sadigh, S.; Mankad, K.; Kapse, N.; Rajeswaran, G. The imaging of osteomyelitis. Quant. Imaging Med. Surg. 2016, 6, 184–198. [Google Scholar] [CrossRef]
- Foti, G.; Catania, M.; Caia, S.; Romano, L.; Beltramello, A.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema of the ankle: Diagnostic accuracy of dual-energy CT in comparison with MRI. Radiol. Med. 2019, 124, 1028–1036. [Google Scholar] [CrossRef]
- Booz, C.; Nöske, J.; Albrecht, M.H.; Lenga, L.; Martin, S.S.; Wichmann, J.L.; Huizinga, N.A.; Eichler, K.; Nour-Eldin, N.E.; Vogl, T.J.; et al. Traumatic bone marrow edema of the calcaneus: Evaluation of color-coded virtual non-calcium dual-energy CT in a multi-reader diagnostic accuracy study. Eur. J. Radiol. 2019, 118, 207–214. [Google Scholar] [CrossRef]
- Deng, K.; Sun, C.; Liu, C.; Ma, R. Initial experience with visualizing hand and foot tendons by dual-energy computed tomography. Clin. Imaging 2009, 33, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Fighera, A.; Campacci, A.; Natali, S.; Guerriero, M.; Zorzi, C.; Carbognin, G. Diagnostic Performance of Dual-Energy CT for Detecting Painful Hip Prosthesis Loosening. Radiology 2021, 300, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Foti, G.; Longo, C.; D’Onofrio, M.; Natali, S.; Piovan, G.; Oliboni, E.; Iacono, V.; Guerriero, M.; Zorzi, C. Dual-Energy CT for Detecting Painful Knee Prosthesis Loosening. Radiology 2022, 211818. [Google Scholar] [CrossRef]
- Zou, Z.; Yang, M.; Wang, Y.; Zhang, B. Gout of ankle and foot: DECT versus US for crystal detection. Clin. Rheumatol. 2021, 40, 1533–1537. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.H.; Hawkins, R.A.; Katz, R.D. Bacterial osteomyelitis: Findings on plain radiography, CT, MR, and scintigraphy. AJR Am. J. Roentgenol. 1991, 157, 365–370. [Google Scholar] [CrossRef]
- Llewellyn, A.; Jones-Diette, J.; Kraft, J.; Holton, C.; Harden, M.; Simmonds, M. Imaging tests for the detection of osteomyelitis: A systematic review. Health Technol. Assess. 2019, 23, 1. [Google Scholar] [CrossRef]
- Johnson, P.W.; Collins, M.S.; Wenger, D.E. Diagnostic utility of T1-weighted MRI characteristics in evaluation of osteomyelitis of the foot. Am. J. Roentgenol. 2009, 192, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Park, S.; Park, Y.U.; Kwack, K.S.; Jeon, S.W.; Lee, H.Y. Multivariate analyses of MRI findings for predicting osteomyelitis of the foot in diabetic patients. Acta Radiol. 2020, 61, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | No. of Participants (n = 44) |
|---|---|
| Age (y) | 62.5 (18–87) [16.5] |
| Number of men | 32 (72.7%) |
| Number of women | 12 (27.3%) |
| Osteomyelitis | 32 (72.7%) |
| No osteomyelitis | 12 (27.3%) |
| Side | |
| Right | 21 (47.7%) |
| Left | 23 (52.3%) |
| Skeletal segment | |
| Foot | 30 (68.1%) |
| Knee/leg | 9 (20.5%) |
| Hip | 5 (11.4%) |
| Sensitivity | Specificity | AUC | PPV | NPV | |
|---|---|---|---|---|---|
| MRI | 89.1% * [82.3, 93.9] | 87.5% * [71.8, 95.3] | 0.88 * [0.78, 0.98] | 95.0% [89.4, 98.1] | 75.0% [61.6, 85.5] |
| Reader 1 | 93.8% [79.2, 99.2] | 91.7% [61.5, 99.8] | 0.93 [0.84, 1.00] | 96.8% [83.3, 99.9] | 84.6% [54.6, 98.1] |
| Reader 2 | 84.4% [67.2, 94.7] | 91.7% [61.5, 99.8] | 0.88 [0.78, 0.98] | 96.4% [81.7, 99.9] | 68.8% [41.3, 89.0] |
| Reader 3 | 87.5% [71.0, 96.5] | 83.3% [51.6, 97.9] | 0.85 [0.73, 0.98] | 93.3% [77.9, 99.2] | 71.4% [41.9, 91.6] |
| Reader 4 | 90.6% [75.0, 98.0] | 83.3% [51.6, 97.9] | 0.87 [0.75, 0.99] | 93.5% [78.6, 99.2] | 76.9% [46.2, 95.0] |
| DECT | 89.0% * [82.2, 93.8] | 72.9% * [58.2, 84.7] | 0.81 * [0.67, 0.95] | 89.7% [83.0, 94.4] | 71.4% [56.7, 83.4] |
| Reader 1 | 93.8% [79.2, 99.2] | 75.0% [42.8, 94.5] | 0.84 [0.71, 0.98] | 90.9% [75.7, 97.7] | 81.8% [48.2, 97.7] |
| Reader 2 | 84.4% [67.2, 94.7] | 75.0% [42.8, 94.5] | 0.80 [0.65, 0.94] | 90.0% [73.5, 97.9] | 64.3% [35.1, 87.2] |
| Reader 3 | 93.8% [79.2, 99.2] | 66.7% [34.9, 90.1] | 0.80 [0.66, 0.95] | 88.2% [72.5, 96.7] | 80.0% [44.4, 97.5] |
| Reader 4 | 81.2% [63.6, 92.8] | 75.0% [42.8, 94.5] | 0.78 [0.64, 0.93] | 89.7% [72.6, 97.8] | 60.0% [32.2, 83.7] |
| Sensitivity | Specificity | AUC | PPV | NPV | |
|---|---|---|---|---|---|
| DECT | 91.2% * [84.8, 95.5] | 82.4% * [69.1, 91.6] | 90 * [0.84, 0.96] | 92.7% [86.6, 96.6] | 79.2% [65.9, 89.2] |
| Reader 1 | 90.6% [75.0, 98.0] | 83.3% [51.6, 97.9] | 0.87 [0.75, 0.99] | 93.5% [78.6, 99.2] | 76.9% [46.2, 95.0] |
| Reader 2 | 87.1% [77, 96] | 84% [71, 94] | 0.87 [0.80, 0.94] | 96.8% [83.3, 99.9] | 84.6% [54.6, 98.1] |
| Reader 3 | 85% [72, 94] | 96% [85, 100] | 0.90 [0.84, 0.96] | 96.8% [83.3, 99.9] | 84.6% [54.6, 98.1] |
| Reader 4 | 87% [74, 95] | 91% [79, 98] | 0.89 [0.83, 0.96] | 96.8% [83.3, 99.9] | 84.6% [54.6, 98.1] |
| MRI | DECT | |||||
|---|---|---|---|---|---|---|
| Sensitivity | Specificity | AUC | Sensitivity | Specificity | AUC | |
| BME | 94.5% * [79.5, 99.6] | 91.6% * [61.5, 99.8] | 0.93 * | 89.1% * [74.5, 97.8] | 81.2% * [50.3, 96.8] | 0.85 * |
| Reader 1 | 93.8% [79.2, 99.2] | 100% [73.5, 1.00] | 0.97 [0.93, 1.00] | 90.6% [75.0, 98.0] | 83.3% [51.6, 97.7] | 0.87 [0.75, 0.99] |
| Reader 2 | 93.8% [79.2, 99.2] | 91.7% [61.5, 99.8] | 0.93 [0.81, 0.97] | 87.5% [71.0, 96.5] | 83.3% [51.6, 97.9] | 0.85 [0.73, 0.98] |
| Reader 3 | 93.8% [79.2, 99.2] | 91.7% [61.5, 99.8] | 0.93 [0.81, 0.97] | 90.6% [75.0, 98.0] | 75.0% [42.8, 94.5] | 0.82 [0.69, 0.97] |
| Reader 4 | 96.9% [83.8, 99.9] | 83.3% [51.6, 97.9] | 0.90 [0.79, 1.00] | 87.5% [71.0, 96.5] | 83.3% [51.6, 97.7] | 0.85 [0.73, 0.98] |
| Erosions | 49.2% * [40.3, 58.2] | 89.4% * [77.3, 96.5] | 0.53 * | 74.2% * [65.7, 81.5] | 79.2% * [65.0, 89.5] | 0.77 * |
| Reader 1 | 34.4% [18.6, 53.2] | 91.7% [61.5, 99.8] | 0.37 [0.25, 0.49] | 68.8% [50.0, 83.9] | 83.3% [51.6, 97.7] | 0.76 [0.62, 0.90] |
| Reader 2 | 46.9% [29.1, 65.3] | 91.7% [61.5, 99.8] | 0.31 [0.19, 0.43] | 75.0% [56.6, 88.5] | 83.3% [51.6, 97.9] | 0.79 [0.65, 0.93] |
| Reader 3 | 56.3% [37.7, 73.6] | 83.3% [51.6, 97.9] | 0.70 [0.56, 0.84] | 78.1% [60.0, 90.7] | 66.7% [34.9, 90.1] | 0.72 [0.57, 0.88] |
| Reader 4 | 59.4% [40.6, 76.3] | 91.7% [61.5, 99.8] | 0.75 [0.63, 0.87] | 75.0% [56.6, 88.5] | 83.3% [51.6, 97.7] | 0.79 [0.65, 0.93] |
| Abscesses | 31.2% * [23.4, 40.4] | 91.7% * [80.0, 97.7] | 0.39 * | 26.6% * [19.1, 35.1] | 89.6% * [77.3, 96.5] | 0.42 * |
| Reader 1 | 31.2% [16.1, 50.0] | 75.0% [42.8, 94.5] | 0.47 [0.31, 0.62] | 25.0% [11.5, 43.4] | 83.3% [51.6, 97.9] | 0.46 [0.32, 0.59] |
| Reader 2 | 43.8% [26.4, 62.3] | 91.7% [61.5, 99.8] | 0.32 [0.20, 0.44] | 34.4% [18.6, 53.2] | 91.7% [61.5, 99.8] | 0.37 [0.25, 0.49] |
| Reader 3 | 28.1% [13.7, 46.7] | 100% [73.5, 100] | 0.36 [0.28, 0.44] | 25.0% [11.5, 43.4] | 91.7% [61.5, 99.8] | 0.42 [0.30, 0.53] |
| Reader 4 | 21.9% [9.3, 40.0] | 100% [71.5, 100] | 0.39 [0.32, 0.46] | 21.9% [9.3, 40.0] | 91.7% [61.5, 99.8] | 0.43 [0.32, 0.54] |
| Sinus tract | 22.7% * [15.7, 30.9] | 95.8% * [85.7, 99.5] | 0.41 * | 21.9% * [15.1, 30.0] | 93.8% * [82.8, 98.7] | 0.42 * |
| Reader 1 | 28.1% [13.7, 46.7] | 91.7% [61.5, 99.8] | 0.40 [0.29, 0.51] | 25.0% [11.5, 43.4] | 91.7% [61.5, 99.8] | 0.42 [0.31, 0.53] |
| Reader 2 | 25.0% [11.5, 43.4] | 91.7% [61.5, 99.8] | 0.41 [0.31, 0.53] | 28.1% [13.7, 46.7] | 91.7% [61.5, 99.8] | 0.40 [0.29, 0.51] |
| Reader 3 | 25.0% [11.5, 43.4] | 100% [73.5, 100] | 0.38 [0.30, 0.45] | 18.8% [7.2, 36.4] | 91.7% [61.5, 99.8] | 0.45 [0.34, 0.55] |
| Reader 4 | 12.5% [3.5, 29.0] | 100% [73.5, 100] | 0.44 [0.38, 0.50] | 15.6% [5.3, 32.8] | 100% [73.5, 100] | 0.42 [0.36, 0.49] |
| Gas | 12.5% * [7.3, 19.5] | 97.9% * [65.9, 89.2] | 0.45 * | 15.7% * [9.8, 23.1] | 89.6% * [77.3, 96.5] | 0.48 * |
| Reader 1 | 9.4% [2.0, 25.0] | 91.7% [61.5, 99.8] | 0.49 [0.40, 0.59] | 9.4% [2.0, 25.0] | 91.7% [61.5, 99.8] | 0.49 [0.40, 0.59] |
| Reader 2 | 18.8% [7.2, 36.4] | 100% [73.5, 100] | 0.40 [0.34, 0.47] | 18.8% [7.2, 36.4] | 91.7% [61.5, 99.8] | 0.45 [0.34, 0.55] |
| Reader 3 | 9.4% [2.0, 25.0] | 100% [73.5, 100] | 0.45 [0.40, 0.50] | 15.6% [5.3, 32.8] | 83.3% [51.6, 97.9] | 0.51 [0.38, 0.63] |
| Reader 4 | 12.5% [3.5, 29.0] | 100% [73.5, 100] | 0.44 [0.38, 0.50] | 18.8% [7.2, 36.4] | 91.7% [61.5, 99.8] | 0.45 [0.34, 0.55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foti, G.; Longo, C.; Sorgato, C.; Oliboni, E.S.; Mazzi, C.; Motta, L.; Bertoli, G.; Marocco, S. Osteomyelitis of the Lower Limb: Diagnostic Accuracy of Dual-Energy CT versus MRI. Diagnostics 2023, 13, 703. https://doi.org/10.3390/diagnostics13040703
Foti G, Longo C, Sorgato C, Oliboni ES, Mazzi C, Motta L, Bertoli G, Marocco S. Osteomyelitis of the Lower Limb: Diagnostic Accuracy of Dual-Energy CT versus MRI. Diagnostics. 2023; 13(4):703. https://doi.org/10.3390/diagnostics13040703
Chicago/Turabian StyleFoti, Giovanni, Chiara Longo, Claudia Sorgato, Eugenio Simone Oliboni, Cristina Mazzi, Leonardo Motta, Giulia Bertoli, and Stefania Marocco. 2023. "Osteomyelitis of the Lower Limb: Diagnostic Accuracy of Dual-Energy CT versus MRI" Diagnostics 13, no. 4: 703. https://doi.org/10.3390/diagnostics13040703
APA StyleFoti, G., Longo, C., Sorgato, C., Oliboni, E. S., Mazzi, C., Motta, L., Bertoli, G., & Marocco, S. (2023). Osteomyelitis of the Lower Limb: Diagnostic Accuracy of Dual-Energy CT versus MRI. Diagnostics, 13(4), 703. https://doi.org/10.3390/diagnostics13040703







