Heart Rate Lowering Significantly Increases Feasibility in Doppler Recording Blood Flow Velocity in Coronaries during Transthoracic Doppler Echocardiography
Abstract
1. Introduction
2. Materials and Methods
2.1. Echocardiographic Equipment Characteristics and Settings
2.2. Echo Tomographic Planes
2.3. E-Doppler TTE versus Angiography: Pulsed-Wave Doppler Analysis
2.4. Feasibility Study Protocol
2.4.1. Study Protocol
2.4.2. Angiographic Analysis
3. Results
4. Discussion
4.1. Coronary Ultrasound Feasibility
4.2. Previous Studies
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Caiati, C.; Montaldo, C.; Zedda, N.; Bina, A.; Iliceto, S. New noninvasive method for coronary flow reserve assessment—Contrast-enhanced transthoracic second harmonic echo Doppler. Circulation 1999, 99, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Holte, E.; Vegsundvag, J.; Hegbom, K.; Hole, T.; Wiseth, R. Transthoracic Doppler for detection of stenoses in the three main coronary arteries by use of stenotic to prestenotic velocity ratio and aliased coronary flow. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1323–1330. [Google Scholar] [CrossRef][Green Version]
- Caiati, C.; Lepera, M.E.; Pollice, P.; Iacovelli, F.; Favale, S. A new noninvasive method for assessing mild coronary atherosclerosis: Transthoracic convergent color Doppler after heart rate reduction. Validation vs. intracoronary ultrasound. Coron. Artery Dis. 2020, 31, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Zedda, N.; Cadeddu, M.; Chen, L.; Montaldo, C.; Iliceto, S.; Favale, S. Detection, location, and severity assessment of left anterior descending coronary artery stenoses by means of contrast-enhanced transthoracic harmonic echo Doppler. Eur. Heart J. 2009, 30, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Alla, B.; Alexander, V.; Rostislav, K. Transthoracic Echocardiography in the Assessment of Coronary Arteries. In Coronary Angiography; Branislav, B., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 2. [Google Scholar]
- Schroder, J.; Prescott, E. Doppler Echocardiography Assessment of Coronary Microvascular Function in Patients With Angina and No Obstructive Coronary Artery Disease. Front. Cardiovasc. Med. 2021, 8, 723542. [Google Scholar] [CrossRef]
- Lethen, H.H.P.T.; Kersting, S.; Lambertz, H. Validation of noninvasive assessment of coronary flow velocity reserve in the right coronary artery. A comparison of transthoracic echocardiographic results with intracoronary Doppler flow wire measurements. Eur. Heart J. 2003, 24, 1567–1575. [Google Scholar] [CrossRef]
- Lethen, H.; Tries, H.P.; Brechtken, J.; Kersting, S.; Lambertz, H. Comparison of transthoracic Doppler echocardiography to intracoronary Doppler guidewire measurements for assessment of coronary flow reserve in the left anterior descending artery for detection of restenosis after coronary angioplasty. Am. J. Cardiol. 2003, 91, 412–417. [Google Scholar] [CrossRef]
- Hozumi, T.; Yoshida, K.; Akasaka, T.; Asami, Y.; Ogata, Y.; Takagi, T.; Kaji, S.; Kawamoto, T.; Ueda, Y.; Morioka, S. Noninvasive assessment of coronary flow velocity and coronary flow velocity reserve in the left anterior descending coronary artery by Doppler echocardiography: Comparison with invasive technique. J. Am. Coll. Cardiol. 1998, 32, 1251–1259. [Google Scholar] [CrossRef]
- Boudoulas, H.; Rittgers, S.E.; Lewis, R.P.; Leier, C.V.; Weissler, A.M. Changes in diastolic time with various pharmacologic agents: Implication for myocardial perfusion. Circulation 1979, 60, 164–169. [Google Scholar] [CrossRef]
- Shechter, G.; Resar, J.R.; McVeigh, E.R. Displacement and velocity of the coronary arteries: Cardiac and respiratory motion. IEEE Trans. Med. Imaging 2006, 25, 369–375. [Google Scholar] [CrossRef]
- Vembar, M.; Garcia, M.J.; Heuscher, D.J.; Haberl, R.; Matthews, D.; Böhme, G.E.; Greenberg, N.L. A dynamic approach to identifying desired physiological phases for cardiac imaging using multislice spiral CT. Med. Phys. 2003, 30, 1683–1693. [Google Scholar] [CrossRef]
- Caiati, C.; Aragona, P.; Iliceto, S.; Rizzon, P. Improved Doppler detection of proximal left anterior descending coronary artery stenosis after intravenous injection of a lung-crossing contrast agent: A transesophageal Doppler echocardiographic study. J. Am. Coll. Cardiol. 1996, 27, 1413–1421. [Google Scholar] [CrossRef] [PubMed]
- Isaaz, K.; da Costa, A.; de Pasquale, J.P.; Cerisier, A.; Lamaud, M. Use of the continuity equation for transesophageal Doppler assessment of severity of proximal left coronary artery stenosis: A quantitative coronary angiography validation study. J. Am. Coll. Cardiol. 1998, 32, 42–48. [Google Scholar] [CrossRef]
- Campbell, J.; King Iii, S.B.; Douglas, J.S., Jr.; Bradford, J.M. Prevalence and distribution of disease in patients catheterized for suspected coronary artery disease. In Coronary Arteriography and Angioplasty; King, S.B., III, Douglas, J.S., Jr., Eds.; McGraw-Hill Book Company: New York, NY, USA, 1985; p. 359. [Google Scholar]
- Meimoun, P.; Tribouilloy, C. Non-invasive assessment of coronary flow and coronary flow reserve by transthoracic Doppler echocardiography: A magic tool for the real world. Eur. J. Echocardiogr. 2008, 9, 449–457. [Google Scholar] [CrossRef]
- Caiati, C.; Zedda, N.; Montaldo, C.; Montisci, R.; Iliceto, S. Contrast-enhanced transthoracic second harmonic echo Doppler with adenosine: A noninvasive, rapid and effective method for coronary flow reserve assessment. J. Am. Coll. Cardiol. 1999, 34, 122–130. [Google Scholar] [CrossRef]
- McAlpine, W.A. Heart and Coronary Arteries; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1975; pp. 27–56. [Google Scholar]
- Caiati, C.; Lepera, M.; Pollice, P.; Favale, S. Non Invasive Detection of Accelerated Stenotic Flow in The Entire Left Anterior Descending Coronary Artery Provides Insight into the Causes of Impaired Coronary Flow Reserve: A Study Conducted with Enhanced Transthoracic Convergent Color Doppler Echocardiography. J. Am. Coll. Cardiol. 2020, 75 (Suppl. S1), 1784. [Google Scholar]
- Iliceto, S.; Caiati, C.; Aragona, P.; Verde, R.; Schlief, R.; Rizzon, P. Improved Doppler signal intensity in coronary-arteries after intravenous peripheral injection of a lung-crossing contrast agent (SHU-508A). J. Am. Coll. Cardiol. 1994, 23, 184–190. [Google Scholar] [CrossRef]
- Campbell, M.J.; Gardner, M.J. Calculating confidence intervals for some non-parametric analyses. Br. Med. J. (Clin. Res. Ed.) 1988, 296, 1454–1456. [Google Scholar] [CrossRef]
- Iliceto, S.; Marangelli, V.; Memmola, C.; Rizzon, P. Transesophageal Doppler echocardiography evaluation of coronary blood flow velocity in baseline conditions and during dipyridamole-induced coronary vasodilation. Circulation 1991, 83, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Caiati, C.; Montaldo, C.; Zedda, N.; Montisci, R.; Ruscazio, V.; Lai, G.; Cadeddu, M.; Meloni, L.; Iliceto, S. Validation of a new noninvasive, method (contrast-enhanced transthoracic second harmonic echo Doppler) for the evaluation of coronary flow reserve—Comparison with intracoronary Doppler flow wire. J. Am. Coll. Cardiol. 1999, 34, 1193–1200. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Yoshida, K.; Ogata, Y.; Akasaka, T.; Asami, Y.; Takagi, T.; Morioka, S. Noninvasive assessment of significant left anterior descending coronary artery stenosis by coronary flow velocity reserve with transthoracic color Doppler echocardiography. Circulation 1998, 97, 1557–1562. [Google Scholar] [CrossRef]
- Pizzuto, F.; Voci, P.; Mariano, E.; Puddu, P.E.; Sardella, G.; Nigri, A. Assessment of flow velocity reserve by transthoracic Doppler echocardiography and venous adenosine infusion before and after left anterior descending coronary artery stenting. J. Am. Coll. Cardiol. 2001, 38, 155–162. [Google Scholar] [CrossRef]
- Vegsundvåg, J.; Holte, E.; Wiseth, R.; Hegbom, K.; Hole, T. Coronary flow velocity reserve in the three main coronary arteries assessed with transthoracic Doppler: A comparative study with quantitative coronary angiography. J. Am. Soc. Echocardiogr. 2011, 24, 758–767. [Google Scholar] [CrossRef]
- Caiati, C.; Desario, P.; Tricarico, G.; Iacovelli, F.; Pollice, P.; Favale, S.; Lepera, M.E. Wellens’ Syndrome from COVID-19 Infection Assessed by Enhanced Transthoracic Coronary Echo Doppler: A Case Report. Diagnostics 2022, 12, 804. [Google Scholar] [CrossRef]
- Ellis, K.; Ziada, K.M.; Vivekananthan, D.; Latif, A.A.; Shaaraoui, M.; Martin, D.; Grimm, R.A. Transthoracic echocardiographic predictors of left atrial appendage thrombus. Am. J. Cardiol. 2006, 97, 421–425. [Google Scholar] [CrossRef]
- Lambertz, H.; Tries, H.P.; Stein, T.; Lethen, H. Noninvasive assessment of coronary flow reserve with transthoracic signal-enhanced Doppler echocardiography. J. Am. Soc. Echocardiogr. 1999, 12, 186–195. [Google Scholar] [CrossRef]
- Auriti, A.; Cianfrocca, C.; Pristipino, C.; Greco, S.; Galeazzi, M.; Guido, V.; Santini, M. Improving feasibility of posterior descending coronary artery flow recording by transthoracic Doppler echocardiography. Eur. J. Echocardiogr. 2003, 4, 214–220. [Google Scholar] [CrossRef]
- Gould, K.L.; Johnson, N.P. Coronary Physiology Beyond Coronary Flow Reserve in Microvascular Angina: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 2642–2662. [Google Scholar] [CrossRef]
- Caiati, C.; Siena, P.; Iacovelli, F.; Piscitelli, L.; Pollice, P.; Favale, S.; Lepera, M.E. Assessing Diffuse Coronary Atherosclerosis in Subjects with Impaired Coronary Flow Reserve but no Angiographic Critical Stenosis. A Transthoracic Enhanced Color Doppler Echocardiographyc Study. J. Am. Coll. Cardiol. 2021, 77 (Suppl. S1), 1429. [Google Scholar] [CrossRef]
- Fujimoto, K.; Watanabe, H.; Hozumi, T.; Otsuka, R.; Hirata, K.; Yamagishi, H.; Yoshiyama, M.; Yoshikawa, J. New noninvasive diagnosis of myocardial ischemia of the left circumflex coronary artery using coronary flow reserve measurement by transthoracic Doppler echocardiography: Comparison with thallium-201 single photon emission computed tomography. J. Cardiol. 2004, 43, 109–116. [Google Scholar]
- Auriti, A.; Pristipino, C.; Cianfrocca, C.; Granatelli, A.; Guido, V.; Pelliccia, F.; Greco, S.; Richichi, G.; Santini, M. Distal left circumflex coronary artery flow reserve recorded by transthoracic Doppler echocardiography: A comparison with Doppler-wire. Cardiovasc. Ultrasound 2007, 5, 22. [Google Scholar] [CrossRef]
- Murata, E.; Hozumi, T.; Matsumura, Y.; Fujimoto, K.; Sugioka, K.; Takemoto, Y.; Watanabe, H.; Yamagishi, H.; Yoshiyama, M.; Iwao, H.; et al. Coronary flow velocity reserve measurement in three major coronary arteries using transthoracic Doppler echocardiography. Echocardiography 2006, 23, 279–286. [Google Scholar] [CrossRef]
- Primitivo, S.; Santoro, D.; Marzullo, M.; Piscopo, A.; Pollice, P.; Rizzo, C.; De Santis, D.; Lepera, M.; Quagliara, D.; Masi, F.; et al. Non-invasive assessment of coronary flow reserve in distal left circumflex coronary artery in patients with angiographically normal coronary tree. G. Ital. Cardiol. 2011, 12 (Suppl. S3), e157. [Google Scholar]
- Qiu, S.; Shi, S.; Ping, H.; Zhou, S.; Wang, H.; Yang, B. Efficacy of Ivabradine versus beta-Blockers for Heart Rate Reduction during Computed Tomography Coronary Angiography: A Meta-Analysis of Randomized Controlled Trials. Cardiology 2016, 135, 133–140. [Google Scholar] [CrossRef]

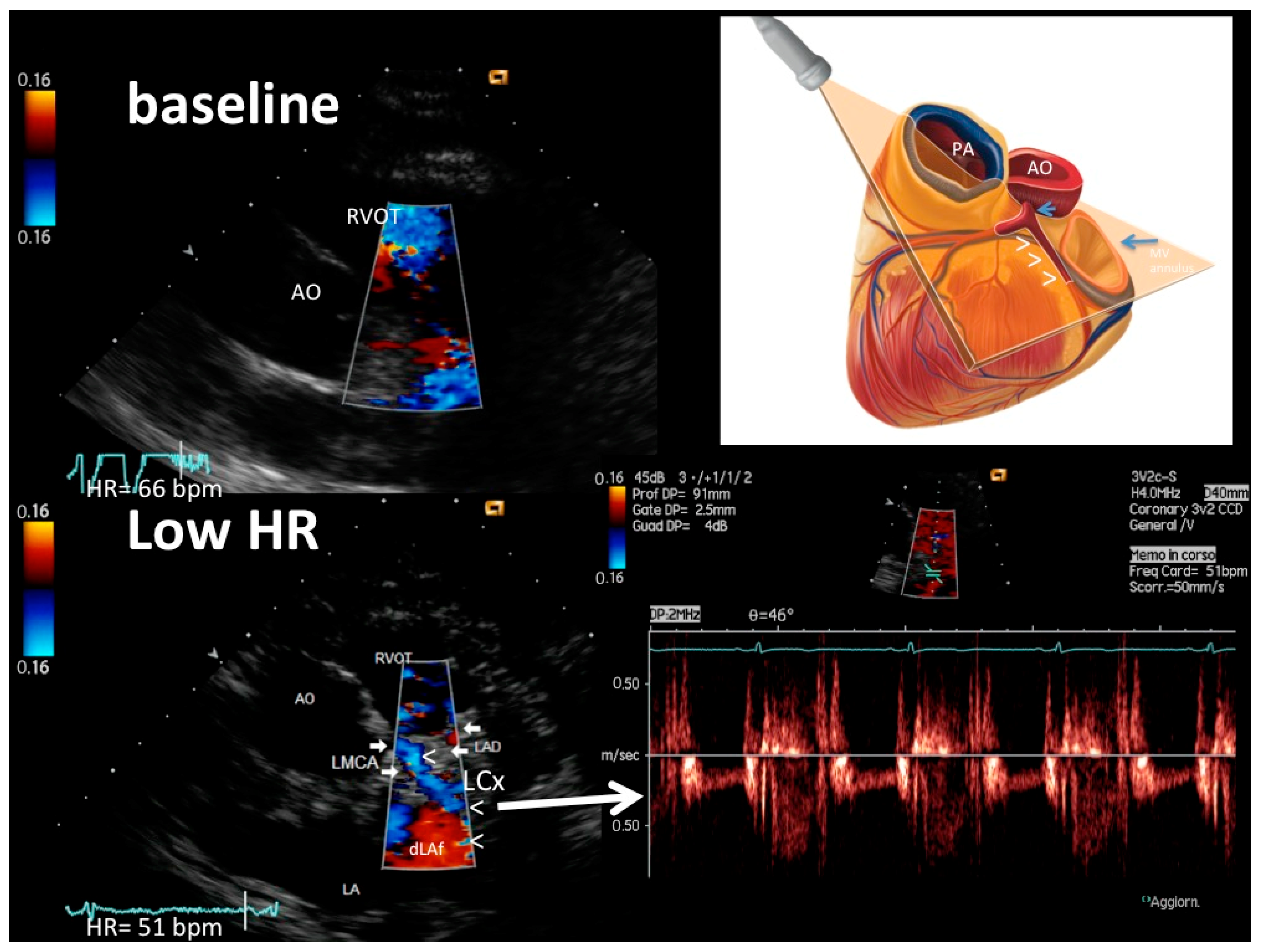


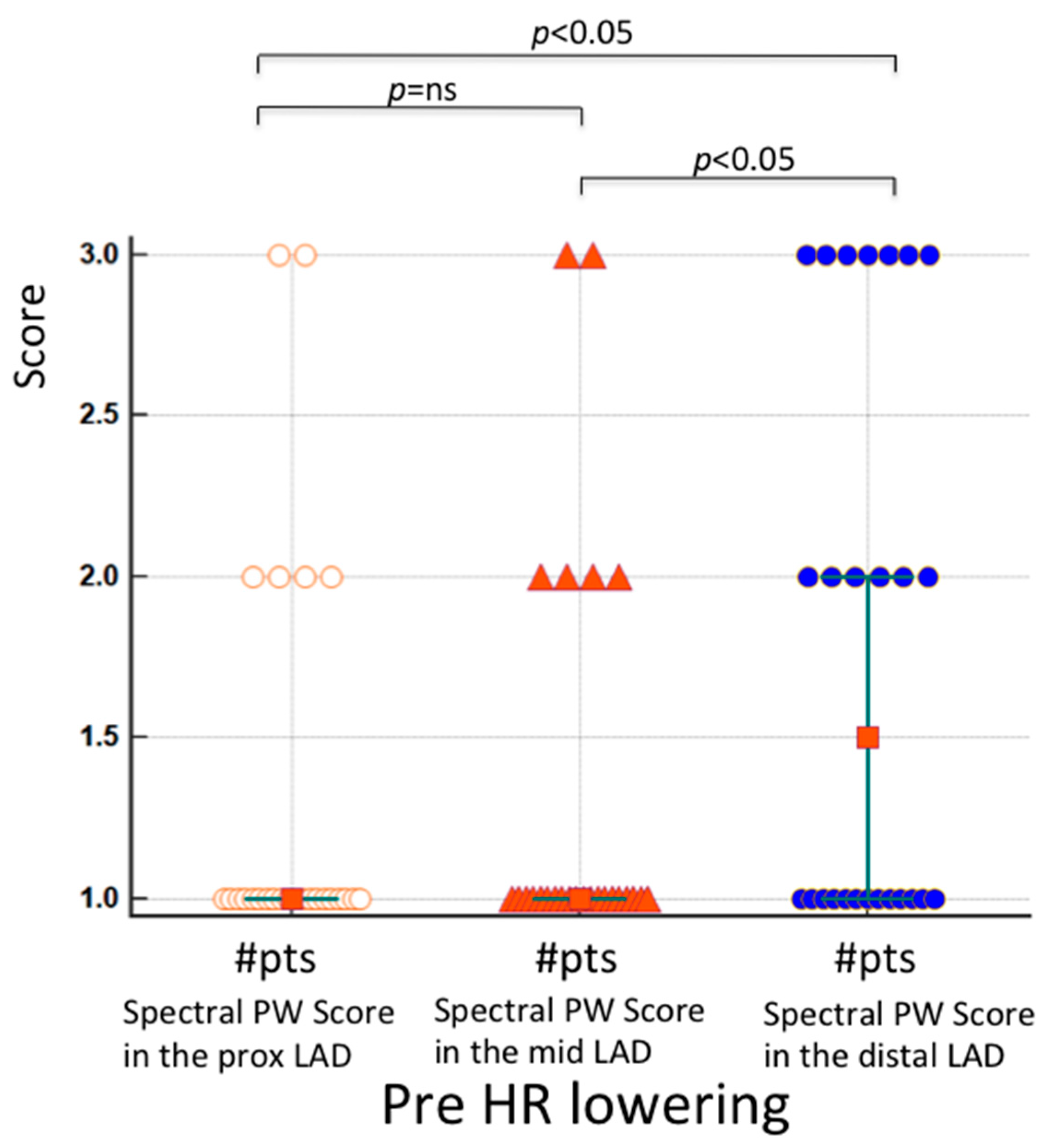
| Age, years (years) | 62 ± 14 |
| Gender | |
| Males, #patients (%) | 17 (65%) |
| Females, #patients (%) | 9 (35%) |
| BMI | 26 ± 4 |
| Diabetes, #patients (%) | 4 (15%) |
| Hypertension, #patients (%) | 18 (69%) |
| Typical Angina, #patients (%) | 3 (12%) |
| Atypical Angina, #patients (%) | 6 (24%) |
| Non-Anginal chest pain, #patients (%) | 1 (4%) |
| Hystory of CAD, #patients (%) | 18 (69%) |
| Previous PTCA, #patients (%) | 2 (8%) |
| Previous myocardial infarction, #patients (%) | 7 (27%) |
| Blood work | |
| Glycemia (mg/dL) | 110 ± 43 |
| Total cholesterol (mg/dL) | 190 ± 45 |
| HDL cholesterol (mg/dL) | 46 ± 14 |
| LDL cholesterol (mg/dL) | 116 ± 46 |
| Triglycerides (mg/dL) | 141 ± 80 |
| Echocardiographic data | |
| LVEDd (mm) | 51 ± 5 |
| LVESd (mm) | 33 ± 6 |
| LVEF, % | 58 ± 12 |
| Color Doppler | PW Doppler | CF Length | |||||
|---|---|---|---|---|---|---|---|
| Score 1 | Score 2 | Score 3 | Score 1 | Score 2 | Score 3 | mm | |
| # (%) | # (%) | # (%) | # (%) | # (%) | # (%) | ||
| LMC b, 26 seg | 24 (92) | 1 (4) | 1 (4) | 24 (92) | 1 (4) | 1 (4) | - |
| LMC a, 26 seg | 0 (0) | 12 (46) | 14 (54) * | 0 (0) | 15 (58) | 10 (38) * | - |
| LAD prx, b 26 seg | 20 (77) | 6 (23) | 0 (0) | 20 (77) | 4 (15) | 2 (8) | - |
| LAD prx, a 26 seg | 0 (0) | 0 (0) | 26 (100) * | 0 (0) | 2 (8) | 24 (92) * | - |
| LAD mid, b 26 seg | 21(81) | 3(11) | 2 (8) | 22 (85) | 4(15) | 0 | |
| LAD mid, a 26 seg | 0 (0) | 3 (11) | 23 (88) * | 0 | 2 (8) | 24 (92) * | - |
| LAD dst, b 26 seg | 14 (54) | 9 (35) | 3 (11) | 13 (50) | 6 (23) | 7 (27) | - |
| LAD dst, a 26 seg | 1 (4) | 1 (4) | 24 (92) * | 1 (4) | 2 (8) | 23 (88) * | - |
| LCx b, 26 seg | 26 (100) | 0 | 0 | 26 (100) | 0 | 0 | 0 |
| LCx a, 26 seg | 0 | 9 (35) | 17 (65) * | 0 | 6 (23) | 20 (77) * | 23 (9.07) ** |
| OM b, 26 seg | 23 (88) | 3 (11) | 0 | 24 (92) | 1 (4) | 1 (4) | 0 |
| OM a, 26 seg | 1 (4) | 12 (46) | 13 (50) * | 1 (4) | 15 (58) | 10 (38) * | 25 (12) ** |
| Coronary Angiography | E-Doppler TTE | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| # of Patients | Prox LAD (% st) | Mid-LAD (% st) | Distal LAD (% st) | Before HR Lowering | After HR Lowering | ||||
| LAD CF Length (mm) | AsF in the LAD (%) | LAD CF Length (mm) | AsF Prox LAD (%) | Asf Mid-LAD (%) | AsF Dis LAD (%) | ||||
| 1 | 0 | 50 | 0 | 13.00 | 0 | 79.00 | 104.55 | 34.38 | 0.00 |
| 2 | 0 | 0 | 0 | 0.00 | 0 | 79.00 | 0.00 | 0.00 | 0.00 |
| 3 | 0 | 0 | 0 | 44.00 | 0 | 70.00 | 0.00 | 0.00 | 0.00 |
| 4 | 0 | 0 | 0 | 0.00 | 0 | 101.00 | 0.00 | 0.00 | 0.00 |
| 5 | 50 | 0 | 0 | 0.00 | 0 | 77.00 | 168.1 | 0.00 | 0.00 |
| 6 | 0 | 0 | 0 | 35.00 | 0 | 81.00 | 0.00 | 0.00 | 0.00 |
| 7 | 40 | 0 | 0 | 9.00 | 0 | 92.00 | 37.14 | 0.00 | 0.00 |
| 8 | 70 | 75 | 0 | 9.00 | 0 | 114.00 | 164.00 | 83.33 | 7.32 |
| 9 | 80 | 0 | 70 | 0.00 | 0 | 72.00 | 971.43 | 0.00 | 0.00 |
| 10 | 0 | 0 | 20 | 18.00 | 0 | 71.00 | 0.00 | 0.00 | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caiati, C.; Pollice, P.; Lepera, M.E. Heart Rate Lowering Significantly Increases Feasibility in Doppler Recording Blood Flow Velocity in Coronaries during Transthoracic Doppler Echocardiography. Diagnostics 2023, 13, 670. https://doi.org/10.3390/diagnostics13040670
Caiati C, Pollice P, Lepera ME. Heart Rate Lowering Significantly Increases Feasibility in Doppler Recording Blood Flow Velocity in Coronaries during Transthoracic Doppler Echocardiography. Diagnostics. 2023; 13(4):670. https://doi.org/10.3390/diagnostics13040670
Chicago/Turabian StyleCaiati, Carlo, Paolo Pollice, and Mario Erminio Lepera. 2023. "Heart Rate Lowering Significantly Increases Feasibility in Doppler Recording Blood Flow Velocity in Coronaries during Transthoracic Doppler Echocardiography" Diagnostics 13, no. 4: 670. https://doi.org/10.3390/diagnostics13040670
APA StyleCaiati, C., Pollice, P., & Lepera, M. E. (2023). Heart Rate Lowering Significantly Increases Feasibility in Doppler Recording Blood Flow Velocity in Coronaries during Transthoracic Doppler Echocardiography. Diagnostics, 13(4), 670. https://doi.org/10.3390/diagnostics13040670






