The Role of Color Doppler Imaging in the Diagnosis of Glaucoma: A Review of the Literature
Abstract
1. Introduction
2. Anatomical Aspects
3. Ocular Perfusion Pressure and Blood Flow Regulation
4. Pathophysiology of Glaucoma
5. Vascular Theory of Glaucoma
6. Ocular Blood Flow Measurement
7. Color Doppler Imaging
7.1. CDI Principles
7.2. Doppler Waveform
7.3. Advantages and Limitations
7.4. Safety of CDI
8. CDI and Glaucoma
8.1. Primary Open-Angle Glaucoma
| Study | Objectives | Sample | Results | Conclusions |
|---|---|---|---|---|
| Meng et al., 2013 [10] | Diagnostic value of CDI in measuring intraocular blood flow in patients with POAG. | 23 studies—approx. 2000 eyes (POAG/control groups) | • ↓PSV and ↓EDV in OA, CRA, SPCA • ↑RI in OA, CRA, SPCA | CDI may be used in the diagnosis of POAG patients. |
| Xu et al., 2015 [11] | Possible diagnostic value of CDI in the assessment of hemodynamic changes in patients with NTG. | 23 studies—approx. 1700 eyes (NTG/control groups) | • ↓PSV and ↓EDV in OA, CRA, SPCA • ↑RI in OA and TSPCA | Ischemia is one of the major effects of NTG. Vascular parameters measured by CDI may be used as criteria in the diagnosis of NTG. |
| Abegão Pinto et al., 2016 [36] | Identify which of the vascular data can be helpful in the clinical practice for screening and disease stratification. | 546 patients: 214 POAG, 192 NTG, 140 control groups | Glaucoma patients showed: • ↓intraocular velocities using CDI • higher venous saturation • asymmetries of choroid thickness | The database aims to introduce vascular parameters into daily clinical practice for the diagnosis of glaucoma. |
| Kurysheva et al., 2017 [40] | Diagnostic value of OBF and choroid thickness parameters in the detection of early glaucoma. | 62 eyes: 32 glaucoma, 30 control groups. | • ↓EDV in CRA, SPCA • ↓mean flow in CRA | Hemodynamic parameters, by measuring velocities with CDI, show better reliability for the diagnosis of early glaucoma, compared to the structural parameters. |
| Eniola et al., 2018 [50] | Comparison of CDI data of CRA and OA in young patients with POAG with normal population. | 200 eyes: 100 with POAG 100 control groups. | • ↓PSV and ↓EDV in OA, CRA • ↑RI in OA and CRA | Patients with POAG show reduced velocities and elevated RI of the intraocular vessels compared to the normal population. |
| Kalayci et al., 2020 [51] | Analyze flow parameters of OA, CRA and ICA using CDI. | 145 patients: 35 POAG, 65 NTG and 45 control groups | • ↑RI, PI, PR in OA • ↑RI in CRA | OA PR (peak ratio) and ICA intima-media thickness may be used as diagnostic tools. |
| Krzyżanowska-Berkowska et al., 2021 [41] | To evaluate association between OBF biomarkers and lamina cribrosa parameters. | 211 patients: 70 POAG, 72 glaucoma suspects, 69 control groups. | • ↓PSV in OA, CRA • ↓MFV in OA, CRA | Impaired OBF was found to be associated with deformed lamina cribrosa in glaucoma patients. |
8.2. NTG versus POAG
| EDV CRA | PSV NSPCA | ||||
|---|---|---|---|---|---|
| Reference | POAG/NTG | POAG | NTG | POAG | NTG |
| [36] | 214/192 | 2.66 ± 0.9 ** | 2.71 ± 0.98 ** | 9.39 ± 3.1 | 9.35 ± 3.3 |
| [42] | 17/28 | 2.4 (0.4) | 2.9 (0.9) | 10.3 (2.0) | 10.1 (3.5) |
| [43] | 74/63 | 2.63 ± 0.8 * | 2.88 ± 0.9 * | 9.17 ± 2.8 | 9.52 ± 3.0 |
| [44] | 86/69 | 2.70 (0.9) | 2.96 (1.1) | 9.51 (2.9) | 9.51 (2.8) |
| [46] | 88/58 | 2.72 ± 0.8 | 2.92 ± 1.0 | 9.68 ± 2.9 | 9.22 ± 2.7 |
| [47] | 102/89 | 2.75 ± 0.95 | 2.87 ± 1.08 | 9.44 ± 2.70 * | 8.58 ± 2.69 * |
| [53] | 49/62 | 2.7 (0.98) | 2.8 (0.98) | 8.9 (2.3) * | 8.2 (2.6) * |
8.3. Pseudoexfoliation Glaucoma (PXG)
8.4. Angle-Closure Glaucoma
9. Waveform Analysis
10. CDI as a Tool to Monitor Progression
11. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weinreb, R.N.; Khaw, P.T. Primary open-angle glaucoma. Lancet 2004, 363, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Aung, T.; Bourne, R.R.; Bron, A.M.; Ritch, R.; Panda-Jonas, S. Glaucoma. Lancet 2017, 390, 2183–2193. [Google Scholar] [CrossRef]
- Tham, Y.C.; Li, X.; Wong, T.Y.; Quigley, H.A.; Aung, T.; Cheng, C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 2014, 121, 2081–2090. [Google Scholar] [CrossRef]
- Zhang, N.; Wang, J.; Li, Y.; Jiang, B. Prevalence of primary open angle glaucoma in the last 20 years: A meta-analysis and systematic review. Sci. Rep. 2021, 11, 13762. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Park, K.H. Update on the prevalence, etiology, diagnosis, and monitoring of normal-tension glaucoma. Asia Pac. J. Ophthalmol. 2016, 5, 23–31. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, S.K.; Agarwal, P.; Saxena, R.; Agarwal, S.S. Current concepts in the pathophysiology of glaucoma. Indian J. Ophthalmol. 2009, 57, 257–266. [Google Scholar] [CrossRef]
- Weinreb, R.N.; Aung, T.; Medeiros, F.A. The pathophysiology and treatment of glaucoma: A review. JAMA 2014, 311, 1901–1911. [Google Scholar] [CrossRef]
- Yanagi, M.; Kawasaki, R.; Wang, J.J.; Wong, T.Y.; Crowston, J.; Kiuchi, Y. Vascular risk factors in glaucoma: A review. Clin. Exp. Ophthalmol. 2011, 39, 252–258. [Google Scholar] [CrossRef]
- Harris, A.; Guidoboni, G.; Siesky, B.; Mathew, S.; Verticchio Vercellin, A.C.; Rowe, L.; Arciero, J. Ocular blood flow as a clinical observation: Value, limitations and data analysis. Prog. Retin. Eye Res. 2020, 78, 100841, Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Zhang, P.; Huang, H.; Ma, J.; Zhang, Y.; Li, H.; Qu, Y. Color Doppler imaging analysis of retrobulbar blood flow velocities in primary open-angle glaucomatous eyes: A meta-analysis. PLoS ONE 2013, 8, e62723. [Google Scholar] [CrossRef]
- Xu, S.; Huang, S.; Lin, Z.; Liu, W.; Zhong, Y. Color Doppler imaging analysis of ocular blood flow velocities in normal tension glaucoma patients: A meta-analysis. J. Ophthalmol. 2015, 2015, 919610. [Google Scholar] [CrossRef]
- Barbosa-Breda, J.; Van Keer, K.; Abegão-Pinto, L.; Nassiri, V.; Molenberghs, G.; Willekens, K.; Vandewalle, E.; Rocha-Sousa, A.; Stalmans, I. Improved discrimination between normal-tension and primary open-angle glaucoma with advanced vascular examinations–the Leuven Eye Study. Acta Ophthalmol. 2019, 97, 50–56. [Google Scholar] [CrossRef]
- Flammer, J.; Orgül, S.; Costa, V.P.; Orzalesi, N.; Krieglstein, G.K.; Serra, L.M.; Renard, J.P.; Stefánsson, E. The impact of ocular blood flow in glaucoma. Prog. Retin. Eye Res. 2002, 21, 359–393. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, P.J.; Cioffi, G.A. Vascular anatomy of the optic nerve head. Can. J. Ophthalmol. 2008, 43, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.P.; Harris, A.; Anderson, D.; Stodtmeister, D.; Cremasco, F.; Kergoat, H.; Lovasik, J.; Stalmans, I.; Zeitz, O.; Lanzl, I.; et al. Ocular perfusion pressure in glaucoma. Acta Ophthalmol. 2014, 92, 252–266. [Google Scholar] [CrossRef]
- Ahmad, S.S. Controversies in the vascular theory of glaucomatous optic nerve degeneration. Taiwan J. Ophthalmol. 2016, 6, 182–186. [Google Scholar] [CrossRef]
- Mursch-Edlmayr, A.S.; Bolz, M.; Strohmaier, C. Vascular Aspects in Glaucoma: From Pathogenesis to Therapeutic Approaches. Int. J. Mol. Sci. 2021, 22, 4662. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.; Harris, A.; Wudunn, D.; Kheradiya, N.; Siesky, B. Dysfunctional regulation of ocular blood flow: A risk factor for glaucoma? Clin. Ophthalmol. 2008, 2, 849–861. [Google Scholar] [CrossRef]
- Luo, X.; Shen, Y.M.; Jiang, M.N.; Lou, X.F.; Shen, Y. Ocular Blood Flow Autoregulation Mechanisms and Methods. J. Ophthalmol. 2015, 2015, 864871. [Google Scholar] [CrossRef]
- Fan, N.; Wang, P.; Tang, L.; Liu, X. Ocular Blood Flow and Normal Tension Glaucoma. Biomed Res. Int. 2015, 2015, 308505. [Google Scholar] [CrossRef]
- Kim, K.E.; Oh, S.; Baek, S.U.; Ahn, S.J.; Park, K.H.; Jeoung, J.W. Ocular Perfusion Pressure and the Risk of Open-Angle Glaucoma: Systematic Review and Meta-analysis. Sci. Rep. 2020, 10, 10056. [Google Scholar] [CrossRef]
- Flammer, J.; Konieczka, K.; Flammer, A.J. The Role of Ocular Blood Flow in the Pathogenesis of Glaucomatous Damage. US Ophthalmic Rev. 2011, 4, 84–87. [Google Scholar] [CrossRef]
- Killer, H.E.; Pircher, A. Normal tension glaucoma: Review of current understanding and mechanisms of the pathogenesis. Eye 2018, 32, 924–930. [Google Scholar] [CrossRef] [PubMed]
- Trivli, A.; Koliarakis, I.; Terzidou, C.; Goulielmos, G.N.; Siganos, C.S.; Spandidos, D.A.; Dalianis, G.; Detorakis, E.T. Normal-tension glaucoma: Pathogenesis and genetics. Exp. Ther. Med. 2019, 17, 563–574. [Google Scholar] [CrossRef]
- Funk, R.O.; Hodge, D.O.; Kohli, D.; Roddy, G.W. Multiple Systemic Vascular Risk Factors Are Associated With Low-Tension Glaucoma. J. Glaucoma 2022, 31, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Hsu, M.Y.; Lin, C.H.; Lin, C.C.; Wang, C.Y.; Shen, Y.C.; Wang, I.J. Risk of developing open-angle glaucoma in patients with carotid artery stenosis: A nationwide cohort study. PLoS ONE 2018, 13, e0194533. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Liu, S.; Zhang, X.; Hu, Y.; Shang, X.; Zhu, Z.; Huang, Y.; Wu, G.; Xiao, Y.; Du, Z.; et al. Shared genetic architecture between the two neurodegenerative diseases: Alzheimer’s disease and glaucoma. Front. Aging Neurosci. 2022, 14, 880576. [Google Scholar] [CrossRef]
- Tian, T.; Liu, Y.H. Normal-tension glaucoma and Alzheimer’s disease: Retinal vessel signs as a possible common underlying risk factor. Med. Hypotheses 2011, 77, 466. [Google Scholar] [CrossRef]
- Mohindroo, C.; Ichhpujani, P.; Kumar, S. Current Imaging Modalities for assessing Ocular Blood Flow in Glaucoma. J. Curr. Glaucoma Pract. 2016, 10, 104–112. [Google Scholar] [CrossRef]
- Modrzejewska, M. Guidelines for ultrasound examination in ophthalmology. Part III: Color Doppler ultrasonography. J. Ultrason. 2019, 19, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Tranquart, F.; Bergès, O.; Koskas, P.; Arsene, S.; Rossazza, C.; Pisella, P.J.; Pourcelot, L. Color Doppler imaging of orbital vessels: Personal experience and literature review. J. Clin. Ultrasound 2003, 31, 258–273. [Google Scholar] [CrossRef]
- Stalmans, I.; Vandewalle, E.; Anderson, D.R.; Costa, V.P.; Frenkel, R.E.P.; Garhofer, G.; Grunwald, J.; Gugleta, K.; Harris, A.; Hudson, C.; et al. Use of colour Doppler imaging in ocular blood flow research. Acta Ophthalmol. 2011, 89, 609–630. [Google Scholar] [CrossRef]
- Revzin, M.V.; Imanzadeh, A.; Menias, C.; Pourjabbar, S.; Mustafa, A.; Nezami, N.; Spektor, M.; Pellerito, J.S. Optimizing Image Quality When Evaluating Blood Flow at Doppler US: A Tutorial. Radiographics 2019, 39, 1501–1523. [Google Scholar] [CrossRef]
- Abramowicz, J.S.; Adhikari, S.; Dickman, E.; Estroff, J.A.; Harris, G.R.; Nomura, J.; Silverman, R.H.; Taylor, L.A.; Barr, R.G. Ocular Ultrasound: Review of Bioeffects and Safety, Including Fetal and Point of Care Perspective. J. Ultrasound Med. 2022, 41, 1609–1622. [Google Scholar] [CrossRef] [PubMed]
- Rusia, D.; Harris, A.; Pernic, A.; Williamson, K.M.; Moss, A.M.; Shoshani, Y.Z.; Siesky, B. Feasibility of creating a normative database of colour doppler imaging parameters in glaucomatous eyes and controls. Br. J. Ophthalmol. 2011, 95, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Abegão Pinto, L.; Willekens, K.; Van Keer, K.; Shibesh, A.; Molenberghs, G.; Vandewalle, E.; Stalmans, I. Ocular blood flow in glaucoma—the Leuven Eye Study. Acta Ophthalmol. 2016, 94, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, R.; Harris, A.; Siesky, B.; Moss, A.; Ramanathan, M.; Pickett, M.; Wudunn, D.; Hawkes, M.; Shoshani, Y. Repeatability of retrobulbar blood flow velocity measured using color doppler imaging in the Indianapolis glaucoma progression study. J. Glaucoma 2011, 20, 540–547. [Google Scholar] [CrossRef]
- Founti, P.; Harris, A.; Papadopoulou, D.; Emmanouilidis, P.; Siesky, B.; Kilintzis, V.; Anastasopoulos, E.; Salonikiou, A.; Pappas, T.; Topouzis, F. Agreement among three examiners of colour Doppler imaging retrobulbar blood flow velocity measurements. Acta Ophthalmol. 2011, 89, 631–634. [Google Scholar] [CrossRef]
- Laurel, M.D. Medical Ultrasound Safety, 4th ed.; American Institute of Ultrasound in Medicine: West Laurel, MD, USA, 2020. [Google Scholar]
- Kurysheva, N.I.; Parshunina, O.A.; Shatalova, E.O.; Kiseleva, T.N.; Lagutin, M.B.; Fomin, A.V. Value of Structural and Hemodynamic Parameters for the Early Detection of Primary Open-Angle Glaucoma. Curr. Eye Res. 2017, 42, 411–417. [Google Scholar] [CrossRef]
- Krzyżanowska-Berkowska, P.; Czajor, K.; Iskander, D.R. Associating the biomarkers of ocular blood flow with lamina cribrosa parameters in normotensive glaucoma suspects. Comparison to glaucoma patients and healthy controls. PLoS ONE 2021, 16, 0248851. [Google Scholar] [CrossRef]
- Stalmans, I.; Harris, A.; Fieuws, S.; Zeyen, T.; Vanbellinghen, V.; McCranor, L.; Siesky, B. Color Doppler imaging and ocular pulse amplitude in glaucomatous and healthy eyes. Eur. J. Ophthalmol. 2009, 19, 580–587. [Google Scholar] [CrossRef]
- Abegão Pinto, L.; Vandewalle, E.; Willekens, K.; Marques-Neves, C.; Stalmans, I. Ocular pulse amplitude and Doppler waveform analysis in glaucoma patients. Acta Ophthalmol. 2014, 92, 280–285. [Google Scholar] [CrossRef]
- Abegão Pinto, L.; Vandewalle, E.; De Clerck, E.; Marques-Neves, C.; Stalmans, I. Lack of spontaneous venous pulsation: Possible risk indicator in normal tension glaucoma? Acta Ophthalmol. 2013, 91, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Véronique, P.; Daouk, J.; Bouzerar, R.; Jany, B.; Milazzo, S.; Balédent, O. Ocular blood flow and cerebrospinal fluid pressure in glaucoma. Acta Radiol. Open 2016, 5, 2058460115624275. [Google Scholar] [CrossRef]
- Willekens, K.; Abegão Pinto, L.; Vandewalle, E.; Stalmans, I. Higher optic nerve sheath diameters are associated with lower ocular blood flow velocities in glaucoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2014, 252, 477–483. [Google Scholar] [CrossRef]
- Abegão Pinto, L.; Vandewalle, E.; De Clerck, E.; Marques-Neves, C.; Stalmans, I. Ophthalmic artery Doppler waveform changes associated with increased damage in glaucoma patients. Invest. Ophthalmol. Vis. Sci. 2012, 53, 2448–2453. [Google Scholar] [CrossRef]
- Galambos, P.; Vafiadis, J.; Vilchez, S.E.; Wagenfeld, L.; Matthiessen, E.T.; Richard, G.; Klemm, M.; Zeitz, O. Compromised autoregulatory control of ocular hemodynamics in glaucoma patients after postural change. Ophthalmology 2006, 113, 1832–1836. [Google Scholar] [CrossRef] [PubMed]
- Garhöfer, G.; Fuchsjäger-Mayrl, G.; Vass, C.; Pemp, B.; Hommer, A.; Schmetterer, L. Retrobulbar blood flow velocities in open angle glaucoma and their association with mean arterial blood pressure. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6652–6657. [Google Scholar] [CrossRef]
- Eniola, M.A.; Adeyomoye, A.A.O.; Musa, K.O.; Ishola, A.A.S.; Olatunji, O.O. Ophthalmic artery and central retinal artery Doppler patterns in primary open angle glaucoma patients at the Lagos University teaching Hospital, Nigeria. J. West Afr. Coll. Surg. 2018, 8, 1–21. [Google Scholar] [PubMed]
- Kalayci, M.; Tahtabasi, M. Assessment of Doppler flow parameters of the retrobulbar arteries and internal carotid artery in patients with glaucoma: The significance of ophthalmic artery peak ratio and the intima-media thickness of the internal carotid artery. Int. Ophthalmol. 2020, 40, 3337–3348. [Google Scholar] [CrossRef]
- Fan, N.; Tan, J.; Liu, X. Is “normal tension glaucoma” glaucoma? Med. Hypotheses 2019, 133, 109405. [Google Scholar] [CrossRef] [PubMed]
- Abegão Pinto, L.; Vandewalle, E.; Stalmans, I. Disturbed correlation between arterial resistance and pulsatility in glaucoma patients. Acta Ophthalmol. 2012, 90, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Plateroti, P.; Plateroti, A.M.; Abdolrahimzadeh, S.; Scuderi, G. Pseudoexfoliation Syndrome and Pseudoexfoliation Glaucoma:A Review of the Literature with Updates on Surgical Management. J. Ophthalmol. 2015, 2015, 370371. [Google Scholar] [CrossRef]
- Martinez, A.; Sanchez, M. Retrobulbar hemodynamic parameters in pseudoexfoliation syndrome and pseudoexfoliative glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Yuksel, N.; Karabas, V.L.; Arslan, A.; Demirci, A.; Caglar, Y. Ocular hemodynamics in pseudoexfoliation syndrome and pseudoexfoliation glaucoma. Ophthalmology 2001, 108, 1043–1049. [Google Scholar] [CrossRef]
- Sekeroglu, M.A.; Irkec, M.; Mocan, M.C.; Ileri, E.; Dikmenoglu, N.; Seringec, N.; Karaosmanoglu, D.; Orhan, M. The association of ocular blood flow with haemorheological parameters in primary open-angle and exfoliative glaucoma. Acta Ophthalmol. 2011, 89, 429–434. [Google Scholar] [CrossRef]
- Kocaturk, T.; Isikligil, I.; Uz, B.; Dayanir, V.; Dayanir, Y.O. Ophthalmic artery blood flow parameters in pseudoexfoliation glaucoma. Eur. J. Ophthalmol. 2016, 26, 124–127. [Google Scholar] [CrossRef]
- Kocaturk, T.; Abdullayev, O.K.; Bekmez, S.; Polat, Y.D. Arterial hemodynamics and its correlation with retinal microarchitecture in pseudoexfoliation glaucoma. Arq. Bras. Oftalmol. 2022, 86, S0004-27492022005006211, Online ahead of print. [Google Scholar] [CrossRef]
- Yuksel, N.; Karabas, V.L.; Demirci, A.; Arslan, A.; Altintas, O.; Caglar, Y. Comparison of blood flow velocities of the extraocular vessels in patients with pseudoexfoliation or primary open-angle glaucoma. Ophthalmologica 2001, 215, 424–429. [Google Scholar] [CrossRef]
- Dogan, N.C.; Ozdemir, N.; Aikimbaev, K.; Ciloglu, E. Retrobulbar Short Posterior Ciliary Artery Hemodynamics in Patients with Pseudoexfoliation Glaucoma and Primary Open-Angle Glaucoma. J. Curr. Ophthalmol. 2022, 34, 25–29. [Google Scholar] [CrossRef]
- Martinez, A.; Sanchez, M. Ocular haemodynamics in pseudoexfoliative and primary open-angle glaucoma. Eye 2008, 22, 515–520. [Google Scholar] [CrossRef]
- Galassi, F.; Giambene, B.; Menchini, U. Ocular perfusion pressure and retrobulbar haemodynamics in pseudoexfoliative glaucoma. Graefes Arch. Clin. Exp. Ophthalmol. 2008, 246, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Marjanovic, I.; Milic, N.; Martinez, A.; Benitez-del-Castillo, J. Retrobulbar hemodynamic parameters in open-angle and angle-closure glaucoma patients. Eye 2012, 26, 523–528. [Google Scholar] [CrossRef]
- Cheng, C.Y.; Liu, C.J.; Chiou, H.J.; Chou, J.C.; Hsu, W.M.; Liu, J.H. Color Doppler imaging study of retrobulbar hemodynamics in chronic angle-closure glaucoma. Ophthalmology 2001, 108, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Nong, T.; Ninghua, F. Color Doppler imaging in the study of retrobulbar hemodynamic changes of primary angle-closure glaucoma. Yan Ke Xue Bao 1997, 13, 113–115. [Google Scholar]
- Carichino, L.; Harris, A.; Lapin, S.; Guidoboni, G.; Cassani, S.; De Silvestri, A.; Tinelli, C.; Milano, G.; Siesky, B.; Verticchio Vercellin, A.C. Waveform parameters of retrobulbar vessels in glaucoma patients with different demographics and disease severity. Eur. J. Ophthalmol. 2020, 30, 1019–1027. [Google Scholar] [CrossRef]
- Zegadlo, A.; Wierzbowska, J. Colour Doppler imaging of retrobulbar circulation in different severity of glaucoma optic neuropathy. Med. Ultrason. 2021, 23, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Ferreras, A.; Polo, V.; Güerri, N.; Seral, P.; Fuertes-Lazaro, I.; Pablo, L.E. Predictive value of retrobulbar blood flow velocities in glaucoma suspects. Invest Ophthalmol. Vis. Sci. 2012, 53, 3875–3884. [Google Scholar] [CrossRef]
- Martínez, A.; Sánchez, M. Predictive value of colour Doppler imaging in a prospective study of visual field progression in primary open-angle glaucoma. Acta Ophthalmol. Scand. 2005, 83, 716–722. [Google Scholar] [CrossRef]
- Magureanu, M.; Stanila, A.; Bunescu, L.V.; Armeanu, C. Color Doppler imaging of the retrobulbar circulation in progressive glaucoma optic neuropathy. Rom. J. Ophthalmol. 2016, 60, 237–248. [Google Scholar]
- Suprasanna, K.; Shetty, C.M.; Charudutt, S.; Kadavigere, R. Doppler evaluation of ocular vessels in patients with primary open angle glaucoma. J. Clin. Ultrasound. 2014, 42, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aragon, F.; Garcia-Martin, E.; Larrosa-Lopez, R.; Artigas-Martín, J.M.; Seral-Moral, P.; Pablo, L.E. Role of color Doppler imaging in early diagnosis and prediction of progression in glaucoma. BioMed Res. Int. 2013, 2013, 871689. [Google Scholar] [CrossRef] [PubMed]
- Zeitz, O.; Galambos, P.; Wagenfeld, L.; Wiermann, A.; Wlodarsch, P.; Praga, R.; Matthiessen, E.T.; Richard, G.; Klemm, M. Glaucoma progression is associated with decreased blood flow velocities in the short posterior ciliary artery. Br. J. Ophthalmol. 2006, 90, 1245–1248. [Google Scholar] [CrossRef] [PubMed]
- Galassi, F.; Sodi, A.; Ucci, F.; Renieri, G.; Pieri, B.; Baccini, M. Ocular hemodynamics and glaucoma prognosis. Arch. Ophthalmol. 2003, 121, 1711–1715. [Google Scholar] [CrossRef] [PubMed]

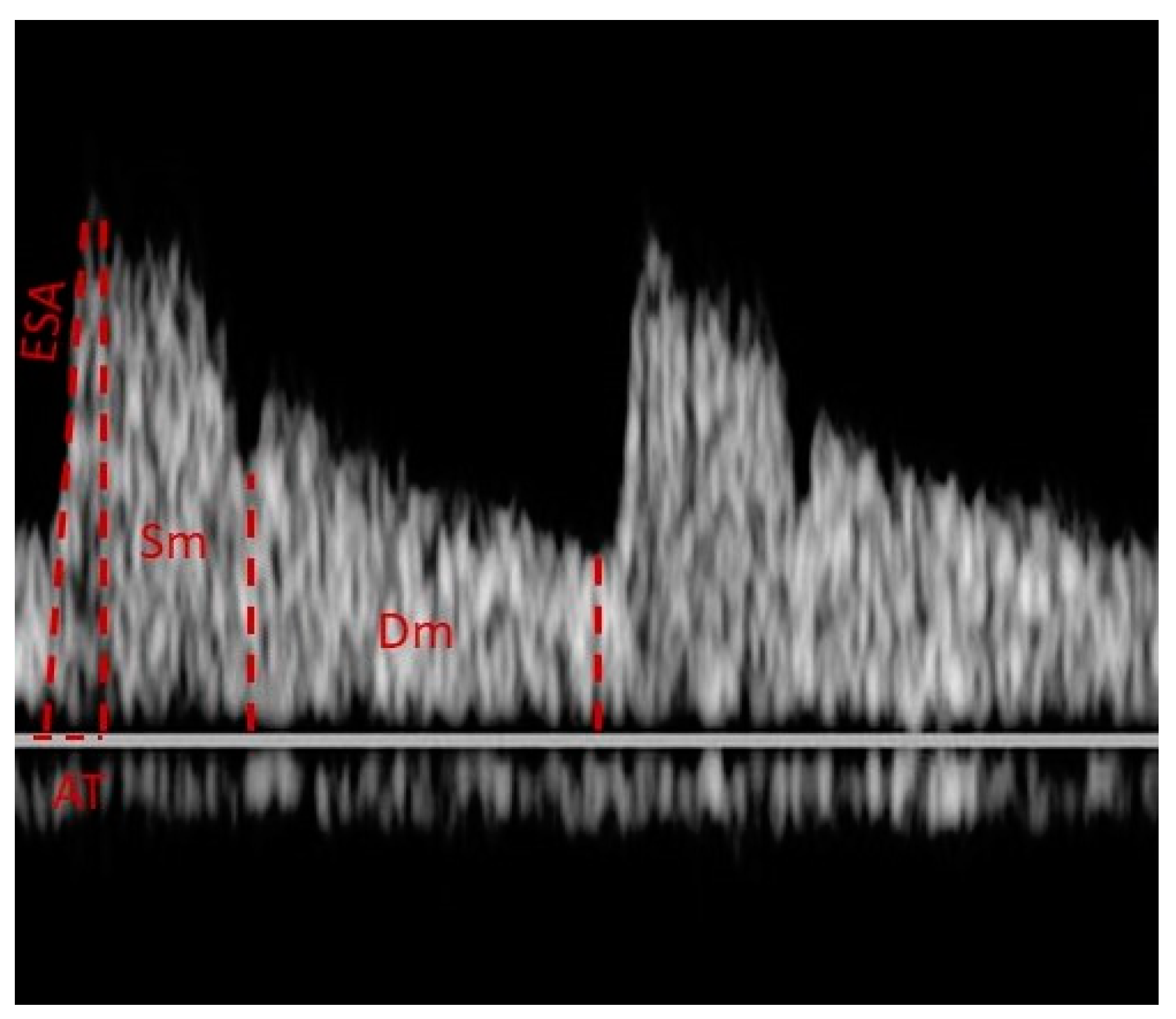
| PSV | Peak Systolic Velocity |
| EDV | End Diastolic Velocity |
| RI | Resistive Index, RI = (PSV − EDV)/PSV. |
| MFV | Mean Flow Velocity |
| PI | Pulsatility Index, PI = (PSV-EDV)/mean velocity |
| ESA | Early Systolic Acceleration |
| AT | Acceleration Time |
| Sm | Mean velocity of the systolic wave components |
| Dm | Mean velocity of the diastolic wave components |
| Sm/Dm | The ratio of average velocities |
| Vmax | Maximum venous velocity |
| Vmin | Minimum venous velocity |
| PXG vs. Control Groups | PXG vs. POAG | |
|---|---|---|
| Kocaturk et al., 2021 [59] | • ↑RI in OA, CRA | - |
| Dogan et al., 2021 [61] | • ↓PSV, ↓EDV in TSPCAs | No statistical differences |
| Kocaturk et al., 2016 [58] | • ↓PSV, ↓EDV, ↑RI in OA | - |
| Sekeroglu et al., 2011 [57] | • ↓PSV, ↓EDV, ↑RI (all vessels) | - |
| Martinez et al., 2008 [55] | • ↓EDV, ↑RI (all vessels) | - |
| Martinez et al., 2008 (Eye) [62] | - | •↓PSV, ↓EDV, ↑RI in POAG (vs. PXG) |
| Galassi et al., 2008 [63] | - | •↓PSV, ↓EDV, ↑RI In PXG (vs. POAG) |
| Yuksel et al., 2001 [56] | • ↓PSV, ↓EDV, ↑RI (all vessels) | - |
| Yuksel et al., 2001 [60] | • ↓PSV, ↓EDV, ↑RI (all vessels) | No statistical differences |
| Study | Sample | Results | Conclusions |
|---|---|---|---|
| Zegadlo et al., 2021 [68] | 89 eyes studied: • 31 preperimetric • 29 early glaucoma • 12 moderate glaucoma • 17 advanced glaucoma | • ↓PSV OA and CRA in advanced than in preperimetric glaucoma • ↑RI CRA in advanced/moderate than in preperimetric glaucoma | CDI may be used as a diagnostic tool for the control or treatment of patients at increased risk of a more aggressive optic neuropathy |
| Kalayci et al., 2020 [51] | 145 patients: • 35 POAG • 65 NTG • 45 control groups | • ↑RI, PI, PR in OA • ↑RI in CRA | OBF measurements may be used to determine the severity of the damage and monitor the progression of the disease |
| Magureanu et al., 2016 [71] | • 102 patients -202 eyes- with a confirmed diagnosis of GON. | • ↓PSV in CRA was relevant in glaucoma progression | CDI would represent an important diagnosis method, whose results could help adopt more or less aggressive therapeutic measures in conflicted cases |
| Suprasanna et al., 2014 [72] | • 78 eyes with established POAG -25 with progressive visual field loss and 53 with stable visual field- • 78 control eyes | • ↓EDV in OA and • ↑RI in OA and PCAs in glaucomatous eyes with progressive than with stable visual field loss | OBF appears compromised in eyes with POAG, particularly in those with progressive visual field loss |
| Jimenez-Aragon F. et al., 2013 [73] | • 71 patients categorized as “Progression” or “No Progression” (5-year follow-up) | Progression group presented: • ↓EDV in OA, CRA • ↑RI in OA, CRA compared to the “No Progression” group | Orbital hemodynamics studied by CDI may represent an important biomarker to discriminate glaucoma patients with higher risk for progression |
| Calvo et al., 2012 [69] | • 262 glaucoma suspects (48-month follow-up) | • ↑RI > 0.75 in OA was associated with the development of glaucoma | Abnormal OBF velocities measured by CDI may be a risk factor for conversion to glaucoma |
| Zeitz et al., 2006 [74] | • 114 patients with glaucoma • 40 healthy volunteers | • ↓PSV in CRA • ↓PSV and ↓EDV in SPCAs in patients with progressive glaucoma | Progressive glaucoma is associated with decreased blood flow velocities |
| Martínez et al., 2005 [70] | • 49 POAG patients (36-month follow-up) | • ↑RI in OA, SPCAs in the eyes that progressed (23 out of 36) | In eyes with POAG and elevated IOP, the RIs of the OA or SPCAs may reliably predict visual field progression |
| Galassi et al., 2003 [75] | • 44 POAG patients (7-year follow-up) | • ↑RI in OA in patients with visual field loss | CDI variables of OA correlate with the risk of visual field deterioration in patients with POAG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banou, L.; Dastiridou, A.; Giannoukas, A.; Kouvelos, G.; Baros, C.; Androudi, S. The Role of Color Doppler Imaging in the Diagnosis of Glaucoma: A Review of the Literature. Diagnostics 2023, 13, 588. https://doi.org/10.3390/diagnostics13040588
Banou L, Dastiridou A, Giannoukas A, Kouvelos G, Baros C, Androudi S. The Role of Color Doppler Imaging in the Diagnosis of Glaucoma: A Review of the Literature. Diagnostics. 2023; 13(4):588. https://doi.org/10.3390/diagnostics13040588
Chicago/Turabian StyleBanou, Lamprini, Anna Dastiridou, Athanasios Giannoukas, Georgios Kouvelos, Christos Baros, and Sofia Androudi. 2023. "The Role of Color Doppler Imaging in the Diagnosis of Glaucoma: A Review of the Literature" Diagnostics 13, no. 4: 588. https://doi.org/10.3390/diagnostics13040588
APA StyleBanou, L., Dastiridou, A., Giannoukas, A., Kouvelos, G., Baros, C., & Androudi, S. (2023). The Role of Color Doppler Imaging in the Diagnosis of Glaucoma: A Review of the Literature. Diagnostics, 13(4), 588. https://doi.org/10.3390/diagnostics13040588








