Depth-Resolved Attenuation Mapping of the Vaginal Wall under Prolapse and after Laser Treatment Using Cross-Polarization Optical Coherence Tomography: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Samples
2.2. Intravaginal Nd:YAG Laser Treatment Protocol
2.3. OCT System
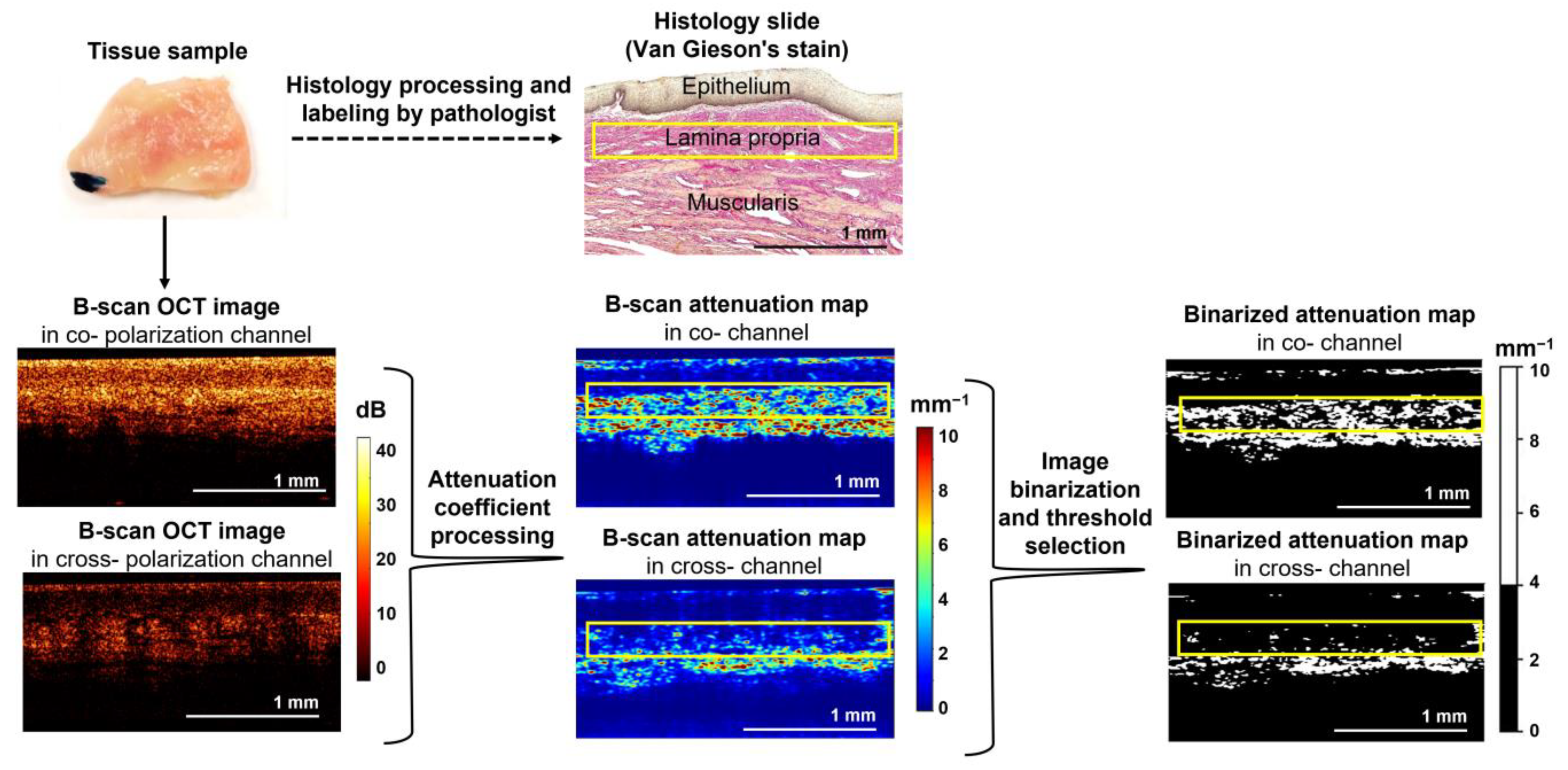
2.4. CP OCT Data Processing and Quantitative Analysis
2.5. Histological Examination
2.6. Statistics
3. Results
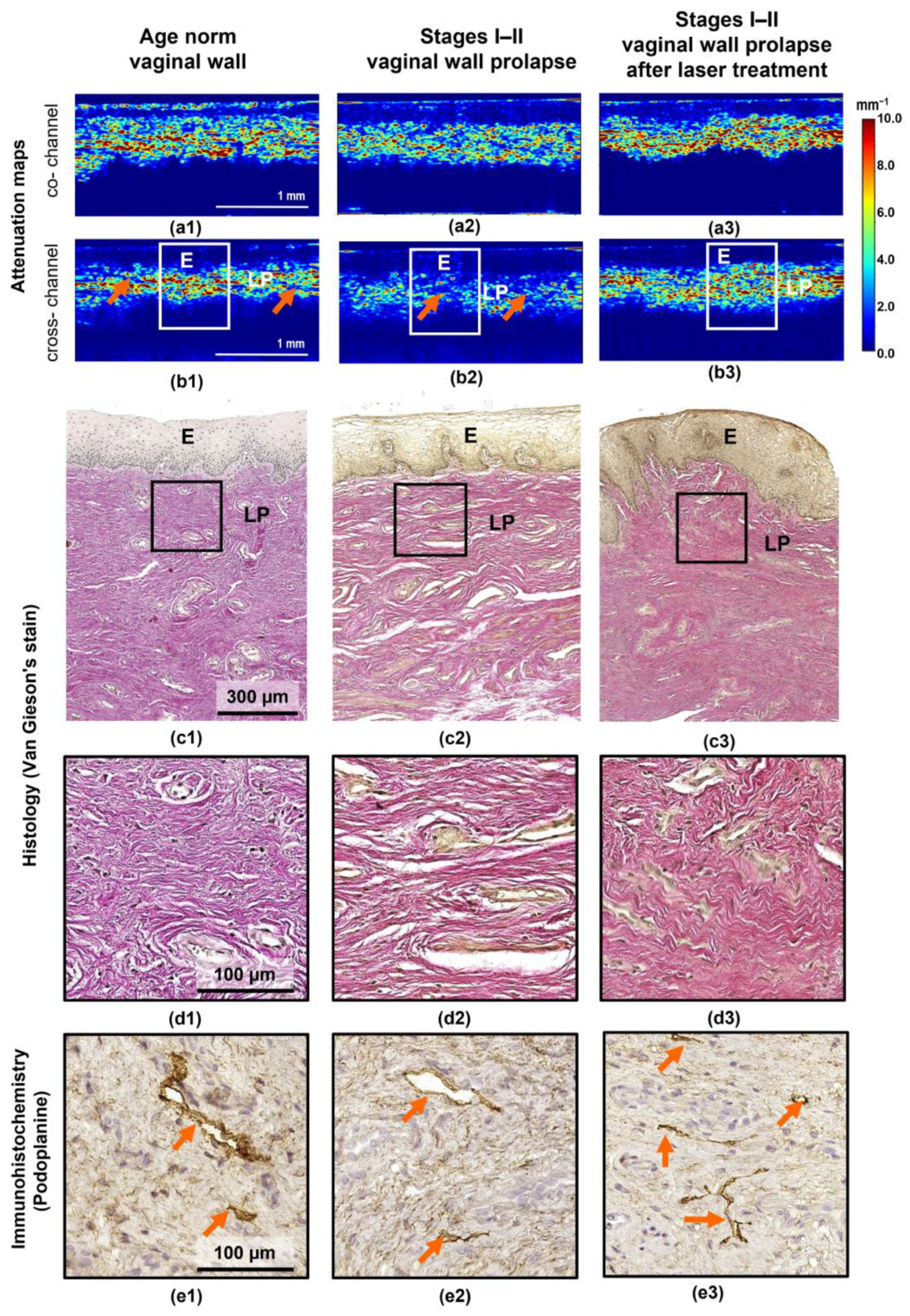
3.1. Correspondence of Depth-Resolved Attenuation Maps with the Histology for Vaginal Wall at Different States
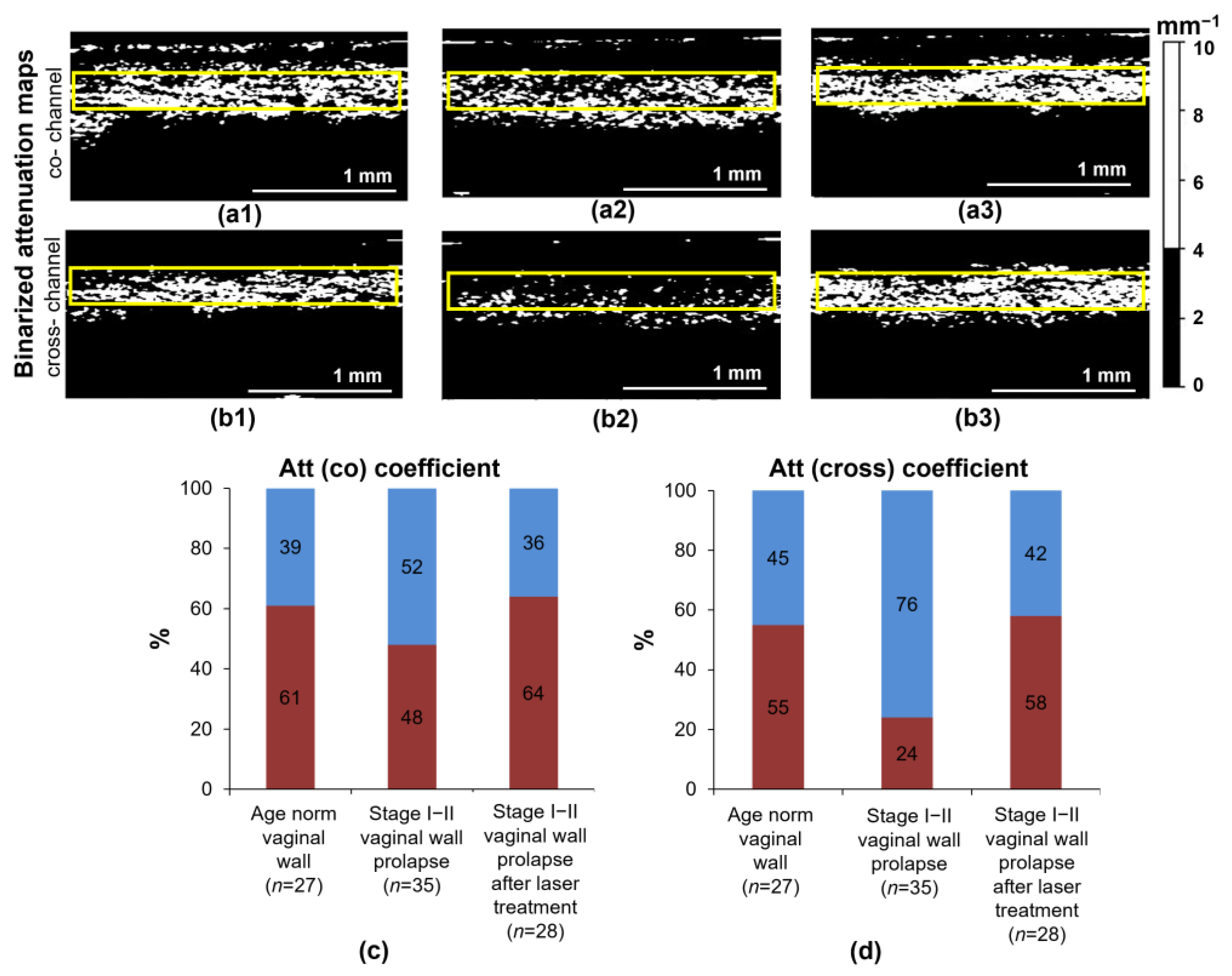
3.2. Quantitative Analysis of Attenuation Maps under the Studied Conditions of the Vaginal Wall
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barber, M.D.; Maher, C. Epidemiology and Outcome Assessment of Pelvic Organ Prolapse. Int. Urogynecol. J. 2013, 24, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Iglesia, C.B.; Smithling, K.R. Pelvic Organ Prolapse. Am. Fam. Physician 2017, 96, 179–185. [Google Scholar] [PubMed]
- Chi, N.; Lozo, S.; Rathnayake, R.A.C.; Botros-Brey, S.; Ma, Y.; Damaser, M.; Wang, R.R. Distinctive structure, composition and biomechanics of collagen fibrils in vaginal wall connective tissues associated with pelvic organ prolapse. Acta Biomater. 2022, 152, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Collins, S.; Lewicky-Gaupp, C. Pelvic Organ Prolapse. Gastroenterol. Clin. N. Am. 2022, 51, 177–193. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.Y.; Glinter, H.; Marcus-Braun, N. Narrative Review of the Epidemiology, Diagnosis and Pathophysiology of Pelvic Organ Prolapse. Int. Braz. J. Urol. 2020, 46, 5–14. [Google Scholar] [CrossRef] [PubMed]
- DeLancey, J.O.L. What’s New in the Functional Anatomy of Pelvic Organ Prolapse? Curr. Opin. Obstet. Gynecol. 2016, 28, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Razzak, L. Pathophysiology of Pelvic Organ Prolapse. In Pelvic Floor Disorders; Rizvi, R.M., Ed.; InTech: London, UK, 2018; ISBN 9781789232448. [Google Scholar]
- Kim, T.; Sridharan, I.; Ma, Y.; Zhu, B.; Chi, N.; Kobak, W.; Rotmensch, J.; Schieber, J.D.; Wang, R. Identifying Distinct Nanoscopic Features of Native Collagen Fibrils towards Early Diagnosis of Pelvic Organ Prolapse. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Xia, Z. Collagen Changes in Pelvic Support Tissues in Women with Pelvic Organ Prolapse. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 185–189. [Google Scholar] [CrossRef]
- Bray, R.; Derpapas, A.; Fernando, R.; Khullar, V.; Panayi, D.C. Does the Vaginal Wall Become Thinner as Prolapse Grade Increases? Int. Urogynecol. J. 2017, 28, 397–402. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, J.; Wang, H.; Zhou, Y.; Wu, J.; Yan, G. Correlation Between Autophagy and Collagen Deposition in Patients with Pelvic Organ Prolapse. Female Pelvic Med. Reconstr. Surg. 2018, 24, 213–221. [Google Scholar] [CrossRef]
- De Landsheere, L.; Munaut, C.; Nusgens, B.; Maillard, C.; Rubod, C.; Nisolle, M.; Cosson, M.; Foidart, J.-M. Histology of the Vaginal Wall in Women with Pelvic Organ Prolapse: A Literature Review. Int. Urogynecol. J. 2013, 24, 2011–2020. [Google Scholar] [CrossRef]
- Dwyer, L.; Kearney, R. Conservative Management of Pelvic Organ Prolapse. Obstet. Gynaecol. Reprod. Med. 2018, 28, 15–21. [Google Scholar] [CrossRef]
- Baessler, K.; Christmann-Schmid, C.; Maher, C.; Haya, N.; Crawford, T.J.; Brown, J. Surgery for Women with Pelvic Organ Prolapse with or without Stress Urinary Incontinence. Cochrane Database Syst. Rev. 2018, 8, CD013108. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Ding, J.; Ai, F.; Zhu, L. Successful Use of the Gellhorn Pessary as a Second-Line Pessary in Women with Advanced Pelvic Organ Prolapse. Menopause 2017, 24, 1277–1281. [Google Scholar] [CrossRef] [PubMed]
- Friedman, T.; Eslick, G.D.; Dietz, H.P. Risk Factors for Prolapse Recurrence: Systematic Review and Meta-Analysis. Int. Urogynecol. J. 2018, 29, 13–21. [Google Scholar] [CrossRef] [PubMed]
- González Isaza, P.; Jaguszewska, K.; Cardona, J.L.; Lukaszuk, M. Long-Term Effect of Thermoablative Fractional CO2 Laser Treatment as a Novel Approach to Urinary Incontinence Management in Women with Genitourinary Syndrome of Menopause. Int. Urogynecol. J. 2018, 29, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Paraiso, M.F.R.; Ferrando, C.A.; Sokol, E.R.; Rardin, C.R.; Matthews, C.A.; Karram, M.M.; Iglesia, C.B. A Randomized Clinical Trial Comparing Vaginal Laser Therapy to Vaginal Estrogen Therapy in Women with Genitourinary Syndrome of Menopause: The VeLVET Trial. Menopause 2020, 27, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Mothes, A.R.; Runnebaum, M.; Runnebaum, I.B. An Innovative Dual-Phase Protocol for Pulsed Ablative Vaginal Erbium:YAG Laser Treatment of Urogynecological Symptoms. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 229, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fistonić, N.; Fistonić, I.; Guštek, Š.F.; Turina, I.S.B.; Marton, I.; Vižintin, Z.; Kažič, M.; Hreljac, I.; Perhavec, T.; Lukač, M. Minimally Invasive, Non-Ablative Er:YAG Laser Treatment of Stress Urinary Incontinence in Women—A Pilot Study. Lasers Med. Sci. 2016, 31, 635–643. [Google Scholar] [CrossRef]
- Gaspar, A.; Brandi, H.; Gomez, V.; Luque, D. Efficacy of Erbium:YAG Laser Treatment Compared to Topical Estriol Treatment for Symptoms of Genitourinary Syndrome of Menopause: Efficacy of Erbium:Yag Laser Treatment of Gsm. Lasers Surg. Med. 2017, 49, 160–168. [Google Scholar] [CrossRef]
- Sajjadi, A.Y.; Mitra, K.; Grace, M. Expression of Heat Shock Proteins 70 and 47 in Tissues Following Short-Pulse Laser Irradiation: Assessment of Thermal Damage and Healing. Med. Eng. Phys. 2013, 35, 1406–1414. [Google Scholar] [CrossRef] [PubMed]
- Souil, E.; Capon, A.; Mordon, S.; Dinh-Xuan, A.T.; Polla, B.S.; Bachelet, M. Treatment with 815-Nm Diode Laser Induces Long-Lasting Expression of 72-KDa Heat Shock Protein in Normal Rat Skin. Br. J. Dermatol. 2001, 144, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.J.; Silapunt, S. Histologic Evaluation of a Q-Switched Nd:YAG Laser in the Nonablative Treatment of Wrinkles. Dermatol. Surg. 2001, 27, 744–746. [Google Scholar] [CrossRef] [PubMed]
- Gambacciani, M.; Palacios, S. Laser Therapy for the Restoration of Vaginal Function. Maturitas 2017, 99, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dang, Y.; Wang, Z.; Chai, X.; Ren, Q. Laser Induced Collagen Remodeling: A Comparative Study in Vivo on Mouse Model. Lasers Surg. Med. 2008, 40, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, I.A.; Apolikhina, I.A.; Spokoinyi, L.B. Vozmozhnosti primeneniia neodimovogo lazera (ND:YAG) v ginekologii. Metamorfozy 2019, 26, 26–31. (In Russian) [Google Scholar]
- Pauzina, O.A.; Apolikhina, I.A.; Malyshkina, D.A. Possibilities of Using Neodymium Laser (Nd:YAG) in Patients with Concomitant Diseases Which Are Accompanied by Pathological Discharge from the Genital Tract. Gynecology 2020, 22, 75–81. [Google Scholar] [CrossRef]
- Gubarkova, E.; Potapov, A.; Krupinova, D.; Shatilova, K.; Karabut, M.; Khlopkov, A.; Loginova, M.; Sovetsky, A.; Zaitsev, V.; Radenska-Lopovok, S.; et al. Compression Optical Coherence Elastography for Assessing Elasticity of the Vaginal Wall under Prolapse after Neodymium Laser Treatment. Photonics 2022, 10, 6. [Google Scholar] [CrossRef]
- Duan, L.; McRaven, M.D.; Liu, W.; Shu, X.; Hu, J.; Sun, C.; Veazey, R.S.; Hope, T.J.; Zhang, H.F. Colposcopic Imaging Using Visible-Light Optical Coherence Tomography. J. Biomed. Opt. 2017, 22, 056003. [Google Scholar] [CrossRef][Green Version]
- Vincent, K.L.; Stanberry, L.R.; Moench, T.R.; Breitkopf, C.R.; Loza, M.L.; Wei, J.; Grady, J.; Paull, J.; Motamedi, M.; Rosenthal, S.L. Optical Coherence Tomography Compared with Colposcopy for Assessment of Vaginal Epithelial Damage: A Randomized Controlled Trial. Obstet. Gynecol. 2011, 118, 1354–1361. [Google Scholar] [CrossRef]
- Nandy, S.; Sanders, M.; Zhu, Q. Classification and Analysis of Human Ovarian Tissue Using Full Field Optical Coherence Tomography. Biomed. Opt. Express 2016, 7, 5182. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, T.; Biswal, N.C.; Wang, X.; Sanders, M.; Brewer, M.; Zhu, Q. Optical Scattering Coefficient Estimated by Optical Coherence Tomography Correlates with Collagen Content in Ovarian Tissue. J. Biomed. Opt. 2011, 16, 090504. [Google Scholar] [CrossRef] [PubMed]
- Sudol, N.T.; Miao, Y.; Li, Y.; Chen, J.J.; Jing, J.C.; Zhu, J.; Tadir, Y.; Chen, Z.; Lane, F. Optical Vaginal Biopsy Using Optical Coherence Tomography. Female Pelvic Med. Reconstr. Surg. 2020, 26, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Sudol, N.T.; Li, Y.; Chen, J.J.; Arthur, R.A.; Qiu, S.; Jiang, Y.; Tadir, Y.; Lane, F.; Chen, Z. Optical Coherence Tomography Evaluation of Vaginal Epithelial Thickness during CO2 Laser Treatment: A Pilot Study. J. Biophotonics 2022, 15, e202200052. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical Coherence Tomography Today: Speed, Contrast, and Multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.; Placzek, F.; Rank, E.; Krainz, L.; Haindl, R.; Li, Q.; Liu, M.; Andreana, M.; Unterhuber, A.; Schmoll, T.; et al. Enhanced Medical Diagnosis for dOCTors: A Perspective of Optical Coherence Tomography. J. Biomed. Opt. 2021, 26, 100601. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and Elasticity Imaging in Compression Optical Coherence Elastography: The Two-decade Perspective and Recent Advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, E.; Kirillin, M.; Feldchtein, F.; Vitkin, A.; Sergeeva, E.; Zagaynova, E.; Streltzova, O.; Shakhov, B.; Gubarkova, E.; Gladkova, N. Differential Diagnosis of Human Bladder Mucosa Pathologies in Vivo with Cross-Polarization Optical Coherence Tomography. Biomed. Opt. Express 2015, 6, 1464. [Google Scholar] [CrossRef]
- Gubarkova, E.; Kiseleva, E.; Moiseev, A.; Vorontsov, D.; Kuznetsov, S.; Plekhanov, A.; Karabut, M.; Sirotkina, M.; Gelikonov, G.; Gamayunov, S.; et al. Intraoperative Assessment of Breast Cancer Tissues after Breast-Conserving Surgery Based on Mapping the Attenuation Coefficients in 3D Cross-Polarization Optical Coherence Tomography. Cancers 2023, 15, 2663. [Google Scholar] [CrossRef]
- Potapov, A.L.; Loginova, M.M.; Moiseev, A.A.; Radenska-Lopovok, S.G.; Kuznetsov, S.S.; Kuznetsova, I.A.; Mustafina, N.N.; Safonov, I.K.; Gladkova, N.D.; Sirotkina, M.A. Cross-Polarization Optical Coherence Tomography for Clinical Evaluation of Dermal Lesion Degrees in Vulvar Lichen Sclerosus. Sovrem. Tehnol. Med. 2023, 15, 53. [Google Scholar] [CrossRef] [PubMed]
- Kiseleva, E.B.; Ryabkov, M.G.; Moiseev, A.A.; Sizov, M.A.; Bederina, E.L.; Korzhimanova, Y.V.; Gelikonov, G.V.; Gelikonov, V.M.; Gladkova, N.D. Attenuation Coefficient for Layer-by-Layer Assessment of the Intestinal Wall in Acute Ischemia According to Optical Coherence Tomography. Laser Phys. Lett. 2022, 19, 075605. [Google Scholar] [CrossRef]
- Li, S.; Luo, H.; Kou, S.; Hagemann, I.S.; Zhu, Q. Depth-resolved Attenuation Mapping of the Human Ovary and Fallopian Tube Using Optical Coherence Tomography. J. Biophotonics 2023, 16, e202300002. [Google Scholar] [CrossRef] [PubMed]
- Persu, C.; Chapple, C.R.; Cauni, V.; Gutue, S.; Geavlete, P. Pelvic Organ Prolapse Quantification System (POP-Q)—A new era in pelvic prolapse staging. J. Med. Life 2011, 4, 75–81. [Google Scholar] [PubMed]
- Gelikonov, V.M.; Romashov, V.N.; Shabanov, D.V.; Ksenofontov, S.Y.; Terpelov, D.A.; Shilyagin, P.A.; Gelikonov, G.V.; Vitkin, I.A. Cross-Polarization Optical Coherence Tomography with Active Maintenance of the Circular Polarization of a Sounding Wave in a Common Path System. Radiophys. Quantum Electron. 2018, 60, 897–911. [Google Scholar] [CrossRef]
- Gong, P.; Almasian, M.; Van Soest, G.; De Bruin, D.M.; Van Leeuwen, T.G.; Sampson, D.D.; Faber, D.J. Parametric Imaging of Attenuation by Optical Coherence Tomography: Review of Models, Methods, and Clinical Translation. J. Biomed. Opt. 2020, 25, 040901. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; De Boer, J.F. Depth-Resolved Model-Based Reconstruction of Attenuation Coefficients in Optical Coherence Tomography. Biomed. Opt. Express 2014, 5, 322–337. [Google Scholar] [CrossRef]
- Moiseev, A.; Sherstnev, E.; Kiseleva, E.; Achkasova, K.; Potapov, A.; Yashin, K.; Sirotkina, M.; Gelikonov, G.; Matkivsky, V.; Shilyagin, P.; et al. Depth-resolved Method for Attenuation Coefficient Calculation from Optical Coherence Tomography Data for Improved Biological Structure Visualization. J. Biophotonics 2023, e202100392. [Google Scholar] [CrossRef]
- Moiseev, A.; Potapov, A.; Sherstnev, E.; Gelikonov, G.; Gelikonov, V.; Sirotkina, M.; Shilyagin, P.; Ksenofontov, S.; Gladkova, N. Depth-Resolved Attenuation Coefficient Estimation from Optical Coherence Tomography Data in Case of Incomplete Signal Attenuation in the Imaging Depth Range. Laser Phys. Lett. 2023, 20, 075601. [Google Scholar] [CrossRef]
- Mutter, G.L.; Prat, J. (Eds.) Pathology of the Female Reproductive Tract, 3rd ed.; Churchill Livingstone Elsevier: London, UK, 2014; ISBN 9780702044977. [Google Scholar]
- Han, L.; Wang, L.; Wang, Q.; Li, H.; Zang, H. Association between Pelvic Organ Prolapse and Stress Urinary Incontinence with Collagen. Exp. Ther. Med. 2014, 7, 1337–1341. [Google Scholar] [CrossRef]
- Salman, M.C.; Ozyuncu, O.; Sargon, M.F.; Kucukali, T.; Durukan, T. Light and Electron Microscopic Evaluation of Cardinal Ligaments in Women with or without Uterine Prolapse. Int. Urogynecol. J. 2010, 21, 235–239. [Google Scholar] [CrossRef]
- Rentchler, E.C.; Gant, K.L.; Drapkin, R.; Patankar, M.J.; Campagnola, P. Imaging Collagen Alterations in STICs and High Grade Ovarian Cancers in the Fallopian Tubes by Second Harmonic Generation Microscopy. Cancers 2019, 11, 1805. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gubarkova, E.; Potapov, A.; Moiseev, A.; Kiseleva, E.; Krupinova, D.; Shatilova, K.; Karabut, M.; Khlopkov, A.; Loginova, M.; Radenska-Lopovok, S.; et al. Depth-Resolved Attenuation Mapping of the Vaginal Wall under Prolapse and after Laser Treatment Using Cross-Polarization Optical Coherence Tomography: A Pilot Study. Diagnostics 2023, 13, 3487. https://doi.org/10.3390/diagnostics13223487
Gubarkova E, Potapov A, Moiseev A, Kiseleva E, Krupinova D, Shatilova K, Karabut M, Khlopkov A, Loginova M, Radenska-Lopovok S, et al. Depth-Resolved Attenuation Mapping of the Vaginal Wall under Prolapse and after Laser Treatment Using Cross-Polarization Optical Coherence Tomography: A Pilot Study. Diagnostics. 2023; 13(22):3487. https://doi.org/10.3390/diagnostics13223487
Chicago/Turabian StyleGubarkova, Ekaterina, Arseniy Potapov, Alexander Moiseev, Elena Kiseleva, Darya Krupinova, Ksenia Shatilova, Maria Karabut, Andrey Khlopkov, Maria Loginova, Stefka Radenska-Lopovok, and et al. 2023. "Depth-Resolved Attenuation Mapping of the Vaginal Wall under Prolapse and after Laser Treatment Using Cross-Polarization Optical Coherence Tomography: A Pilot Study" Diagnostics 13, no. 22: 3487. https://doi.org/10.3390/diagnostics13223487
APA StyleGubarkova, E., Potapov, A., Moiseev, A., Kiseleva, E., Krupinova, D., Shatilova, K., Karabut, M., Khlopkov, A., Loginova, M., Radenska-Lopovok, S., Gelikonov, G., Grechkanev, G., Gladkova, N., & Sirotkina, M. (2023). Depth-Resolved Attenuation Mapping of the Vaginal Wall under Prolapse and after Laser Treatment Using Cross-Polarization Optical Coherence Tomography: A Pilot Study. Diagnostics, 13(22), 3487. https://doi.org/10.3390/diagnostics13223487








