Abstract
For women achieving clinical remission after the completion of initial treatment for epithelial ovarian cancer, 80% with advanced-stage disease will develop recurrence. However, the standard treatment of women with recurrent platinum-sensitive diseases remains poorly defined. Secondary (SCS), tertiary (TCS) or quaternary (QCS) cytoreduction surgery for recurrence has been suggested to be associated with increased overall survival (OS). We searched five databases for studies reporting death rate, OS, cytoreduction rates, post-operative morbidity/mortality and diagnostic models predicting complete cytoreduction in a platinum-sensitive disease recurrence setting. Death rates calculated from raw data were pooled based on a random-effects model. Meta-regression/linear regression was performed to explore the role of complete or optimal cytoreduction as a moderator. Pooled death rates were 45%, 51%, 66% for SCS, TCS and QCS, respectively. Median OS for optimal cytoreduction ranged from 16–91, 24–99 and 39–135 months for SCS, TCS and QCS, respectively. Every 10% increase in complete cytoreduction rates at SCS corresponds to a 7% increase in median OS. Complete cytoreduction rates ranged from 9–100%, 35–90% and 33–100% for SCS, TCS and QCS, respectively. Major post-operative thirty-day morbidity was reported to range from 0–47%, 13–33% and 15–29% for SCS, TCS and QCS, respectively. Thirty-day post-operative mortality was 0–6%, 0–3% and 0–2% for SCS, TCS and QCS, respectively. There were two externally validated diagnostic models predicting complete cytoreduction at SCS, but none for TCS and QCS. In conclusion, our data confirm that maximal effort higher order cytoreductive surgery resulting in complete cytoreduction can improve survival.
1. Introduction
Advances in ovarian cancer chemotherapeutics and ultra-radical surgery have led to a growing number of long-term survivors. In 1975, the 5-year survival rates were 34% in comparison to 51% in 2015 [1]. 80% with FIGO stage III–IV disease will develop recurrent disease despite primary cytoreduction surgery followed by platinum and taxane-based chemotherapy [1]. At present, there is no standard of care for the management of recurrence. Whilst treatment for recurrent ovarian cancer usually requires multiple lines of systemic therapy, additional cytoreduction surgery may be beneficial.
The principles used to explain the survival benefit of primary cytoreduction surgery are also thought to apply to recurrent cytoreduction and are believed to be related to theories of tumour cell kinetics and the development of drug resistance. The Gompertz cell growth curve model shows an increased growth rate in the earlier part of the curve when tumours are relatively small [2]; therefore, the log-kill of tumours by chemotherapy is greater in small-volume tumours made up of rapidly growing and dividing cells. Cytoreduction surgery works by removing large tumours with a relatively small growth fraction and leaving behind smaller (or microscopic) tumours with a relatively greater growth fraction (higher proportion of actively dividing cells), making them more susceptible to the effects of cytotoxic chemotherapy. Also, a reduction in the tumour size decreases the adverse metabolic effects of the tumour, leading to symptom relief and improved performance status. Moreover, tumour debulking may enhance tumour perfusion, resulting in improved drug delivery to the target tissues. Another important concept is that decreasing the number of viable tumour cells decreases the rate of somatic mutations that often perpetuate drug resistance [3]. Hence, cytoreduction surgery removes existing resistant tumour cells and decreases the spontaneous development of resistant cells.
Randomised trials have generated conflicting data, exacerbating the controversy of secondary cytoreduction surgery [4,5,6], and there remains a paucity of data on the role of tertiary and quaternary surgery. Therefore, this systematic review and meta-analysis aims to present data in relation to prognosis following recurrent cytoreduction surgery, and diagnostic criteria predictive of complete cytoreduction, to inform patient selection and aid in counselling in this setting of ovarian cancer recurrence.
2. Materials and Methods
2.1. Search Strategy and Selection Criteria
This systematic review and meta-analysis was performed in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) and Preferred Re-porting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Details of the protocol were registered prospectively on the international PROSPERO database. Five databases were searched from inception to August 2023 using a common search strategy (Supplementary Table S1): Medline, Embase, PubMed, Prospero, Cochrane. Additionally, web-based platforms were searched, including specialised journals, Google searches for grey literature, conference proceedings and clinical trial registries (ISRCTN-registry/ClinicalTrials.gov registry). Searches were not restricted by geographical location, publication year or study design, but were limited to human studies and the English language. The search was re-run prior to final analyses to capture recently published studies.
Predefined inclusion criteria were women >18 years old with platinum-sensitive recurrent epithelial ovarian cancer undergoing recurrence cytoreduction surgery. We excluded women undergoing primary or delayed cytoreduction surgery following initial diagnosis; recurrence surgery for non-epithelial ovarian cancer or platinum-resistant disease; duplicated studies; and studies with abstracts alone with no full text. Outcome measures included death rate (the number of deaths divided by the number of patients in the cohort), median overall survival (OS, defined from date of diagnosis to date of death), complete cytoreduction rate (no macroscopic residual disease), thirty-day post-operative major morbidity (Clavien-Dindo grades III–IV) and mortality, and diagnostic criteria predictive of achieving complete cytoreduction.
2.2. Data Extraction, Quality Assessment and Analysis
Data were extracted using a standardised, predesigned formatted sheet (following piloting and refinement) in Microsoft Excel 2013 by two independent investigators (FG and DC). Inter-rater reliability was analysed using quantity (Q) and allocation (A) disagreements [7]. Any discrepancies were referred to investigators EB and SP for discussion and consensus. Three main categories of data were extracted: methodological characteristics, interventions (secondary (SCS), tertiary (TCS) and quaternary (QCS) cytoreduction surgery defined as cytoreduction surgery after first, second and third recurrence, respectively) and reported outcome measures. The risk of bias was assessed using the Newcastle Ottawa Scale (NOS) [8]. GRADE (Grading of Recommendations Assessment Development and Evaluations) was used to assess the overall quality of evidence for each outcome. No studies were excluded from data synthesis based on quality assessment scores. We tabulated the characteristics and reported outcome measures of all studies for qualitative synthesis. In instances where two or more studies had overlapping datasets, the study with the least risk of bias or highest quality was used for pooling. The decision to conduct a meta-analysis (quantitative data synthesis) was made a posteriori to ensure sufficient studies with similar characteristics were available. As studies varied in their outcome measures, to ensure comparability between studies, the death rate was calculated using raw data independently extracted by authors FG and DC. The authors of studies in which raw data were missing from the published manuscript were contacted.
Since studies differed by year, geographical location, confounders and reported measurements of effect size, the death rate and 95% confidence intervals calculated from raw data were pooled based on a random effects model. The Der Simonian Laird estimate was used to assess study variance. To determine the extent of inter-study variations, we conducted heterogeneity tests with Higgins’ I2 statistic to measure the proportion of the observed variance that reflects true effect sizes [9]. An I2 ≥ 50% was considered to represent a significant inter-study variation [10]. Meta-regression analysis for the death rate was performed to further explore the role of complete or optimal cytoreduction as a moderator in univariable and multivariable models. Additionally, simple and multiple linear regression analyses were conducted to investigate the association between log-transformed median OS time and the proportion of complete cytoreduction, the proportion of optimal cytoreduction and other clinically important covariates (e.g., age, disease-free interval, post-operative morbidity). A two-sided p-value of less than 0.05 was considered statistically significant, and all statistical analyses were conducted using R version 3.5.1.
3. Results
Supplementary Figure S1 provides the flow chart outlining the search outcomes and the study selection process. Searches of electronic databases and reference lists generated 623 references. Upon evaluation of the titles and abstracts, 100 articles were potentially eligible for detailed assessment, of which 76 met our inclusion criteria for qualitative synthesis (Supplementary Table S2). There were high levels of agreement between the reviewers (Q = 1/76, A = 2/76). A total of 64 studies [4,5,6,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71] (4 randomised controlled trials, 11 prospective cohort studies and 49 retrospective cohort studies) pertained to SCS, 8 [72,73,74,75,76,77,78,79] (all retrospective cohort studies) to TCS and 4 [80,81,82,83] (all retrospective cohort studies) to QCS. The mean follow-up ranged from 16–88, 13–35 and 18 months for SCS, TCS and QCS, respectively; the median age ranged from 51–64, 51–58 and 54–61 years for SCS, TCS and QCS, respectively. The median OS was reported as 16–91 months for SCS. For patients who had achieved optimal cytoreduction, OS ranged from 24–99 and 39–135 months for TCS and QCS, respectively, while for those who had achieved suboptimal cytoreduction, the OS range was 6–79 and 10–13 months for TCS and QCS, respectively. The definition for optimal cytoreduction varied across studies from no macroscopic disease to <2.5 cm of disease. A range of 14–73% of women undergoing SCS had solitary sites of disease, compared with 17–91% in TCS. One of the four studies reporting QCS reported the proportion of patients with a solitary site of disease (45%). Complete cytoreduction (no macroscopic disease) rates ranged from 9–100%, 35–90% and 33–100% for SCS, TCS and QCS, respectively. The major post-operative thirty-day morbidity was reported to range from 0–47%, 13–33% and 15–29% for SCS, TCS and QCS, respectively. Thirty-day post-operative mortality was 0–6%, 0–3% and 0–2% for SCS, TCS and QCS, respectively. The disease-free interval (DFI, time after the primary treatment until first recurrence) was 10–43 months for SCS, while the treatment free interval (TFI, time without any treatment after recurrent cytoreduction surgery until second/third recurrence) was 4–22 and 14–20 months for TCS and QCS, respectively. Table 1 summarises the diagnostic criteria reported as predictors for achieving complete cytoreduction.

Table 1.
Diagnostic criteria predictive of achieving complete cytoreduction at recurrence surgery.
Supplementary Table S3 summarises the risk of bias assessment and Supplementary Table S4 the GRADE assessment for certainty of evidence per outcome. The GRADE certainty for evidence for SCS was high, and moderate for TCS and QCS. According to GRADE, all observational studies have an initial low level of evidence. For SCS, the certainty of evidence was upgraded due to large and consistent size effects and randomised controlled trial data. For TCS and QCS, the certainty of evidence was downgraded due to serious risks of bias and no prospective data, but was upgraded considering the consistent size effect.
Figure 1, Figure 2 and Figure 3 summarise the death rate for each study pertaining to SCS, TCS and QCS. For SCS, the pooled death rate from 36 studies of 2791 patients was 0.45 (95%CI 0.39–0.50). For TCS, the pooled death rate from five studies of 643 patients was 0.51 (95%CI 0.48–0.55). For QCS, the pooled death rate from two studies of 69 patients was 0.66 (95%CI 0.38–0.89).
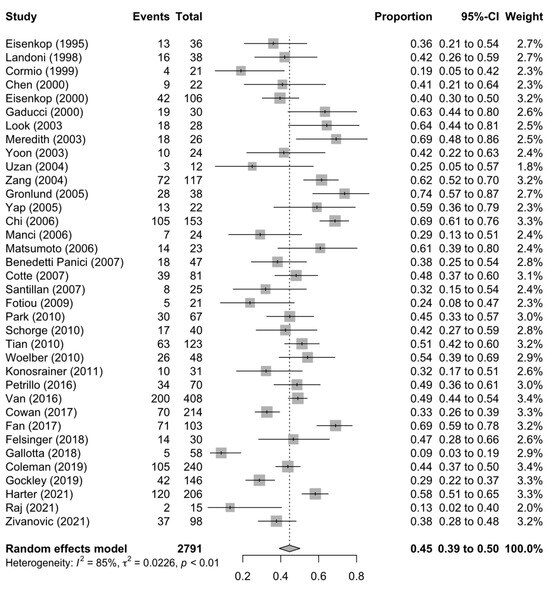
Figure 1.
Forest plot of the death rate for secondary cytoreduction surgery [4,6,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,29,30,31,32,33,34,35,37,38,39,40,41,42,43,71].
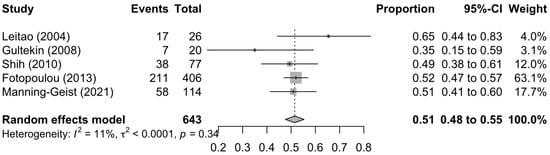
Figure 2.
Forest plot of the death rate for tertiary cytoreduction surgery [72,73,76,78,79].
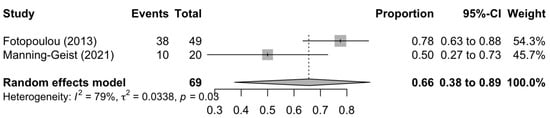
Figure 3.
Forest plot of the death rate for quaternary cytoreduction surgery [80,83].
The heterogeneity of the models as measured by I2 ranged from 11–85%. The SCS and QCS models had high heterogeneity (I2 ≥ 50%), while the TCS model had low heterogeneity (I2 < 50%). For SCS, fourteen studies were located outside the funnel plot, but these were equally distributed on both sides of the plot, indicating low publication bias (Supplementary Figure S2). It was not possible to assess publication bias for TCS and QCS due to a paucity of studies (<10).
A univariate meta-regression analysis of the death rate for SCS conducted to determine the cause of model heterogeneity showed a statistically significant proportion of complete and optimal cytoreduction (Table 2). Age, DFI and pattern of recurrence and post-operative major morbidity had no impact on the death rate. In the multivariable analysis, complete and optimal cytoreduction remained significant moderators of survival, even after adjusting for age, DFI and major post-operative morbidity. DFI had no impact on the death rate for complete cytoreduction, but a longer DFI was associated with an increase in median OS for optimal cytoreduction. It was not possible to perform a meta-regression for the TCS and QCS models due to a paucity of studies (<10).

Table 2.
Meta-regression analysis of the death rate for secondary cytoreduction surgery.
The results of the univariable linear regression model for median OS time (Table 3) demonstrated that a higher proportion of complete cytoreduction and a more advanced age were significantly associated with longer OS. Optimal cytoreduction, type of study design (retrospective versus prospective), length of DFI, major post-operative morbidity and patterns of relapse (solitary versus multifocal) did not significantly affect OS.

Table 3.
Linear regression model for median overall survival time for secondary cytoreduction surgery.
The multivariable effect of the proportion of complete and optimal cytoreduction was also evaluated, adjusting for age, DFI and thirty-day post-operative major morbidity. The median OS time increased by 7.43% when the proportion of patients achieving complete cytoreduction increased by 10%, after adjusting for age, DFI and post-operative major morbidity. For complete cytoreduction, for every 1 month increase in DFI, the median OS increased by 2.46%. The change in median OS time was not statistically significant for optimal cytoreduction. Again, it was not possible to perform a linear regression for the TCS and QCS models due to a paucity of studies (<10).
4. Discussion
In this systematic review and meta-analysis, we showed a pooled death rate of 45%, 51%, and 66% for SCS, TCS, and QCS, respectively in a platinum-sensitive relapse setting. In addition, results from our meta-regression analysis showed that every 10% increase in complete clearance rates at SCS led to a 7% increase in median OS across all types of study designs. An increase in DFI by 1 month increased the median OS for complete cytoreduction at SCS by 2%. Patterns of relapse (solitary versus multifocal recurrence) failed to significantly affect OS.
The three RCTs that have published mature data (GOG-213 [4], SOC-1 [5] and DESKTOP III [6]) all demonstrated that patients who have undergone complete cytoreduction at SCS have a significantly longer progression-free survival (PFS) compared with those treated with chemotherapy alone. However, opposing data were generated regarding the impact of SCS on OS. The three-year OS in the tumour-free surgery arm of GOG-213 was 76%, the lowest amongst the three studies (84% DESKTOP III and 78% SOC-1). However, the three-year OS in the non-surgery arm of GOG-213 was the highest at 75% (62% DESKTOP III and 66% in SOC-1). These differences may be due to the lack of standardisation and surgical quality assurance of participating centres, differences in study design and heterogeneous patient profiles between studies. For example, 84% of the study cohort in the GOG-213 received concomitant and maintenance bevacizumab, while it was only used in 23% and 1% of patients in DESKTOP III and SOC-1, respectively, in the non-surgical chemotherapy group. Study data have shown that the use of adjuvant and maintenance bevacizumab among women treated with second-line, platinum-based chemotherapy increases PFS and OS [84,85]. This in part, may explain the similar OS between the surgery and non-surgery arms of the GOG-213 trial. Both the SOC-1 and DESKTOP trials had standardised patient selection criteria for surgery (iMODEL and AGO criteria, respectively), unlike the GOG-213, where patient selection was determined by the surgeon.
In total, we identified eight retrospective studies evaluating TCS and four retrospective studies evaluating QCS. There remains a paucity of high-quality prospective data. However, the reported OS from these studies was similar to that reported by retrospective studies evaluating SCS with comparable short-term morbidity and mortality data (Supplementary Table S2).
Most studies evaluating SCS, TCS and QCS did not incorporate novel targeted agents (anti-angiogenic agents and PARP inhibitors). The GOG-213 study, which included bevacizumab in both the SCS study arm and systemic therapy arm in 84% of the patient sample, showed similar OS. Therefore, the question of whether routine implementation of these novel agents reduces the impact of cytoreduction recurrence surgery remains to be addressed. We would argue that a multimodal personalised collective therapeutic effort involving shared decision-making with the patient, surgeon and oncologist instead of a single treatment modality is vital to optimise survival. For instance, patients with extensive yet operable bowel disease at recurrence would, without surgery, usually not receive bevacizumab due to the risk of perforation. However, they may become eligible by having the bowel disease resected, thereby restoring anatomy and function before commencing systemic treatment. Hence, surgery is able to complement systemic therapy, enabling maximal therapeutic effort to improve survival. The impact of multimodal therapy is indirectly reflected by more recently published studies using anti-angiogenic and targeted therapies alongside surgery, showing improved survival outcomes when compared with older studies [86].
Both the iMODEL and AGO risk prediction models have been externally validated in clinical studies to identify patients suitable for achieving complete cytoreduction at SCS. The AGO model was validated in the DESKTOP II trial of 516 patients with a complete cytoreduction rate of 76%. However, the negative predictive value was 38% and the specificity 53% [87]. The iMODEL was externally validated on 117 patients. Complete cytoreduction was achieved in 40% of the cohort, with sensitivity and specificity values of 83.3% and 57.6%, respectively [87]. Limitations of both models include the fact that neither considered surgical ability as an evaluation parameter. Patients with the same prediction scores undergoing SCS performed by different surgeons may obtain different complete cytoreduction rates. They also do not consider BRCA/homologous recombination deficiency (HRD) status or the use of PARP inhibitors and/or bevacizumab. Artificial intelligence models have also been developed to assess the importance of the clinical variables predicting complete cytoreduction at SCS. Three main factors have been proposed to predict complete cytoreduction using artificial neuronal network analysis: DFI (importance = 0.231), retroperitoneal recurrence (importance = 0.178) and residual disease at primary surgical treatment (importance = 0.138) [88]. However, these predictors have not yet been modelled and lack external validity. There are currently no externally validated risk prediction models available to identify patients suitable for achieving complete cytoreduction at TCS and QCS.
The strengths of our systematic review and meta-analysis include a comprehensive search strategy identifying all the relevant literature for inclusion, and methodologically rigorous pooled estimates of death rates following recurrence surgery from raw data resulting in standardised measures of effect sizes from included studies. Overlapping datasets with a greater risk of bias were excluded to ensure no particular dataset was over-represented in our analyses. To limit the risk of reporting bias-influencing findings, all published studies of platinum-sensitive disease recurrence were included. Due to the paucity of studies, meta-regression analysis was not possible for TCS and QCS. There was a large amount of statistical heterogeneity (I2 ≥ 50%), and so a random-effects meta-analysis was performed, which produces more conservative confidence intervals. This only partly removed the effects of heterogeneity. Studies across four decades were included in our meta-analysis, resulting in a broad difference in defining optimal residual disease, with residual disease of up to 2.5 cm in older studies considered optimum. Therefore, bulkier disease in the meta-regression analysis might have underestimated the overall survival benefit of <1 cm residual disease. It was not possible to evaluate the impact of hormone receptor status on survival, as this was not reported in the majority of papers included in the meta-analysis.
Our findings are similar to those of previous systematic reviews and meta-analyses evaluating complete cytoreduction at SCS and showing increased OS [86,89]. However, to the best of our knowledge, ours is the first meta-analysis evaluating the death rate for TCS and QCS.
5. Conclusions
Multimodal treatment, incorporating both medical chemotherapeutic advances and recurrence surgery, is key to improving survival in a platinum-sensitive recurrence setting. The strongest predictor of survival in all types of recurrence surgery remains the achievement of complete macroscopic cytoreduction irrespective of the number of sites of recurrence and the length of time between relapses. Particularly, for highly symptomatic patients with recurrent disease, surgery provides a much more rapid relief of symptoms compared with systemic therapy options.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13223484/s1, Figure S1: PRISMA flow diagram for study selection; Figure S2: Funnel plot of secondary cytoreduction studies for publication bias; Table S1: Search strategy for literature review; Table S2: Qualitative data synthesis of studies reporting the overall survival, cytoreduction rates and 30-day major post-operative morbidity and mortality following recurrence surgery in platinum-sensitive epithelial ovarian carcinoma; Table S3: Risk of bias assessment; Table S4: GRADE assessment of certainty of evidence per outcome.
Author Contributions
Conception: F.G. Design and development: F.G. and O.B. Review activities: F.G., D.C., E.B. and S.P. Analysis: F.G. and O.B. Preparation of tables and figures: F.G. and O.B. Initial draft of manuscript: F.G. Writing and approval of manuscript: O.B., N.B., B.R., D.B., T.I., M.N., J.B., O.H., A.J., A.L., R.M. and J.D. All authors had full access to all the data in this study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used or analysed in this study are publicly available. Data generated from the analysis are presented. Any additional data needed can be made available upon reasonable request from the corresponding author.
Acknowledgments
GO SOAR Collaborators: Karen Ash, Paul Kamfwa.
Conflicts of Interest
F.G. declares funding from The NHS Grampian Endowment Fund, Medtronic and the British Gynaecological Cancer Society outside of this work and an honorarium from Astra Zeneca. All other authors declare no conflicts of interest.
References
- GLOBOCAN. Cancer over Time. Available online: https://gco.iarc.fr/overtime/en/dataviz/trends?populations=75200&sexes=1_2&types=1&multiple_populations=0&mode=cancer&multiple_cancers=1&cancers=18 (accessed on 30 August 2023).
- Norton, L. Theoretical concepts and the emerging role of taxanes in adjuvant therapy. Oncologist 2001, 6 (Suppl. S3), 30–35. [Google Scholar] [CrossRef] [PubMed]
- Goldie, J.H.; Coldman, A.J. A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate. Cancer Treat. Rep. 1979, 63, 1727–1733. [Google Scholar] [PubMed]
- Coleman, R.L.; Spirtos, N.M.; Enserro, D.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Kim, J.W.; Park, S.Y.; Kim, B.G.; Nam, J.H.; et al. Secondary Surgical Cytoreduction for Recurrent Ovarian Cancer. N. Engl. J. Med. 2019, 381, 1929–1939. [Google Scholar] [CrossRef]
- Shi, T.; Zhu, J.; Feng, Y.; Tu, D.; Zhang, Y.; Zhang, P.; Jia, H.; Huang, X.; Cai, Y.; Yin, S.; et al. Secondary cytoreduction followed by chemotherapy versus chemotherapy alone in platinum-sensitive relapsed ovarian cancer (SOC-1): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Vergote, I.; Ferron, G.; Reuss, A.; Meier, W.; Greggi, S.; Mosgaard, B.J.; Selle, F.; Guyon, F.; et al. Randomized Trial of Cytoreductive Surgery for Relapsed Ovarian Cancer. N. Engl. J. Med. 2021, 385, 2123–2131. [Google Scholar] [CrossRef] [PubMed]
- Warrens, M.J. Properties of the quantity disagreement and the allocation disagreement. Int. J. Remote Sens. 2015, 36, 1439–1446. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef]
- Borenstein, M.; Hedges, L.V.; Higgins, J.P.; Rothstein, H.R. Introduction to Meta-Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Kunz, R.; Woodcock, J.; Brozek, J.; Helfand, M.; Alonso-Coello, P.; Glasziou, P.; Jaeschke, R.; Akl, E.A.; et al. GRADE guidelines: 7. Rating the quality of evidence—Inconsistency. J. Clin. Epidemiol. 2011, 64, 1294–1302. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Friedman, R.L.; Wang, H.J. Secondary cytoreductive surgery for recurrent ovarian cancer. A prospective study. Cancer 1995, 76, 1606–1614. [Google Scholar] [CrossRef]
- Landoni, F.; Pellegrino, A.; Cormio, G.; Milani, R.; Maggioni, A.; Mangioni, C. Platin-based chemotherapy and salvage surgery in recurrent ovarian cancer following negative second-look laparotomy. Acta Obstet. Gynecol. Scand. 1998, 77, 233–237. [Google Scholar]
- Cormio, G.; di Vagno, G.; Cazzolla, A.; Bettocchi, S.; di Gesu, G.; Loverro, G.; Selvaggi, L. Surgical treatment of recurrent ovarian cancer: Report of 21 cases and a review of the literature. Eur. J. Obstet. Gynecol. Reprod. Biol. 1999, 86, 185–188. [Google Scholar] [CrossRef]
- Eisenkop, S.M.; Friedman, R.L.; Spirtos, N.M. The role of secondary cytoreductive surgery in the treatment of patients with recurrent epithelial ovarian carcinoma. Cancer 2000, 88, 144–153. [Google Scholar] [CrossRef]
- Gadducci, A.; Iacconi, P.; Cosio, S.; Fanucchi, A.; Cristofani, R.; Riccardo Genazzani, A. Complete salvage surgical cytoreduction improves further survival of patients with late recurrent ovarian cancer. Gynecol. Oncol. 2000, 79, 344–349. [Google Scholar] [CrossRef]
- Chen, L.M.; Leuchter, R.S.; Lagasse, L.D.; Karlan, B.Y. Splenectomy and surgical cytoreduction for ovarian cancer. Gynecol. Oncol. 2000, 77, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.S.; Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Barakat, R.R.; Blumgart, L.H.; Chi, D.S. Resection of recurrent ovarian or fallopian tube carcinoma involving the liver. Gynecol. Oncol. 2003, 91, 383–388. [Google Scholar] [CrossRef]
- Merideth, M.A.; Cliby, W.A.; Keeney, G.L.; Lesnick, T.G.; Nagorney, D.M.; Podratz, K.C. Hepatic resection for metachronous metastases from ovarian carcinoma. Gynecol. Oncol. 2003, 89, 16–21. [Google Scholar] [CrossRef]
- Look, M.; Chang, D.; Sugarbaker, P.H. Long-term results of cytoreductive surgery for advanced and recurrent epithelial ovarian cancers and papillary serous carcinoma of the peritoneum. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2004, 14, 35–41. [Google Scholar] [CrossRef]
- Uzan, C.; Morice, P.; Rey, A.; Pautier, P.; Camatte, S.; Lhommé, C.; Haie-Meder, C.; Duvillard, P.; Castaigne, D. Outcomes after combined therapy including surgical resection in patients with epithelial ovarian cancer recurrence(s) exclusively in lymph nodes. Ann. Surg. Oncol. 2004, 11, 658–664. [Google Scholar] [CrossRef]
- Zang, R.Y.; Li, Z.T.; Tang, J.; Cheng, X.; Cai, S.M.; Zhang, Z.Y.; Teng, N.N. Secondary cytoreductive surgery for patients with relapsed epithelial ovarian carcinoma: Who benefits? Cancer 2004, 100, 1152–1161. [Google Scholar] [CrossRef]
- Gronlund, B.; Lundvall, L.; Christensen, I.J.; Knudsen, J.B.; Høgdall, C. Surgical cytoreduction in recurrent ovarian carcinoma in patients with complete response to paclitaxel-platinum. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2005, 31, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Yap, O.W.; Kapp, D.S.; Teng, N.N.; Husain, A. Intraoperative radiation therapy in recurrent ovarian cancer. Int. J. Radiat. Oncol. Biol. Phys. 2005, 63, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Manci, N.; Bellati, F.; Muzii, L.; Calcagno, M.; Alon, S.A.; Pernice, M.; Angioli, R.; Panici, P.B. Splenectomy during secondary cytoreduction for ovarian cancer disease recurrence: Surgical and survival data. Ann. Surg. Oncol. 2006, 13, 1717–1723. [Google Scholar] [CrossRef]
- Matsumoto, A.; Higuchi, T.; Yura, S.; Mandai, M.; Kariya, M.; Takakura, K.; Fujii, S. Role of salvage cytoreductive surgery in the treatment of patients with recurrent ovarian cancer after platinum-based chemotherapy. J. Obstet. Gynaecol. Res. 2006, 32, 580–587. [Google Scholar] [CrossRef]
- Chi, D.S.; McCaughty, K.; Diaz, J.P.; Huh, J.; Schwabenbauer, S.; Hummer, A.J.; Venkatraman, E.S.; Aghajanian, C.; Sonoda, Y.; Abu-Rustum, N.R.; et al. Guidelines and selection criteria for secondary cytoreductive surgery in patients with recurrent, platinum-sensitive epithelial ovarian carcinoma. Cancer 2006, 106, 1933–1939. [Google Scholar] [CrossRef] [PubMed]
- Santillan, A.; Karam, A.K.; Li, A.J.; Giuntoli, R., 2nd; Gardner, G.J.; Cass, I.; Karlan, B.Y.; Bristow, R.E. Secondary cytoreductive surgery for isolated nodal recurrence in patients with epithelial ovarian cancer. Gynecol. Oncol. 2007, 104, 686–690. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Perniola, G.; Angioli, R.; Zullo, M.A.; Manci, N.; Palaia, I.; Bellati, F.; Plotti, F.; Calcagno, M.; Basile, S. Bulky lymph node resection in patients with recurrent epithelial ovarian cancer: Impact of surgery. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2007, 17, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Benedetti Panici, P.; De Vivo, A.; Bellati, F.; Manci, N.; Perniola, G.; Basile, S.; Muzii, L.; Angioli, R. Secondary cytoreductive surgery in patients with platinum-sensitive recurrent ovarian cancer. Ann. Surg. Oncol. 2007, 14, 1136–1142. [Google Scholar] [CrossRef]
- Cotte, E.; Glehen, O.; Mohamed, F.; Lamy, F.; Falandry, C.; Golfier, F.; Gilly, F.N. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: Prospective study of 81 patients. World J. Surg. 2007, 31, 1813–1820. [Google Scholar] [CrossRef]
- Fotiou, S.; Aliki, T.; Petros, Z.; Ioanna, S.; Konstantinos, V.; Vasiliki, M.; George, C. Secondary cytoreductive surgery in patients presenting with isolated nodal recurrence of epithelial ovarian cancer. Gynecol. Oncol. 2009, 114, 178–182. [Google Scholar] [CrossRef]
- Tian, W.J.; Jiang, R.; Cheng, X.; Tang, J.; Xing, Y.; Zang, R.Y. Surgery in recurrent epithelial ovarian cancer: Benefits on Survival for patients with residual disease of 0.1–1 cm after secondary cytoreduction. J. Surg. Oncol. 2010, 101, 244–250. [Google Scholar] [CrossRef]
- Park, J.Y.; Eom, J.M.; Kim, D.Y.; Kim, J.H.; Kim, Y.M.; Kim, Y.T.; Nam, J.H. Secondary cytoreductive surgery in the management of platinum-sensitive recurrent epithelial ovarian cancer. J. Surg. Oncol. 2010, 101, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Woelber, L.; Jung, S.; Eulenburg, C.; Mueller, V.; Schwarz, J.; Jaenicke, F.; Mahner, S. Perioperative morbidity and outcome of secondary cytoreduction for recurrent epithelial ovarian cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2010, 36, 583–588. [Google Scholar] [CrossRef]
- Schorge, J.O.; Wingo, S.N.; Bhore, R.; Heffernan, T.P.; Lea, J.S. Secondary cytoreductive surgery for recurrent platinum-sensitive ovarian cancer. Int. J. Gynaecol. Obstet. Off. Organ Int. Fed. Gynaecol. Obstet. 2010, 108, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Königsrainer, I.; Beckert, S.; Becker, S.; Zieker, D.; Fehm, T.; Grischke, E.M.; Lauk, O.; Glatzle, J.; Brücher, B.; Wallwiener, D.; et al. Cytoreductive surgery and HIPEC in peritoneal recurrent ovarian cancer: Experience and lessons learned. Langenbeck’s Arch. Surg. 2011, 396, 1077–1081. [Google Scholar] [CrossRef] [PubMed]
- van de Laar, R.; Kruitwagen, R.F.; IntHout, J.; Zusterzeel, P.L.; Van Gorp, T.; Massuger, L.F. Surgery for Recurrent Epithelial Ovarian Cancer in the Netherlands: A Population-Based Cohort Study. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2016, 26, 268–275. [Google Scholar] [CrossRef]
- Petrillo, M.; De Iaco, P.; Cianci, S.; Perrone, M.; Costantini, B.; Ronsini, C.; Scambia, G.; Fagotti, A. Long-Term Survival for Platinum-Sensitive Recurrent Ovarian Cancer Patients Treated with Secondary Cytoreductive Surgery Plus Hyperthermic Intraperitoneal Chemotherapy (HIPEC). Ann. Surg. Oncol. 2016, 23, 1660–1665. [Google Scholar] [CrossRef]
- Cowan, R.A.; Eriksson, A.G.Z.; Jaber, S.M.; Zhou, Q.; Iasonos, A.; Zivanovic, O.; Leitao, M.M., Jr.; Abu-Rustum, N.R.; Chi, D.S.; Gardner, G.J. A comparative analysis of prediction models for complete gross resection in secondary cytoreductive surgery for ovarian cancer. Gynecol. Oncol. 2017, 145, 230–235. [Google Scholar] [CrossRef]
- Fan, X.M.; Zhang, J.; Niu, S.H.; Li, K.X.; Song, C.Z. Secondary cytoreductive surgery in recurrent epithelial ovarian cancer: A prognostic analysis with 103 cases. Int. J. Surg. 2017, 38, 61–66. [Google Scholar] [CrossRef]
- Gallotta, V.; Conte, C.; Giudice, M.T.; Nero, C.; Vizzielli, G.; Gueli Alletti, S.; Cianci, S.; Lodoli, C.; Di Giorgio, A.; De Rose, A.M.; et al. Secondary Laparoscopic Cytoreduction in Recurrent Ovarian Cancer: A Large, Single-Institution Experience. J. Minim. Invasive Gynecol. 2018, 25, 644–650. [Google Scholar] [CrossRef]
- Felsinger, M.; Minar, L.; Weinberger, V.; Rovny, I.; Zlamal, F.; Bienertova-Vasku, J. Secondary cytoreductive surgery-viable treatment option in the management of platinum-sensitive recurrent ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 228, 154–160. [Google Scholar] [CrossRef]
- Gockley, A.; Melamed, A.; Cronin, A.; Bookman, M.A.; Burger, R.A.; Cristae, M.C.; Griggs, J.J.; Mantia-Smaldone, G.; Matulonis, U.A.; Meyer, L.A.; et al. Outcomes of secondary cytoreductive surgery for patients with platinum-sensitive recurrent ovarian cancer. Am. J. Obstet. Gynecol. 2019, 221, 625.e1–625.e14. [Google Scholar] [CrossRef]
- Morris, M.; Gershenson, D.M.; Wharton, J.T.; Copeland, L.J.; Edwards, C.L.; Stringer, C.A. Secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Gynecol. Oncol. 1989, 34, 334–338. [Google Scholar] [CrossRef]
- Jänicke, F.; Hölscher, M.; Kuhn, W.; von Hugo, R.; Pache, L.; Siewert, J.R.; Graeff, H. Radical surgical procedure improves survival time in patients with recurrent ovarian cancer. Cancer 1992, 70, 2129–2136. [Google Scholar] [CrossRef]
- Segna, R.A.; Dottino, P.R.; Mandeli, J.P.; Konsker, K.; Cohen, C.J. Secondary cytoreduction for ovarian cancer following cisplatin therapy. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1993, 11, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Lichtenegger, W.; Sehouli, J.; Buchmann, E.; Karajanev, C.; Weidemann, H. Operative results after primary and secondary debulking-operations in advanced ovarian cancer (AOC). J. Obstet. Gynaecol. Res. 1998, 24, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tay, E.H.; Grant, P.T.; Gebski, V.; Hacker, N.F. Secondary cytoreductive surgery for recurrent epithelial ovarian cancer. Obstet. Gynecol. 2002, 99, 1008–1013. [Google Scholar] [CrossRef]
- Loizzi, V.; Chan, J.K.; Osann, K.; Cappuccini, F.; DiSaia, P.J.; Berman, M.L. Survival outcomes in patients with recurrent ovarian cancer who were treated with chemoresistance assay-guided chemotherapy. Am. J. Obstet. Gynecol. 2003, 189, 1301–1307. [Google Scholar] [CrossRef]
- Zanon, C.; Clara, R.; Chiappino, I.; Bortolini, M.; Cornaglia, S.; Simone, P.; Bruno, F.; De Riu, L.; Airoldi, M.; Pedani, F. Cytoreductive surgery and intraperitoneal chemohyperthermia for recurrent peritoneal carcinomatosis from ovarian cancer. World J. Surg. 2004, 28, 1040–1045. [Google Scholar] [CrossRef]
- Güngör, M.; Ortaç, F.; Arvas, M.; Kösebay, D.; Sönmezer, M.; Köse, K. The role of secondary cytoreductive surgery for recurrent ovarian cancer. Gynecol. Oncol. 2005, 97, 74–79. [Google Scholar] [CrossRef]
- Onda, T.; Yoshikawa, H.; Yasugi, T.; Yamada, M.; Matsumoto, K.; Taketani, Y. Secondary cytoreductive surgery for recurrent epithelial ovarian carcinoma: Proposal for patients selection. Br. J. Cancer 2005, 92, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Gultekin, M.; Taskiran, C.; Aksan, G.; Celik, N.Y.; Dursun, P.; Salman, M.C.; Yuce, K.; Kucukali, T. The role of secondary cytoreduction in the treatment of ovarian cancer: Hacettepe University experience. Am. J. Obstet. Gynecol. 2006, 194, 49–56. [Google Scholar] [CrossRef]
- Harter, P.; du Bois, A.; Hahmann, M.; Hasenburg, A.; Burges, A.; Loibl, S.; Gropp, M.; Huober, J.; Fink, D.; Schröder, W.; et al. Surgery in recurrent ovarian cancer: The Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) DESKTOP OVAR trial. Ann. Surg. Oncol. 2006, 13, 1702–1710. [Google Scholar] [CrossRef]
- Rufián, S.; Muñoz-Casares, F.C.; Briceño, J.; Díaz, C.J.; Rubio, M.J.; Ortega, R.; Ciria, R.; Morillo, M.; Aranda, E.; Muntané, J.; et al. Radical surgery-peritonectomy and intraoperative intraperitoneal chemotherapy for the treatment of peritoneal carcinomatosis in recurrent or primary ovarian cancer. J. Surg. Oncol. 2006, 94, 316–324. [Google Scholar] [CrossRef]
- Park, J.Y.; Seo, S.S.; Kang, S.; Lee, K.B.; Lim, S.Y.; Choi, H.S.; Park, S.Y. The benefits of low anterior en bloc resection as part of cytoreductive surgery for advanced primary and recurrent epithelial ovarian cancer patients outweigh morbidity concerns. Gynecol. Oncol. 2006, 103, 977–984. [Google Scholar] [CrossRef]
- Salani, R.; Santillan, A.; Zahurak, M.L.; Giuntoli, R.L., 2nd; Gardner, G.J.; Armstrong, D.K.; Bristow, R.E. Secondary cytoreductive surgery for localized, recurrent epithelial ovarian cancer: Analysis of prognostic factors and survival outcome. Cancer 2007, 109, 685–691. [Google Scholar] [CrossRef]
- Cheng, X.; Jiang, R.; Li, Z.T.; Tang, J.; Cai, S.M.; Zhang, Z.Y.; Tian, W.J.; Zang, R.Y. The role of secondary cytoreductive surgery for recurrent mucinous epithelial ovarian cancer (mEOC). Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2009, 35, 1105–1108. [Google Scholar] [CrossRef]
- Bae, J.; Lim, M.C.; Choi, J.H.; Song, Y.J.; Lee, K.S.; Kang, S.; Seo, S.S.; Park, S.Y. Prognostic factors of secondary cytoreductive surgery for patients with recurrent epithelial ovarian cancer. J. Gynecol. Oncol. 2009, 20, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Bristow, R.E.; Peiretti, M.; Gerardi, M.; Zanagnolo, V.; Ueda, S.; Diaz-Montes, T.; Giuntoli, R.L., 2nd; Maggioni, A. Secondary cytoreductive surgery including rectosigmoid colectomy for recurrent ovarian cancer: Operative technique and clinical outcome. Gynecol. Oncol. 2009, 114, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Hahmann, M.; Lueck, H.J.; Poelcher, M.; Wimberger, P.; Ortmann, O.; Canzler, U.; Richter, B.; Wagner, U.; Hasenburg, A.; et al. Surgery for recurrent ovarian cancer: Role of peritoneal carcinomatosis: Exploratory analysis of the DESKTOP I Trial about risk factors, surgical implications, and prognostic value of peritoneal carcinomatosis. Ann. Surg. Oncol. 2009, 16, 1324–1330. [Google Scholar] [CrossRef]
- Goto, T.; Takano, M.; Watanabe, A.; Miyamoto, M.; Kato, M.; Hirata, J.; Sasa, H.; Furuya, K. Potential survival benefit of secondary cytoreductive surgery for recurrent epithelial ovarian, tubal, and peritoneal cancers. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Fagotti, A.; Costantini, B.; Vizzielli, G.; Perelli, F.; Ercoli, A.; Gallotta, V.; Scambia, G.; Fanfani, F. HIPEC in recurrent ovarian cancer patients: Morbidity-related treatment and long-term analysis of clinical outcome. Gynecol. Oncol. 2011, 122, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Frederick, P.J.; Ramirez, P.T.; McQuinn, L.; Milam, M.R.; Weber, D.M.; Coleman, R.L.; Gershenson, D.M.; Landen, C.N., Jr. Preoperative factors predicting survival after secondary cytoreduction for recurrent ovarian cancer. Int. J. Gynecol. Cancer Off. J. Int. Gynecol. Cancer Soc. 2011, 21, 831–836. [Google Scholar] [CrossRef]
- Burton, E.; Chase, D.; Yamamoto, M.; de Guzman, J.; Imagawa, D.; Berman, M.L. Surgical management of recurrent ovarian cancer: The advantage of collaborative surgical management and a multidisciplinary approach. Gynecol. Oncol. 2011, 120, 29–32. [Google Scholar] [CrossRef] [PubMed]
- Ceelen, W.P.; Van Nieuwenhove, Y.; Van Belle, S.; Denys, H.; Pattyn, P. Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann. Surg. Oncol. 2012, 19, 2352–2359. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Kai, K.; Hirakawa, T.; Nishida, M.; Matsumoto, H.; Kawano, Y.; Narahara, H. Retrospective analysis of outcomes of secondary debulking surgery for recurrent epithelial ovarian cancer with favorable prognostic factors. J. Obstet. Gynaecol. Res. 2014, 40, 791–796. [Google Scholar] [CrossRef]
- Lee, C.K.; Lord, S.; Grunewald, T.; Gebski, V.; Hardy-Bessard, A.C.; Sehouli, J.; Woie, K.; Heywood, M.; Schauer, C.; Vergote, I.; et al. Impact of secondary cytoreductive surgery on survival in patients with platinum sensitive recurrent ovarian cancer: Analysis of the CALYPSO trial. Gynecol. Oncol. 2015, 136, 18–24. [Google Scholar] [CrossRef]
- Minaguchi, T.; Satoh, T.; Matsumoto, K.; Sakurai, M.; Ochi, H.; Onuki, M.; Oki, A.; Yoshikawa, H. Proposal for selection criteria of secondary cytoreductive surgery in recurrent epithelial ovarian, tubal, and peritoneal cancers. Int. J. Clin. Oncol. 2016, 21, 573–579. [Google Scholar] [CrossRef]
- So, M.; Miyamoto, T.; Murakami, R.; Abiko, K.; Hamanishi, J.; Baba, T.; Mandai, M. The efficacy of secondary cytoreductive surgery for recurrent ovarian, tubal, or peritoneal cancer in Tian-model low-risk patients. J. Gynecol. Oncol. 2019, 30, e100. [Google Scholar] [CrossRef]
- Zivanovic, O.; Chi, D.S.; Zhou, Q.; Iasonos, A.; Konner, J.A.; Makker, V.; Grisham, R.N.; Brown, A.K.; Nerenstone, S.; Diaz, J.P.; et al. Secondary Cytoreduction and Carboplatin Hyperthermic Intraperitoneal Chemotherapy for Platinum-Sensitive Recurrent Ovarian Cancer: An MSK Team Ovary Phase II Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2021, 39, 2594–2604. [Google Scholar] [CrossRef]
- Leitao, M.M., Jr.; Kardos, S.; Barakat, R.R.; Chi, D.S. Tertiary cytoreduction in patients with recurrent ovarian carcinoma. Gynecol. Oncol. 2004, 95, 181–188. [Google Scholar] [CrossRef]
- Shih, K.K.; Chi, D.S.; Barakat, R.R.; Leitao, M.M., Jr. Tertiary cytoreduction in patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer: An updated series. Gynecol. Oncol. 2010, 117, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Fotopoulou, C.; Richter, R.; Braicu, I.E.; Schmidt, S.C.; Neuhaus, P.; Lichtenegger, W.; Sehouli, J. Clinical outcome of tertiary surgical cytoreduction in patients with recurrent epithelial ovarian cancer. Ann. Surg. Oncol. 2011, 18, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Karam, A.K.; Santillan, A.; Bristow, R.E.; Giuntoli, R., 2nd; Gardner, G.J.; Cass, I.; Karlan, B.Y.; Li, A.J. Tertiary cytoreductive surgery in recurrent ovarian cancer: Selection criteria and survival outcome. Gynecol. Oncol. 2007, 104, 377–380. [Google Scholar] [CrossRef] [PubMed]
- Gultekin, M.; Velipaşaoğlu, M.; Aksan, G.; Dursun, P.; Dogan, N.U.; Yuce, K.; Ayhan, A. A third evaluation of tertiary cytoreduction. J. Surg. Oncol. 2008, 98, 530–534. [Google Scholar] [CrossRef]
- Hızlı, D.; Boran, N.; Yılmaz, S.; Turan, T.; Altınbaş, S.K.; Celik, B.; Köse, M.F. Best predictors of survival outcome after tertiary cytoreduction in patients with recurrent platinum-sensitive epithelial ovarian cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 163, 71–75. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Zang, R.; Gultekin, M.; Cibula, D.; Ayhan, A.; Liu, D.; Richter, R.; Braicu, I.; Mahner, S.; Harter, P.; et al. Value of tertiary cytoreductive surgery in epithelial ovarian cancer: An international multicenter evaluation. Ann. Surg. Oncol. 2013, 20, 1348–1354. [Google Scholar] [CrossRef]
- Manning-Geist, B.L.; Chi, D.S.; Long Roche, K.; Zivanovic, O.; Sonoda, Y.; Gardner, G.J.; O’Cearbhaill, R.E.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Tertiary cytoreduction for recurrent ovarian carcinoma: An updated and expanded analysis. Gynecol. Oncol. 2021, 162, 345–352. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Savvatis, K.; Kosian, P.; Braicu, I.E.; Papanikolaou, G.; Pietzner, K.; Schmidt, S.C.; Sehouli, J. Quaternary cytoreductive surgery in ovarian cancer: Does surgical effort still matter? Br. J. Cancer 2013, 108, 32–38. [Google Scholar] [CrossRef][Green Version]
- Bacalbaşa, N.; Balescu, I.; Dima, S.; Brasoveanu, V.; Popescu, I. The Role of Quaternary Cytoreduction in Recurrent Epithelial Ovarian Cancer: A Single-center Experience. Anticancer Res. 2015, 35, 3519–3523. [Google Scholar]
- Fanfani, F.; Fagotti, A.; Ercoli, A.; Gallotta, V.; Chiantera, V.; Restaino, S.; Monterossi, G.; Scambia, G. Is There a Role for Tertiary (TCR) and Quaternary (QCR) Cytoreduction in Recurrent Ovarian Cancer? Anticancer Res. 2015, 35, 6951–6955. [Google Scholar]
- Manning-Geist, B.L.; Chi, D.S.; Long Roche, K.; Zivanovic, O.; Sonoda, Y.; Gardner, G.J.; O’Cearbhaill, R.E.; Abu-Rustum, N.R.; Leitao, M.M., Jr. Quaternary and beyond cytoreduction: An updated and expanded analysis. Gynecol. Oncol. Rep. 2021, 37, 100851. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, C.; Blank, S.V.; Goff, B.A.; Judson, P.L.; Teneriello, M.G.; Husain, A.; Sovak, M.A.; Yi, J.; Nycum, L.R. OCEANS: A randomized, double-blind, placebo-controlled phase III trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent epithelial ovarian, primary peritoneal, or fallopian tube cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2012, 30, 2039–2045. [Google Scholar] [CrossRef]
- Baek, M.H.; Park, E.Y.; Ha, H.I.; Park, S.Y.; Lim, M.C.; Fotopoulou, C.; Bristow, R.E. Secondary Cytoreductive Surgery in Platinum-Sensitive Recurrent Ovarian Cancer: A Meta-Analysis. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Li, Z. Prediction Models for Complete Resection in Secondary Cytoreductive Surgery of Patients with Recurrent Ovarian Cancer. Front. Oncol. 2021, 11, 674637. [Google Scholar] [CrossRef]
- Bogani, G.; Rossetti, D.; Ditto, A.; Martinelli, F.; Chiappa, V.; Mosca, L.; Leone Roberti Maggiore, U.; Ferla, S.; Lorusso, D.; Raspagliesi, F. Artificial intelligence weights the importance of factors predicting complete cytoreduction at secondary cytoreductive surgery for recurrent ovarian cancer. J. Gynecol. Oncol. 2018, 29, e66. [Google Scholar] [CrossRef]
- Ding, T.; Tang, D.; Xi, M. The survival outcome and complication of secondary cytoreductive surgery plus chemotherapy in recurrent ovarian cancer: A systematic review and meta-analysis. J. Ovarian Res. 2021, 14, 93. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).