Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania
Abstract
1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
2.3. Treatment
2.4. Treatment Monitoring
2.5. Study Limitations
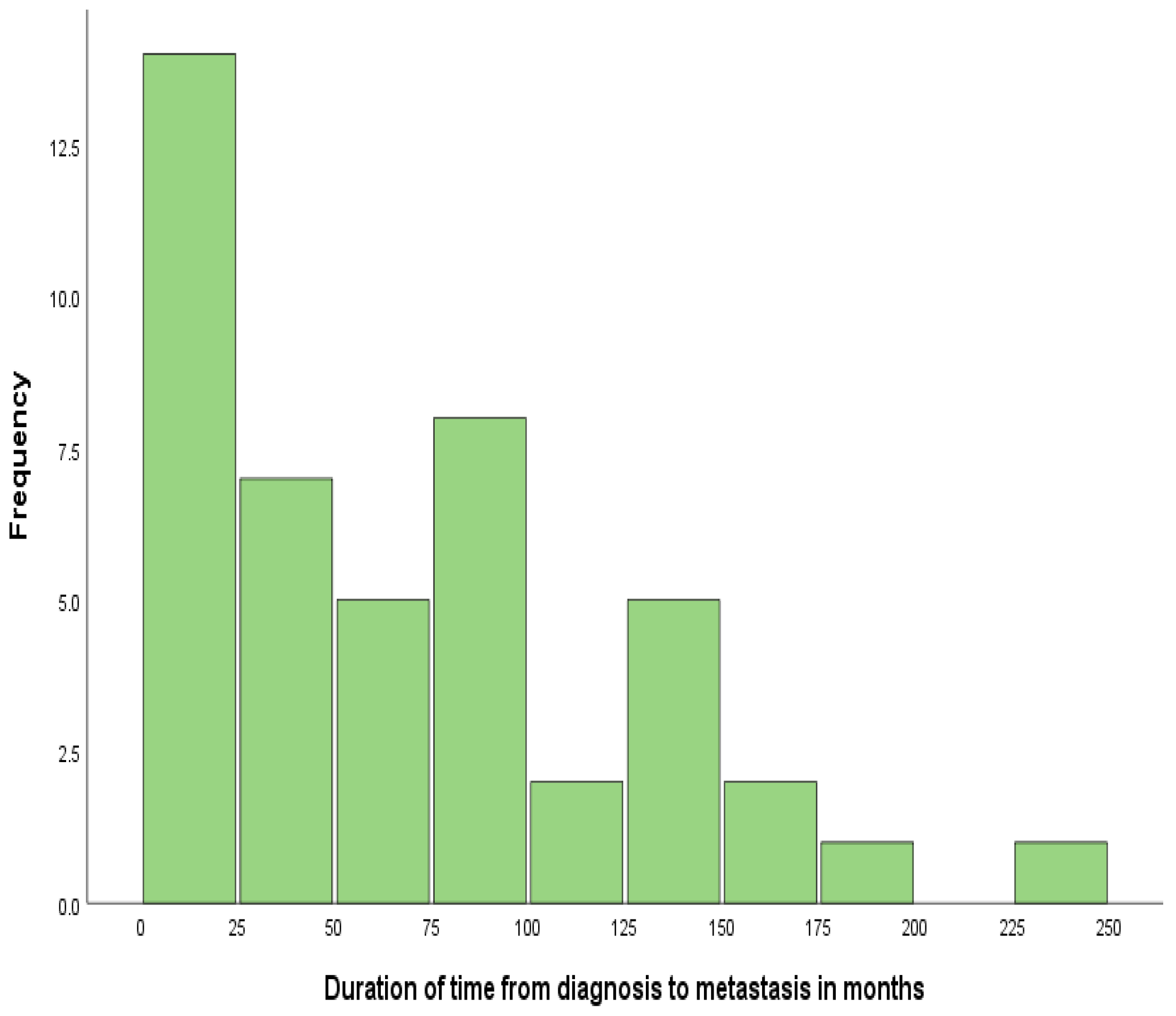
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society: Breast Cancer Facts & Figures 2015–2016. Available online: http://www.cancer.org/acs/groups/content/@research/documents/document/acspc-046381.pdfGoogleScholar (accessed on 5 February 2023).
- Globocan 2020. Available online: https://www.uicc.org/news/globocan-2020-new-global-cancer-data (accessed on 5 February 2023).
- Chia, S.K.; Speers, C.H.; D’yachkova, Y.; Kang, A.; Malfair-Taylor, S.; Barnett, J.; Coldman, A.; Gelmon, K.A.; O’Reilly, S.E.; Olivotto, I.A.; et al. The impact of new chemotherapeutic and hormone agents on survival in a population-based cohort of women with metastatic breast cancer. Cancer 2007, 110, 973. [Google Scholar] [CrossRef] [PubMed]
- Koleva-Kolarova, R.G.; Oktora, M.P.; Robijn, A.L.; Greuter, M.J.; Reyners, A.K.; Buskens, E.; de Bock, G.H. Increased life expectancy as a result of non-hormonal targeted therapies for HER2 or hormone receptor positive metastatic breast cancer: A systematic review and meta-analysis. Cancer Treat. Rev. 2017, 55, 16. [Google Scholar] [CrossRef] [PubMed]
- Dawood, S.; Haaland, B.; Albaracin, C.; Gupta, S.; Cortes, J.; Sim, Y.Y.; Dent, R.A. Is the Proportion of Patients Diagnosed with Synchronous Stage IV Breast Cancer Who Survive More than Two Years Increasing over Time? Oncology 2015, 89, 79. [Google Scholar] [CrossRef] [PubMed]
- Gobbini, E.; Ezzalfani, M.; Dieras, V.; Bachelot, T.; Brain, E.; Debled, M.; Jacot, W.; Mouret-Reynier, M.A.; Goncalves, A.; Dalenc, F.; et al. Time trends of overall survival among metastatic breast cancer patients in the real-life ESME cohort. Eur. J. Cancer 2018, 96, 17. [Google Scholar] [CrossRef]
- Fietz, T.; Tesch, H.; Rauh, J.; Boller, E.; Kruggel, L.; Jänicke, M.; Marschner, N. Palliative systemic therapy and overall survival of 1395 patients with advanced breast cancer—Results from the prospective German TMK cohort study. Breast 2017, 34, 122. [Google Scholar] [CrossRef]
- Greenberg, P.A.; Hortobagyi, G.N.; Smith, T.L.; Ziegler, L.D.; Frye, D.K.; Buzdar, A.U. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J. Clin. Oncol. 1996, 14, 2197. [Google Scholar] [CrossRef]
- Kiely, B.E.; Soon, Y.Y.; Tattersall, M.H.; Stockler, M.R. How much time do I have Estimating typical, best, and worst case scenarios for patients starting first-line chemotherapy for metastatic breast cancer: A systematic review of recent randomized trials. J. Clin. Oncol. 2011, 29, 456. [Google Scholar] [CrossRef]
- Giordano, S.H.; Buzdar, A.U.; Smith, T.L.; Kau, S.-W.; Yang, Y.; Hortobagyi, G.N. Is breast cancer survival improving? Cancer 2004, 100, 44. [Google Scholar] [CrossRef]
- Mauri, D.; Polyzos, N.P.; Salanti, G.; Pavlidis, N.; Ioannidis, J.P.A. Multiple treatment meta-analysis of chemotherapy and targeted therapies in advanced breast cancer. J. Natl. Cancer Inst. 2008, 100, 1780. [Google Scholar] [CrossRef]
- Dawood, S.; Broglio, K.; Buzdar, A.U.; Hortobagyi, G.N.; Giordano, S.H. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional review. J. Clin. Oncol. 2010, 28, 92. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response assessment criteria in solid tumors: The revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228. [Google Scholar] [CrossRef] [PubMed]
- Sledge, G.W.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. MONARCH 2: Abemaciclib in combination with fulvestrant in women with HR+/HER2- advanced breast cancer who had progressed while receiving endocrine therapy. J. Clin. Oncol. 2017, 35, 2875–2884. [Google Scholar] [CrossRef]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [PubMed]
- Knudsen, E.S.; Knudsen, K.E. Tailoring to RB: Tumour suppressor status and therapeutic response. Nat. Rev. Cancer 2008, 8, 714–724. [Google Scholar] [CrossRef]
- VanArsdale, T.; Boshoff, C.; Arndt, K.T.; Abraham, R.T. Molecular pathways: Targeting the cyclin D-CDK4/6 axis for cancer treatment. Clin. Cancer Res. 2015, 21, 2905–2910. [Google Scholar] [CrossRef] [PubMed]
- Torres-Guzman, R.; Calsina, B.; Hermoso, A.; Baquero, C.; Alvarez, B.; Amat, J.; McNulty, A.M.; Gong, X.; Boehnke, K.; Du, J.; et al. Preclinical characterization of abemaciclib in hormone receptor positive breast cancer. Oncotarget 2017, 8, 69493–69507. [Google Scholar] [CrossRef]
- Finn, R.S.; Dering, J.; Conklin, D.; Kalous, O.; Cohen, D.J.; Desai, A.J.; Ginther, C.; Atefi, M.; Chen, I.; Fowst, C.; et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009, 11, R77. [Google Scholar] [CrossRef]
- National Cancer Institute Cancer Therapy Evaluation Program: Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available online: http://Ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htmGoogleScholar (accessed on 3 April 2023).
- Ibrance, INN-Palbociclib (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/ibranceScholar (accessed on 20 February 2023).
- Kisqali, INN-Ribociclib (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/kisqali (accessed on 22 February 2023).
- Verzenios; INN-Abemaciclib (europa.eu). Available online: https://www.ema.europa.eu/en/medicines/human/EPAR/verzenios (accessed on 25 February 2023).
- Scarlat, F.; Scarisoreanu, A.; Verga, N. Absorbed dose distributions using the isodensitometric method for exposures with filter employed for mammographies. Rom. Rep. Phys. 2013, 65, 168–177. [Google Scholar]
- Knudsen, E.S.; Kumarasamy, V.; Nambiar, R.; Pearson, J.D.; Vail, P.; Rosenheck, H.; Wang, J.; Eng, K.; Bremner, R.; Schramek, D.; et al. CDK/cyclin dependencies define extreme cancer cell-cycle heterogeneity and collateral vulnerabilities. Cell Rep. 2022, 38, 110448. [Google Scholar] [CrossRef]
- Al-Qasem, A.J.; Alves, C.L.; Ditzel, H.J. Resistance Mechanisms to Combined CDK4/6 Inhibitors and Endocrine Therapy in ER+/HER2- Advanced Breast Cancer: Biomarkers and Potential Novel Treatment Strategies. Cancers 2021, 13, 5397. [Google Scholar] [CrossRef]
- Asociatia Oncohelp-Centrul de Oncologie Oncohelp-“Study of Real-World Evidence in Patients Treated with Palbociclib during a 2,5 Tears Follow-Up Period (PALBO)”. Available online: https://clinicaltrials.gov/ct2/show/NCT05135104 (accessed on 3 April 2023).
- Stanciu, I.-M.; Parosanu, A.I.; Orlov-Slavu, C.; Iaciu, I.C.; Popa, A.M.; Olaru, C.M.; Pirlog, C.F.; Vrabie, R.C.; Nitipir, C. Mechanisms of Resistance to CDK4/6 Inhibitors and Predictive Biomarkers of Response in HR+/HER2-Metastatic Breast Cancer—A Review of the Literature. Diagnostics 2023, 13, 987. [Google Scholar] [CrossRef] [PubMed]
- Palbociclib (IBRANCE Capsules); FDA: Silver Spring, MD, USA, 2016.
- Syed, Y.Y. Ribociclib: First Global Approval. Drugs 2017, 77, 799–807. [Google Scholar] [CrossRef]
- Royce, M.; Osgood, C.; Mulkey, F.; Bloomquist, E.; Pierce, W.F.; Roy, A.; Shyam, K.; Soma, G.; Reena, P.; Fatima, R.; et al. FDA Approval Summary: Abemaciclib With Endocrine Therapy for High-Risk Early Breast Cancer. J. Clin. Oncol. 2022, 40, 1155–1162. [Google Scholar] [CrossRef]
- Lista Protocoalelor Terapeutice—Iulie 2019.pdf. Available online: http://cas.cnas.ro/casdb/page/protocoale-terapeutice.html (accessed on 3 April 2023).
- Teodorescu, C.O.D.; Șandru, F.; Charkaoui, A.; Teodorescu, A.; Popa, A.R.; Miron, A.-I. The dynamic changes in the pattern of liver function tests in pregnant obese women. Exp. Ther. Med. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- CNAS a Actualizat Din Nou Lista Medicamentelor Decontate Pentru Pacienți în Cadrul Programelor Naționale de Sănătate—360medical.ro. Informare-Clarificare-Numar-Pacienti-Eligibili-DCI-Abemaciclib.pdf. Available online: http://cas.cnas.ro/page/lista-medicamentelor-2021.html (accessed on 3 April 2023).
- Coniac, S.; Costache Outas, M.C.; Pirvu, E.-E.; Patru, R.-I.; Gainariu, E.; Aldea, C.; Iorga, P.G.; Ambroci, M.; Liscu, H.-D.; Miron, A.-I.; et al. Challenges and Limitations of Endocrine Toxicity Evaluation in Non-Small Cell Lung Cancer Patients Treated with Immunotherapy—Retrospective Study from a Tertiary-Level Hospital in Romania. Diagnostics 2023, 13, 1788. [Google Scholar] [CrossRef] [PubMed]
- Groza, A.; Iconaru, S.L.; Jiga, G.; Chapon, P.; Gaiaschi, S.; Verga, N.; Beuran, M.; Prodan, A.M.; Matei, M.; Marinescu, S.A.; et al. The effect of the ionizing radiation on hydroxyapatite–polydimethylsiloxane layers. Polym. Eng. Sci. 2019, 59, 2406–2412. [Google Scholar] [CrossRef]
- Loibl, S.; Turner, N.C.; Ro, J.; Cristofanilli, M.; Iwata, H.; Im, S.A.; Masuda, N.; Loi, S.; André, F.; Harbeck, N.; et al. Palbociclib Combined with Fulvestrant in Premenopausal Women with Advanced Breast Cancer and Prior Progression on Endocrine Therapy: PALOMA-3 Results. Oncologist 2017, 22, 1028–1038. [Google Scholar] [CrossRef]
- Matthew, P.; Toi, G.M.; Campone, M.; Sohn, J.; Paluch-Shimon, S.; Huober, J.; Park, H.; Trédan, O.; Chen, S.-C.; Manso, L.; et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J. Clin. Oncol. 2017, 35, 3638–3646. [Google Scholar]
- Mariana-Iuliana, G.; Ionescu, R.T.; Miron, A.-I. Diversity-Promoting Ensemble for Medical Image Segmentation. arXiv 2022, arXiv:2210.12388. [Google Scholar]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martin, M.; et al. Fasching Updated overall survival (OS) results from the phase III MONALEESA-3 trial of postmenopausal patients (pts) with HR+/HER2- advanced breast cancer (ABC) treated with fulvestrant (FUL) ± ribociclib (RIB). J. Clin. Oncol. 2021, 39 (Suppl. 15), 1001. [Google Scholar] [CrossRef]
- Coniac, S. Actualizări în evenimentele adverse legate de imunitatea endocrină în imunoterapia oncologică. Acta Endocrinol. 2021, 17, 286–289. [Google Scholar] [CrossRef]
- Varlas, V.N.; Angelescu, G. Provocările unei metastate neuroendocrine ovariene ale carcinomului pulmonar cu celule mici avansate—Revizuirea literaturii și raportul de caz. Acta Endocrinol. 2021, 17, 251–258. [Google Scholar] [CrossRef]




| Frequency | Percent | Cumulative Percent | |
|---|---|---|---|
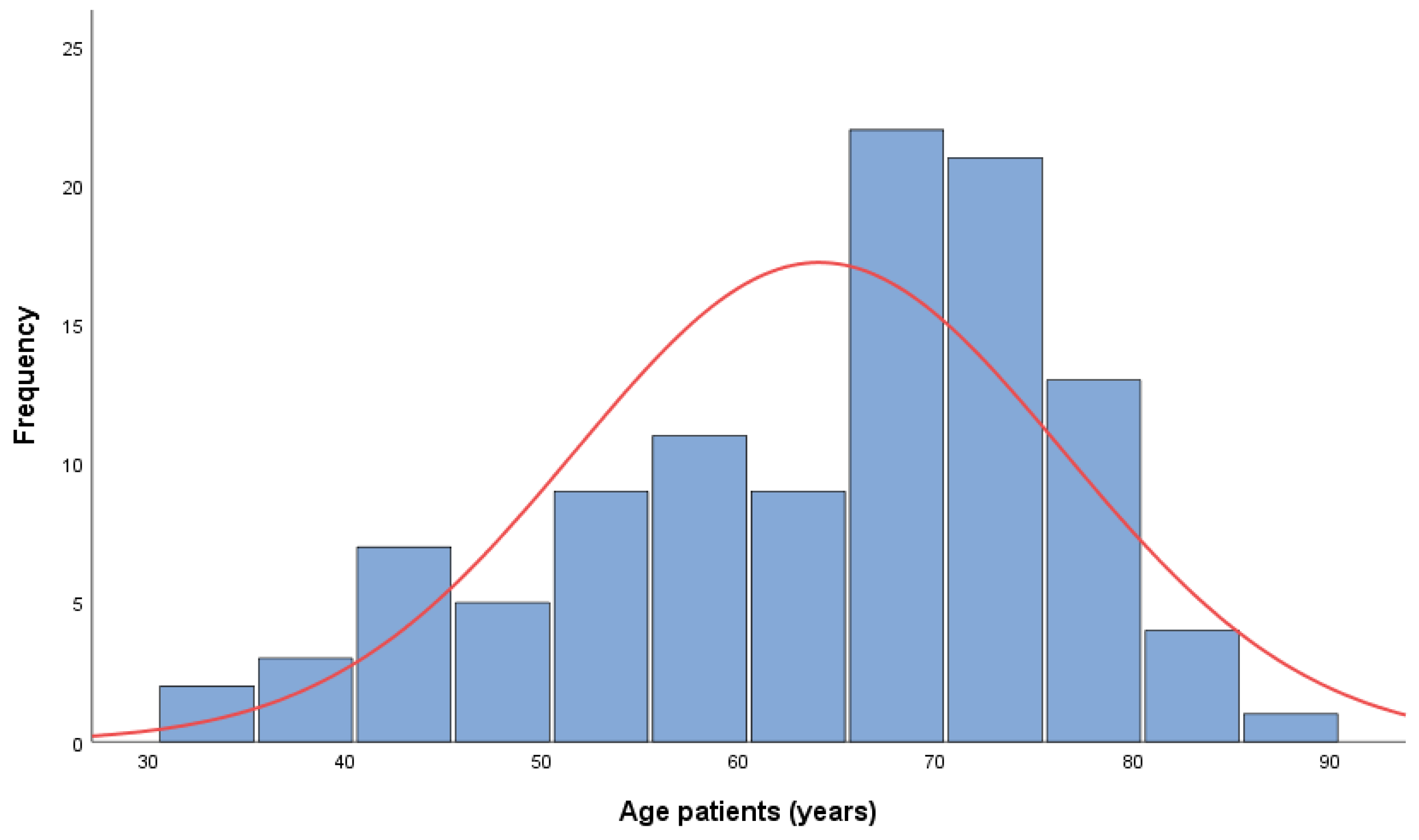
| 18–49 years | 16 | 15.0 | 15.0 |
| 50–64 years | 28 | 26.2 | 41.1 |
| 65–74 years | 42 | 39.3 | 80.4 |
| >75 years | 21 | 19.6 | 100.0 |
| Total | 107 | 100.0 |
| Menopausal Status | Frequency | Percent | Cumulative Percent |
|---|---|---|---|
| Postmenopausal | 85 | 79.4 | 79.4 |
| Premenopausal | 22 | 20.6 | 100.0 |
| Total | 107 | 100.0 |
| IHC | Frequency | Percent | Cumulative Percent |
|---|---|---|---|
| Luminal A | 44 | 42.3 | 42.3 |
| Luminal B | 60 | 57.7 | 100.0 |
| Total | 104 | 100.0 | |
| Test Statistics | |||
| Chi-Square | 2.701 | ||
| Df | 1 | ||
| Asymp. Sig. | 0.100 | ||
| Frequency | Percent | Cumulative Percent | |
|---|---|---|---|
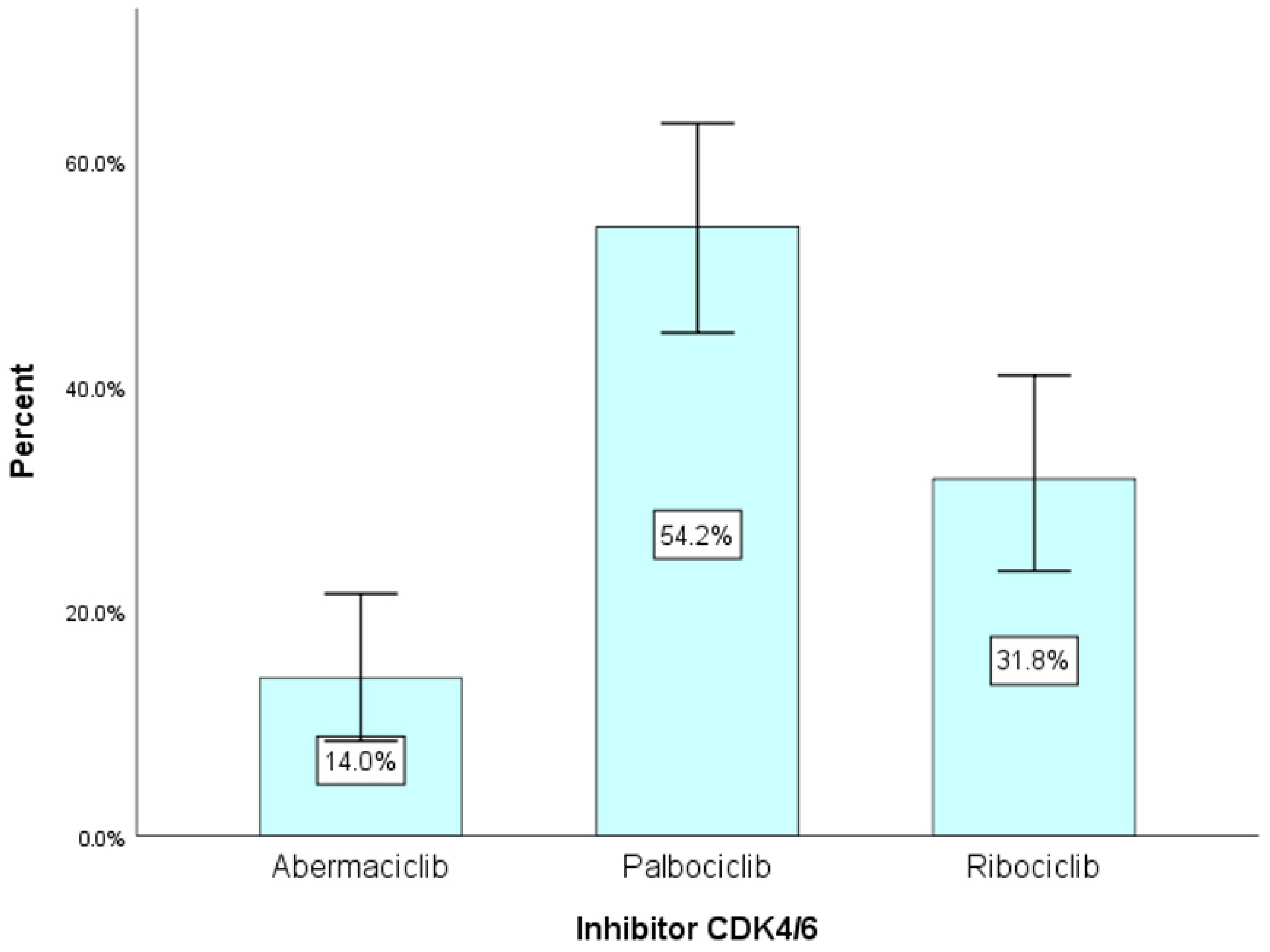
| Abemaciclib | 15 | 14.0 | 14.0 |
| Palbociclib | 58 | 54.2 | 68.2 |
| Ribociclib | 34 | 31.8 | 100 |
| Total | 107 | 100.0 |
| CDK4/6 Inhibitors Endocrine Therapy Crosstabulation | ||||||
|---|---|---|---|---|---|---|
| Endocrine Therapy | Total | |||||
| Anostrozole | Fulvestrant | Letrozole | ||||
| CDK4/6 Inhibitors | Abemaciclib | 15 | 3 | 1 | 11 | 15 |
| 100.0% | 20.0% | 6.7% | 73.3% | 100.0% | ||
| Palbociclib | 58 | 9 | 25 | 24 | 58 | |
| 100.0% | 15.5% | 43.1% | 41.4% | 100.0% | ||
| 2.5 | 4.9 | −7.4 | ||||
| Ribociclib | 34 | 0 | 11 | 23 | 34 | |
| 100.0% | 0.0% | 32.4% | 67.6% | 100.0% | ||
| Total | Count | 107 | 37 | 58 | 107 | |
| % | 100.0% | 34.6% | 54.2% | 100.0% | ||
| Chi-Square Tests | ||||||
| Value | df | Asymptotic Significance (2-sided) | ||||
| Pearson Chi-Square | 14.36 | 4 | 0.006 | |||
| Likelihood Ratio | 19.46 | 4 | 0.001 | |||
| Linear-by-Linear Association | 2.16 | 1 | 0.142 | |||
| N of Valid Cases | 107 | |||||
| Stage | Frequency | Percent | Cumulative Percent | |||
|---|---|---|---|---|---|---|
| IIA | 17 | |||||
| IIB | 8 | 23.4 | 23.4 | |||
| IIIA | 7 | |||||
| IIIB | 11 | |||||
| IIIC | 2 | 18.7 | 42.1 | |||
| IV | 62 | 57.9 | 100 | |||
| Total | 107 | 100.0 | ||||
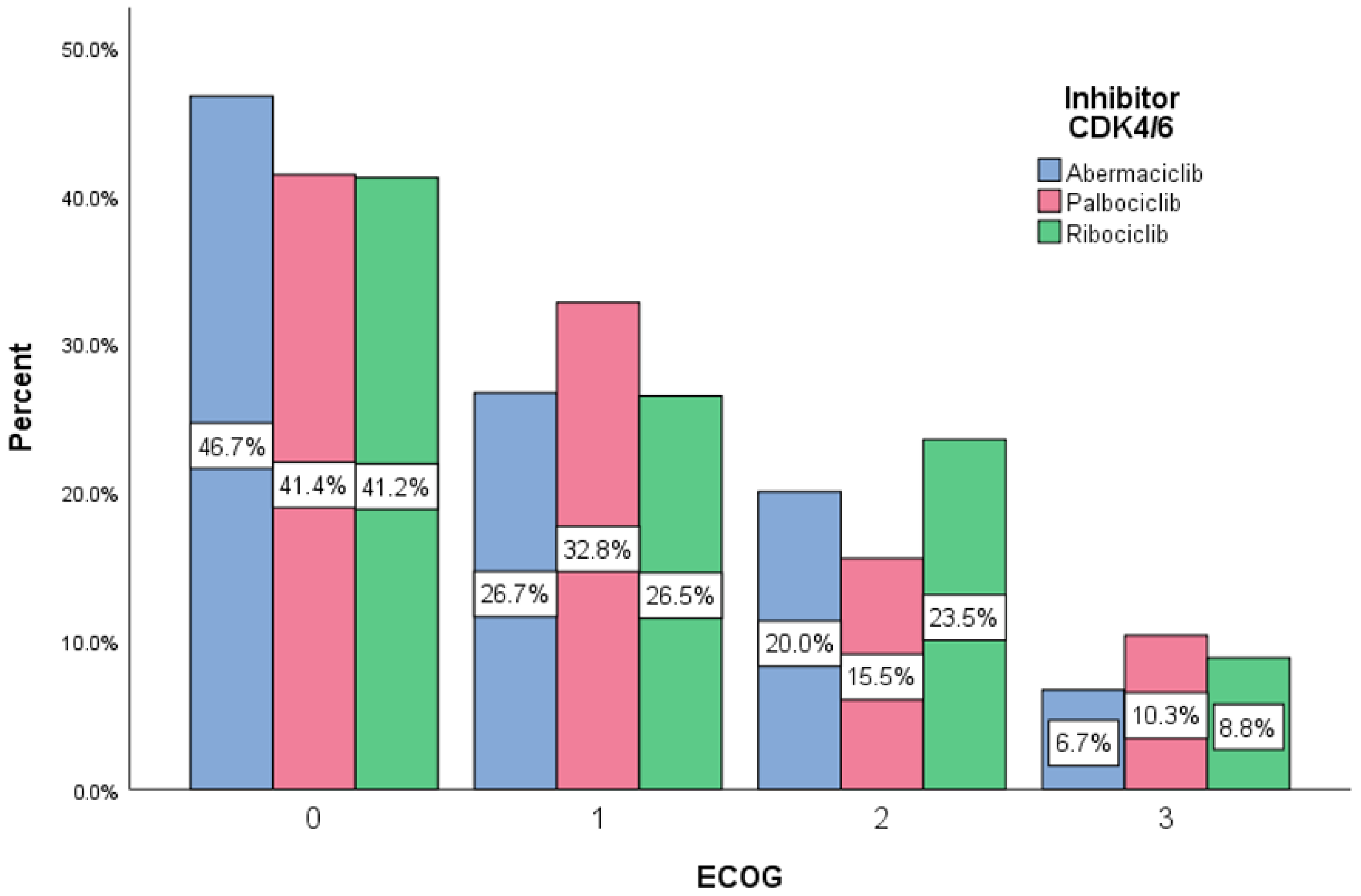
| CDK4/6 Inhibitor STAGE Crosstabulation | Disease stage | Total | ||||
| II | III | IV | ||||
| InhibCDK4/6 | Abemaciclib | Count | 4 | 2 | 9 | 15 |
| % | 26.7 | 13.3 | 60.0 | 100.0% | ||
| Palbociclib | Count | 10 | 13 | 35 | 58 | |
| % | 17.2 | 22.4 | 60.4 | 100.0% | ||
| Ribociclib | Count | 11 | 5 | 18 | 34 | |
| % | 32.4 | 14.7 | 52.9 | 100.0% | ||
| Total | Count | 25 | 20 | 62 | 107 | |
| % | 23.4 | 18.7 | 57.9 | 100.0% | ||
| Chi-Square Tests | Value | df | Asymptotic Significance (2-sided) | |||
| Pearson Chi-Square | 3.34 | 4 | 0.502 | |||
| Likelihood Ratio | 3.33 | 4 | 0.504 | |||
| N of Valid Cases | 107 | |||||
| Visceral | Frequency | Percent | Cumulative Percent | |||||
|---|---|---|---|---|---|---|---|---|
| Yes | 72 | 67.3 | 67.3 | |||||
| No | 35 | 32.7 | 100.0 | |||||
| Non-visceral | ||||||||
| Yes | 78 | 73.6 | 73.6 | |||||
| No | 28 | 26.4 | 100.0 | |||||
| Total | 104 | 100.0 | ||||||
| Visceral Metastasis | Total | |||||||
| No | Yes | |||||||
| CDK4/6 Inhibitors | Abemaciclib | Count | 4 | 11 | 15 | |||
| % | 26.7% | 73.3% | 100.0% | |||||
| Palbociclib | Count | 18 | 40 | 58 | ||||
| % | 31.0% | 69.0% | 100.0% | |||||
| Ribociclib | Count | 13 | 21 | 34 | ||||
| % | 38.2% | 61.8% | 100.0% | |||||
| Total | Count | 35 | 72 | 107 | ||||
| % | 32.7% | 67.3% | 100.0% | |||||
| Chi-Square Tests | Value | Df | Asymptotic Significance (2-sided) | |||||
| Pearson Chi-Square | 0.794 | 2 | 0.672 | |||||
| Likelihood Ratio | 0.792 | 2 | 0.673 | |||||
| N of Valid Cases | 107 | |||||||
| Non-visceral Metastasis | Total | |||||||
| No | Yes | |||||||
| CDK4/6 Inhibitors | Abemaciclib | Count | 6 | 8 | 14 | |||
| % | 46.7% | 53.3% | 100.0% | |||||
| Palbociclib | Count | 13 | 45 | 58 | ||||
| % | 22.4% | 77.6% | 100.0% | |||||
| Ribociclib | Count | 8 | 26 | 34 | ||||
| % | 23.5% | 76.5% | 100.0% | |||||
| Total | Count | 28 | 78 | 106 | ||||
| % | 26.4% | 73.6% | 100.0% | |||||
| Chi-Square Tests | Value | df | Asymptotic Significance (2-sided) | |||||
| Pearson Chi-Square | 4.630 | 2 | 0.099 | |||||
| Likelihood Ratio | 4.167 | 2 | 0.124 | |||||
| N of Valid Cases | 106 | |||||||
| Nr of Sites | Frequency | Percent | Cumulative Percent | ||||
|---|---|---|---|---|---|---|---|
| 1 | 40 | 37.4 | 37.4 | ||||
| 2 | 33 | 30.8 | 68.2 | ||||
| 3 | 18 | 16.8 | 85.0 | ||||
| 4–7 | 16 | 15.0 | 100.0 | ||||
| Total | 104 | 100.0 | |||||
| Inhibitor CDK4/6 Nr of sites | Number of metastatic sites | Total | |||||
| 1 | 2 | 3 | 4 | ||||
| CDK4/6 Inhibitors | Abemaciclib | Count | 9 | 2 | 3 | 1 | 15 |
| % | 60.0% | 13.3% | 20.0% | 6.7% | 100.0% | ||
| Palbociclib | Count | 15 | 24 | 11 | 8 | 58 | |
| % | 25.9% | 41.4% | 19.0% | 13.8% | 100.0% | ||
| Ribociclib | Count | 16 | 7 | 4 | 7 | 34 | |
| % | 47.1% | 20.6% | 11.8% | 20.6% | 100.0% | ||
| Total | Count | 40 | 33 | 18 | 16 | 107 | |
| % | 37.4% | 30.8% | 16.8% | 15.0% | 100.0% | ||
| Chi-Square Tests | Value | Df | Asymptotic Significance (2-sided) | ||||
| Pearson Chi-Square | 11.930 a | 6 | 0.064 | ||||
| Likelihood Ratio | 12.310 | 6 | 0.055 | ||||
| N of Valid Cases | 107 | ||||||
| Parameter | B | Std. Error | Hypothesis Test | Exp(B) | Model Efect | ||
|---|---|---|---|---|---|---|---|
| Wald Chi-Square | df | Sig. | |||||
| (Intercept) | 1.67 | 0.215 | 65.51 | 1 | 0.000 | 5.29 | Wald Chi-square(2) = 3.51 p = 0.173 |
| [Inhibitor CDK4/6 = 3] | 0.19 | 0.25 | 0.54 | 1 | 0.462 | 1.21 | |
| [Inhibitor CDK4/6 = 2] | 0.40 | 0.24 | 2.89 | 1 | 0.089 | 1.50 | |
| [Inhibitor CDK4/6 = 1] | 0 | . | . | . | . | 1 | |
| Means and Medians for Survival Time | |||||||
|---|---|---|---|---|---|---|---|
| Mean a | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | ||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | ||||
| 17.61 | 1.39 | 14.89 | 20.32 | 12.00 | 1.36 | 9.34 | 14.66 |
| Means and Medians for Survival Time | ||||||||
|---|---|---|---|---|---|---|---|---|
| CDK4/6 Inhibitors | Mean a | Median | ||||||
| Estimate | Std. Error | 95% Confidence Interval | Estimate | Std. Error | 95% Confidence Interval | |||
| Lower Bound | Upper Bound | Lower Bound | Upper Bound | |||||
| Abemaciclib | 11.74 | 2.72 | 6.41 | 17.06 | 11.00 | 3.19 | 4.75 | 17.25 |
| Palbociclib | 22.90 | 2.16 | 18.67 | 27.12 | 25.00 | 5.06 | 15.09 | 34.91 |
| Ribociclib | 12.06 | 1.23 | 9.65 | 14.47 | 12.00 | 0.79 | 10.46 | 13.54 |
| Overall | 17.61 | 1.39 | 14.89 | 20.32 | 12.00 | 1.36 | 9.34 | 14.66 |
| Death | Total | ||||
|---|---|---|---|---|---|
| No | Yes | ||||
| CDK4/6 Inhibitors | Abemaciclib | Count | 13 | 2 | 15 |
| % | 86.7% | 13.3% | 100.0% | ||
| Palbociclib | Count | 42 | 16 | 58 | |
| % | 72.4% | 27.6% | 100.0% | ||
| Ribociclib | Count | 28 | 6 | 34 | |
| % | 82.4% | 17.6% | 100.0% | ||
| Total | Count | 83 | 24 | 107 | |
| % | 77.6% | 22.4% | 100.0% | ||
| Chi-Square Tests | Value | Df | Asymptotic Significance (2-sided) | ||
| Pearson Chi-Square | 2.05 | 2 | 0.359 | ||
| Likelihood Ratio | 2.12 | 2 | 0.347 | ||
| N of Valid Cases | 107 | ||||
| Inhibitor | B | Std. Err. | Test | Exp(B) | CI 95% exp(B) | |||
|---|---|---|---|---|---|---|---|---|
| Wald | df | Sig. | Lower | Upper | ||||
| Palbociclib | 0.91 | 0.81 | 1.24 | 1 | 0.266 | 2.48 | 0.50 | 12.22 |
| Ribociclib | 0.33 | 0.88 | 0.14 | 1 | 0.707 | 1.39 | 0.25 | 7.86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miron, A.-I.; Anghel, A.-V.; Barnonschi, A.-A.; Mitre, R.; Liscu, H.-D.; Găinariu, E.; Pătru, R.; Coniac, S. Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics 2023, 13, 1938. https://doi.org/10.3390/diagnostics13111938
Miron A-I, Anghel A-V, Barnonschi A-A, Mitre R, Liscu H-D, Găinariu E, Pătru R, Coniac S. Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics. 2023; 13(11):1938. https://doi.org/10.3390/diagnostics13111938
Chicago/Turabian StyleMiron, Andreea-Iuliana, Alexandra-Valentina Anghel, Andrei-Alexandru Barnonschi, Ruxandra Mitre, Horia-Dan Liscu, Estera Găinariu, Raluca Pătru, and Simona Coniac. 2023. "Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania" Diagnostics 13, no. 11: 1938. https://doi.org/10.3390/diagnostics13111938
APA StyleMiron, A.-I., Anghel, A.-V., Barnonschi, A.-A., Mitre, R., Liscu, H.-D., Găinariu, E., Pătru, R., & Coniac, S. (2023). Real-World Outcomes of CDK4/6 Inhibitors Treatment in Metastatic Breast Cancer in Romania. Diagnostics, 13(11), 1938. https://doi.org/10.3390/diagnostics13111938









