Current Understanding of and Future Directions for Endometriosis-Related Infertility Research with a Focus on Ferroptosis
Abstract
1. Introduction
2. Mechanisms Underlying Endometriosis-Related Infertility
2.1. Morphological Changes
2.2. Poor Oocyte Quality in Endometriosis
3. Oxidative–Antioxidant (Redox) Balance in Endometriosis
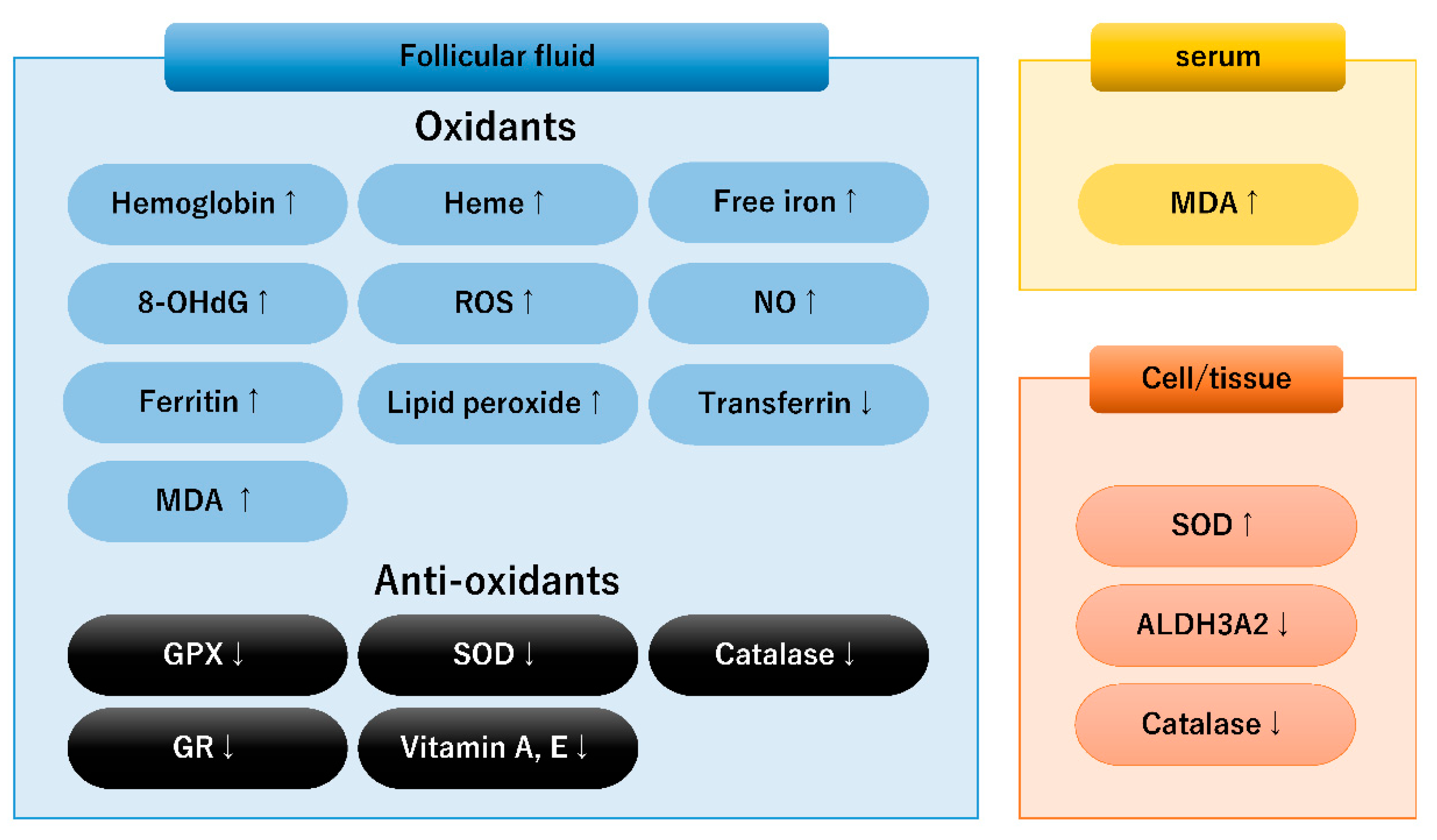
3.1. Biological Markers Involved in Redox Balance in Endometriosis
3.2. Oxidative Stress Caused by Iron Overload
3.3. Dysregulated Antioxidant Systems
4. Ferroptosis
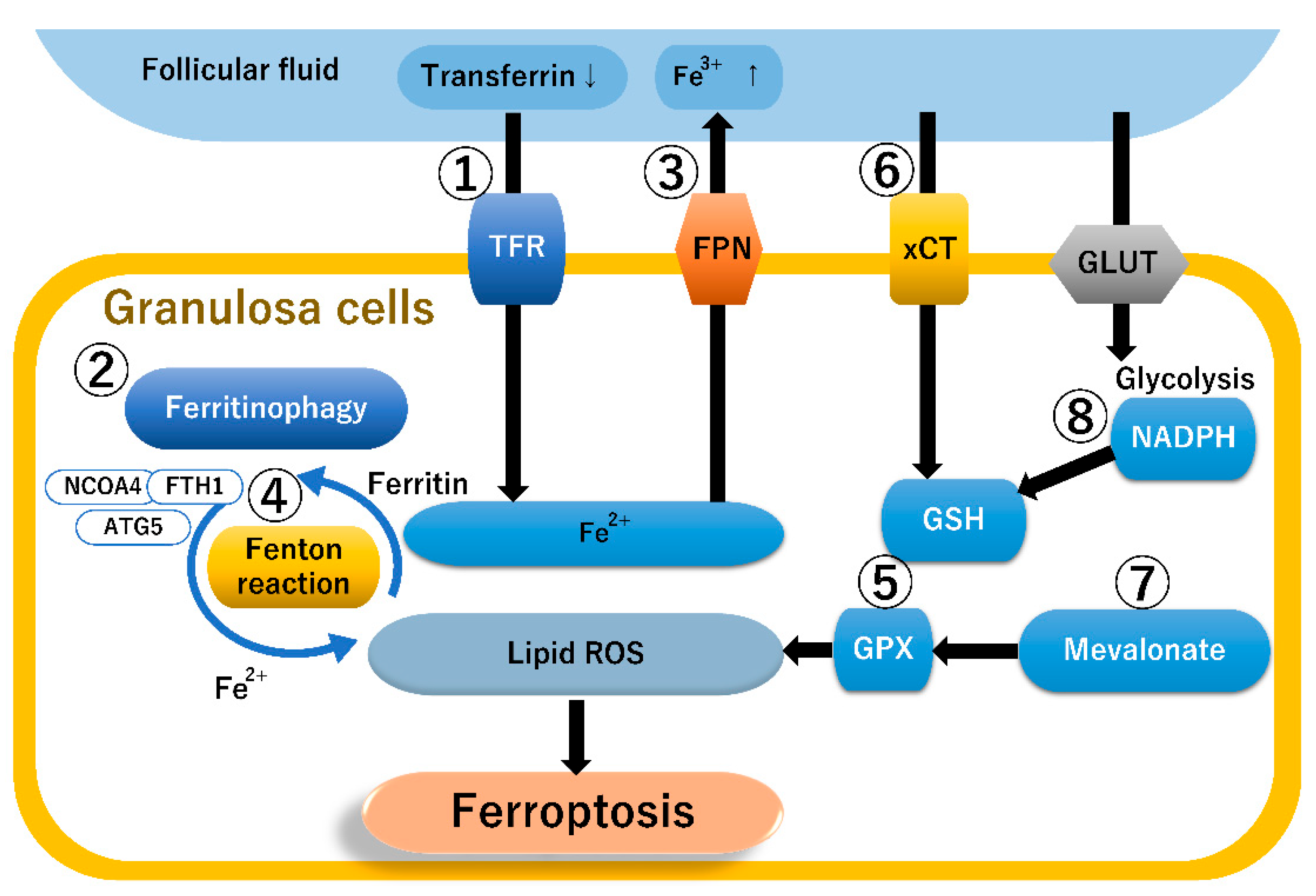
4.1. The Ferroptosis-Related Pathway
4.2. The Ferroptosis Pathway in Endometriosis
4.3. The Ferroptosis Pathway in Granulosa Cells
4.4. The Ferroptosis Pathway in Macrophages
5. Therapeutic Strategies Targeting Ferroptosis
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef] [PubMed]
- Tanbo, T.; Fedorcsak, P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta Obstet. Gynecol. Scand. 2017, 96, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.W. Fibrogenesis resulting from cyclic bleeding: The Holy Grail of the natural history of ectopic endometrium. Hum. Reprod. 2018, 33, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Evans, M.B.; Decherney, A.H. Fertility and Endometriosis. Clin. Obstet. Gynecol. 2017, 60, 497–502. [Google Scholar] [CrossRef]
- Kobayashi, H.; Shigetomi, H.; Imanaka, S. Nonhormonal therapy for endometriosis based on energy metabolism regulation. Reprod. Fertil. 2021, 2, C42–C57. [Google Scholar] [CrossRef]
- Maignien, C.; Santulli, P.; Gayet, V.; Lafay-Pillet, M.C.; Korb, D.; Bourdon, M.; Marcellin, L.; de Ziegler, D.; Chapron, C. Prognostic factors for assisted reproductive technology in women with endometriosis-related infertility. Am. J. Obstet. Gynecol. 2017, 216, 280.e1–280.e9. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Iwabuchi, T.; Yoshimoto, C.; Shigetomi, H.; Kobayashi, H. Cyst fluid hemoglobin species in endometriosis and its malignant transformation: The role of metallobiology. Oncol. Lett. 2016, 11, 3384–3388. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Y.; Huang, Q.; Fu, X.; Zhang, L.; Gao, F.; Jin, Z.; Wu, L.; Shu, C.; Zhang, X.; et al. Iron overload in endometriosis peritoneal fluid induces early embryo ferroptosis mediated by HMOX1. Cell Death Discov. 2021, 7, 355. [Google Scholar] [CrossRef]
- Hayashi, S.; Nakamura, T.; Motooka, Y.; Ito, F.; Jiang, L.; Akatsuka, S.; Iwase, A.; Kajiyama, H.; Kikkawa, F.; Toyokuni, S. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol. 2020, 37, 101726. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, X.; Lu, D.; Yin, M.; Shan, M.; Gao, Y. Erastin induces ferroptosis via ferroportin-mediated iron accumulation in endometriosis. Hum. Reprod. 2021, 36, 951–964. [Google Scholar] [CrossRef]
- Liang, Z.; Wu, Q.; Wang, H.; Tan, J.; Wang, H.; Gou, Y.; Cao, Y.; Li, Z.; Zhang, Z. Silencing of lncRNA MALAT1 facilitates erastin-induced ferroptosis in endometriosis through miR-145-5p/MUC1 signaling. Cell Death Discov. 2022, 8, 190. [Google Scholar] [CrossRef]
- Tonnus, W.; Linkermann, A. The in vivo evidence for regulated necrosis. Immunol. Rev. 2017, 277, 128–149. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. [Google Scholar] [CrossRef]
- Mahoney-Sánchez, L.; Bouchaoui, H.; Ayton, S.; Devos, D.; Duce, J.A.; Devedjian, J.C. Ferroptosis and its potential role in the physiopathology of Parkinson’s Disease. Prog. Neurobiol. 2021, 196, 101890. [Google Scholar] [CrossRef]
- Kitajima, M.; Dolmans, M.M.; Donnez, O.; Masuzaki, H.; Soares, M.; Donnez, J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil. Steril. 2014, 101, 1031–1037. [Google Scholar] [CrossRef]
- Ceviren, A.K.; Ozcelik, N.T.; Urfan, A.; Donmez, L.; Isikoglu, M. Characteristic cytoplasmic morphology of oocytes in endometriosis patients and its effect on the outcome of assisted reproduction treatments cycles. IVF Lite 2014, 1, 88–93. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Vanni, V.S.; Bartiromo, L.; Papaleo, E.; Zilberberg, E.; Candiani, M.; Orvieto, R.; Viganò, P. Is the oocyte quality affected by endometriosis? A review of the literature. J. Ovarian Res. 2017, 10, 43. [Google Scholar] [CrossRef]
- Borges, E., Jr.; Braga, D.P.; Setti, A.S.; Vingris, L.S.; Figueira, R.C.; Iaconelli, A., Jr. Endometriosis affects oocyte morphology in intracytoplasmic sperm injection cycles? JBRA Assist. Reprod. 2015, 19, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Goud, P.T.; Goud, A.P.; Joshi, N.; Puscheck, E.; Diamond, M.P.; Abu-Soud, H.M. Dynamics of nitric oxide, altered follicular microenvironment, and oocyte quality in women with endometriosis. Fertil. Steril. 2014, 102, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Guo, N.; Zhang, X.M.; Shi, W.; Tong, X.H.; Iqbal, F.; Liu, Y.S. Oocyte quality is decreased in women with minimal or mild endometriosis. Sci. Rep. 2015, 5, 10779. [Google Scholar] [CrossRef] [PubMed]
- Van Blerkom, J.; Davis, P.W.; Lee, J. ATP content of human oocytes and developmental potential and outcome after in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 415–424. [Google Scholar] [CrossRef]
- Eichenlaub-Ritter, U.; Wieczorek, M.; Lüke, S.; Seidel, T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 2011, 11, 783–796. [Google Scholar] [CrossRef]
- Fragouli, E.; Spath, K.; Alfarawati, S.; Kaper, F.; Craig, A.; Michel, C.E.; Kokocinski, F.; Cohen, J.; Munne, S.; Wells, D. Altered levels of mitochondrial DNA are associated with female age, aneuploidy, and provide an independent measure of embryonic implantation potential. PLoS Genet. 2015, 11, e1005241. [Google Scholar] [CrossRef]
- Ni, Z.; Li, Y.; Song, D.; Ding, J.; Mei, S.; Sun, S.; Cheng, W.; Yu, J.; Zhou, L.; Kuang, Y.; et al. Iron-overloaded follicular fluid increases the risk of endometriosis-related infertility by triggering granulosa cell ferroptosis and oocyte dysmaturity. Cell Death Dis. 2022, 13, 579. [Google Scholar] [CrossRef]
- Sanchez, A.M.; Papaleo, E.; Corti, L.; Santambrogio, P.; Levi, S.; Viganò, P.; Candiani, M.; Panina-Bordignon, P. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum. Reprod. 2014, 29, 577–583. [Google Scholar] [CrossRef]
- Yang, C.; Geng, Y.; Li, Y.; Chen, C.; Gao, Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: A systematic review and meta-analysis. Reprod. Biomed. Online 2015, 31, 9–19. [Google Scholar] [CrossRef]
- Rossi, A.C.; Prefumo, F. The effects of surgery for endometriosis on pregnancy outcomes following in vitro fertilization and embryo transfer: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 2016, 294, 647–655. [Google Scholar] [CrossRef]
- Ferrero, S.; Scala, C.; Tafi, E.; Racca, A.; Venturini, P.L.; Leone Roberti Maggiore, U. Impact of large ovarian endometriomas on the response to superovulation for in vitro fertilization: A retrospective study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 17–21. [Google Scholar] [CrossRef]
- Harada, M.; Takahashi, N.; Hirata, T.; Koga, K.; Fujii, T.; Osuga, Y. Laparoscopic excision of ovarian endometrioma does not exert a qualitative effect on ovarian function: Insights from in vitro fertilization and single embryo transfer cycles. J. Assist. Reprod. Genet. 2015, 32, 685–689. [Google Scholar] [CrossRef]
- Magnusson, Å.; Källen, K.; Thurin-Kjellberg, A.; Bergh, C. The number of oocytes retrieved during IVF: A balance between efficacy and safety. Hum. Reprod. 2018, 33, 58–64. [Google Scholar] [CrossRef]
- Barnhart, K.; Dunsmoor-Su, R.; Coutifaris, C. Effect of endometriosis on in vitro fertilization. Fertil. Steril. 2002, 77, 1148–1155. [Google Scholar] [CrossRef]
- Shebl, O.; Sifferlinger, I.; Habelsberger, A.; Oppelt, P.; Mayer, R.B.; Petek, E.; Ebner, T. Oocyte competence in in vitro fertilization and intracytoplasmic sperm injection patients suffering from endometriosis and its possible association with subsequent treatment outcome: A matched case-control study. Acta Obstet. Gynecol. Scand. 2016, 96, 736–744. [Google Scholar] [CrossRef]
- Senapati, S.; Sammel, M.D.; Morse, C.; Barnhart, K.T. Impact of endometriosis on in vitro fertilization outcomes: An evaluation of the Society for Assisted Reproductive Technologies Database. Fertil. Steril. 2016, 106, 164–171. [Google Scholar] [CrossRef]
- Hamdan, M.; Dunselman, G.; Li, T.C.; Cheong, Y. The impact of endometrioma on IVF/ICSI outcomes: A systematic review and meta-analysis. Hum. Reprod. Update 2015, 21, 809–825. [Google Scholar] [CrossRef] [PubMed]
- Seyhan, A.; Ata, B.; Uncu, G. The Impact of Endometriosis and Its Treatment on Ovarian Reserve. Semin. Reprod. Med. 2015, 33, 422–428. [Google Scholar] [CrossRef]
- Dong, X.; Liao, X.; Wang, R.; Zhang, H. The impact of endometriosis on IVF/ICSI outcomes. Int. J. Clin. Exp. Pathol. 2013, 6, 1911–1918. [Google Scholar]
- Singh, N.; Lata, K.; Naha, M.; Malhotra, N.; Tiwari, A.; Vanamail, P. Effect of endometriosis on implantation rates when compared to tubal factor in fresh non donor in vitro fertilization cycles. J. Hum. Reprod. Sci. 2014, 7, 143–147. [Google Scholar] [CrossRef]
- Harb, H.M.; Gallos, I.D.; Chu, J.; Harb, M.; Coomarasamy, A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG 2013, 120, 1308–1320. [Google Scholar] [CrossRef] [PubMed]
- Simón, C.; Gutiérrez, A.; Vidal, A.; de los Santos, M.J.; Tarín, J.J.; Remohí, J.; Pellicer, A. Outcome of patients with endometriosis in assisted reproduction: Results from in-vitro fertilization and oocyte donation. Hum. Reprod. 1994, 9, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Pellicer, A.; Oliveira, N.; Gutierrez, A. Implantation in endometriosis: Lessons learned from IVF and oocyte donation. In Progress in Endometriosis; Spinola, P., Coutinho, E.M., Eds.; Parthenon Publishing Group: Carnforth, UK, 1994; pp. 177–183. [Google Scholar]
- Agarwal, A.; Gupta, S.; Sharma, R.K. Role of oxidative stress in female reproduction. Reprod. Biol. Endocrinol. 2005, 3, 28. [Google Scholar] [CrossRef]
- Carvalho, L.F.; Abrão, M.S.; Biscotti, C.; Sharma, R.; Nutter, B.; Falcone, T. Oxidative cell injury as a predictor of endometriosis progression. Reprod. Sci. 2013, 20, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dutta, M.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J. Assist. Reprod. Genet. 2016, 33, 1363–1372. [Google Scholar] [CrossRef]
- Verit, F.F.; Erel, O.; Celik, N. Serum paraoxonase-1 activity in women with endometriosis and its relationship with the stage of the disease. Hum. Reprod. 2008, 23, 100–104. [Google Scholar] [CrossRef]
- Nasiri, N.; Moini, A.; Eftekhari-Yazdi, P.; Karimian, L.; Salman-Yazdi, R.; Arabipoor, A. Oxidative stress statues in serum and follicular fluid of women with endometriosis. Cell J. 2017, 18, 582–587. [Google Scholar] [CrossRef]
- Singh, A.K.; Chattopadhyay, R.; Chakravarty, B.; Chaudhury, K. Markers of oxidative stress in follicular fluid of women with endometriosis and tubal infertility undergoing IVF. Reprod. Toxicol. 2013, 42, 116–124. [Google Scholar] [CrossRef]
- Da Broi, M.G.; de Albuquerque, F.O.; de Andrade, A.Z.; Cardoso, R.L.; Jordão Junior, A.A.; Navarro, P.A. Increased concentration of 8-hydroxy-2′-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res. 2016, 366, 231–242. [Google Scholar] [CrossRef]
- Várnagy, Á.; Kőszegi, T.; Györgyi, E.; Szegedi, S.; Sulyok, E.; Prémusz, V.; Bódis, J. Levels of total antioxidant capacity and 8-hydroxy-2′-deoxyguanosine of serum and follicular fluid in women undergoing in vitro fertilization: Focusing on endometriosis. Hum. Fertil. 2020, 23, 200–208. [Google Scholar] [CrossRef]
- Mansour, G.; Sharma, R.K.; Agarwal, A.; Falcone, T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: A possible cause of infertility. Fertil. Steril. 2010, 94, 1894–1899. [Google Scholar] [CrossRef]
- Regiani, T.; Cordeiro, F.B.; da Costa Ldo, V.; Salgueiro, J.; Cardozo, K.; Carvalho, V.M.; Perkel, K.J.; Zylbersztejn, D.S.; Cedenho, A.P.; Lo Turco, E.G. Follicular fluid alterations in endometriosis: Label-free proteomics by MS(E) as a functional tool for endometriosis. Syst. Biol. Reprod. Med. 2015, 61, 263–276. [Google Scholar] [CrossRef]
- Giacomini, E.; Sanchez, A.M.; Sarais, V.; Beitawi, S.A.; Candiani, M.; Viganò, P. Characteristics of follicular fluid in ovaries with endometriomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 209, 34–38. [Google Scholar] [CrossRef]
- Nakagawa, K.; Hisano, M.; Sugiyama, R.; Yamaguchi, K. Measurement of oxidative stress in the follicular fluid of infertility patients with an endometrioma. Arch. Gynecol. Obstet. 2016, 293, 197–202. [Google Scholar] [CrossRef]
- Nakamura, T.; Naguro, I.; Ichijo, H. Iron homeostasis and iron-regulated ROS in cell death, senescence and human diseases. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 1398–1409. [Google Scholar] [CrossRef]
- Kobayashi, H.; Yamada, Y.; Kanayama, S.; Furukawa, N.; Noguchi, T.; Haruta, S.; Yoshida, S.; Sakata, M.; Sado, T.; Oi, H. The role of iron in the pathogenesis of endometriosis. Gynecol. Endocrinol. 2009, 25, 39–52. [Google Scholar] [CrossRef]
- Defrère, S.; Van Langendonckt, A.; Vaesen, S.; Jouret, M.; González Ramos, R.; Gonzalez, D.; Donnez, J. Iron overload enhances epithelial cell proliferation in endometriotic lesions induced in a murine model. Hum. Reprod. 2006, 21, 2810–2816. [Google Scholar] [CrossRef]
- Reubinoff, B.E.; Har-El, R.; Kitrossky, N.; Friedler, S.; Levi, R.; Lewin, A.; Chevion, M. Increased levels of redox-active iron in follicular fluid: A possible cause of free radical-mediated infertility in beta-thalassemia major. Am. J. Obstet. Gynecol. 1996, 174, 914–918. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Iron overload in the peritoneal cavity of women with pelvic endometriosis. Fertil. Steril. 2002, 78, 712–718. [Google Scholar] [CrossRef]
- Li, A.; Ni, Z.; Zhang, J.; Cai, Z.; Kuang, Y.; Yu, C. Transferrin Insufficiency and Iron Overload in Follicular Fluid Contribute to Oocyte Dysmaturity in Infertile Women with Advanced Endometriosis. Front. Endocrinol. 2020, 11, 391. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mandai, M.; Toyokuni, S.; Hamanishi, J.; Higuchi, T.; Takakura, K.; Fujii, S. Contents of endometriotic cysts, especially the high concentration of free iron, are a possible cause of carcinogenesis in the cysts through the iron-induced persistent oxidative stress. Clin. Cancer Res. 2008, 14, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, A.M.; Viganò, P.; Somigliana, E.; Panina-Bordignon, P.; Vercellini, P.; Candiani, M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum. Reprod. Update 2014, 20, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Benaglia, L.; Paffoni, A.; Mangiarini, A.; Restelli, L.; Bettinardi, N.; Somigliana, E.; Vercellini, P.; Fedele, L. Intrafollicular iron and ferritin in women with ovarian endometriomas. Acta Obstet. Gynecol. Scand. 2015, 94, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Briggs, D.A.; Sharp, D.J.; Miller, D.; Gosden, R.G. Transferrin in the developing ovarian follicle: Evidence for de-novo expression by granulosa cells. Mol. Hum. Reprod. 1999, 5, 1107–1114. [Google Scholar] [CrossRef]
- Vigano, P.; Corti, L.; Berlanda, N. Beyond infertility: Obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 2015, 104, 802–812. [Google Scholar] [CrossRef]
- Zakerkish, F.; Brännström, M.; Carlsohn, E.; Sihlbom, C.; van der Post, S.; Thoroddsen, A. Proteomic analysis of follicular fluid during human ovulation. Acta Obstet. Gynecol. Scand. 2020, 99, 917–924. [Google Scholar] [CrossRef]
- Choi, Y.S.; Cho, S.; Seo, S.K.; Park, J.H.; Kim, S.H.; Lee, B.S. Alteration in the intrafollicular thiol-redox system in infertile women with endometriosis. Reproduction 2015, 149, 155–162. [Google Scholar] [CrossRef]
- Ngô, C.; Chereau, C.; Nicco, C.; Weill, B.; Chapron, C.; Batteux, F. Reactive oxygen species controls endometriosis progression. Am. J. Pathol. 2009, 175, 225–234. [Google Scholar] [CrossRef]
- Ota, H.; Igarashi, S.; Kato, N.; Tanaka, T. Aberrant expression of glutathione peroxidase in eutopic and ectopic endometrium in endometriosis and adenomyosis. Fertil. Steril. 2000, 74, 313–318. [Google Scholar] [CrossRef]
- Oner-Iyidoğan, Y.; Koçak, H.; Gürdöl, F.; Korkmaz, D.; Buyru, F. Indices of oxidative stress in eutopic and ectopic endometria of women with endometriosis. Gynecol. Obstet. Investig. 2004, 57, 214–217. [Google Scholar] [CrossRef]
- Prieto, L.; Quesada, J.F.; Cambero, O.; Pacheco, A.; Pellicer, A.; Codoceo, R.; Garcia-Velasco, J.A. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil. Steril. 2012, 98, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Matos, L.; Stevenson, D.; Gomes, F.; Silva-Carvalho, J.L.; Almeida, H. Superoxide dismutase expression in human cumulus oophorus cells. Mol. Hum. Reprod. 2009, 15, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Ekart, J.; McNatty, K.; Hutton, J.; Pitman, J. Ranking and selection of MII oocytes in human ICSI cycles using gene expression levels from associated cumulus cells. Hum. Reprod. 2013, 28, 2930–2942. [Google Scholar] [CrossRef] [PubMed]
- Donabela, F.C.; Meola, J.; Padovan, C.C.; de Paz, C.C.; Navarro, P.A. Higher SOD1 Gene Expression in Cumulus Cells from Infertile Women with Moderate and Severe Endometriosis. Reprod. Sci. 2015, 22, 1452–1460. [Google Scholar] [CrossRef]
- González-Fernández, R.; Hernández, J.; Martín-Vasallo, P.; Puopolo, M.; Palumbo, A.; Ávila, J. Expression Levels of the Oxidative Stress Response Gene ALDH3A2 in Granulosa-Lutein Cells Are Related to Female Age and Infertility Diagnosis. Reprod. Sci. 2016, 23, 604–609. [Google Scholar] [CrossRef]
- Lousse, J.C.; Van Langendonckt, A.; Defrere, S.; Ramos, R.G.; Colette, S.; Donnez, J. Peritoneal endometriosis is an inflammatory disease. Front. Biosci.-Elite 2012, 4, 23–40. [Google Scholar] [CrossRef]
- Li, G.; Lin, Y.; Zhang, Y.; Gu, N.; Yang, B.; Shan, S.; Liu, N.; Ouyang, J.; Yang, Y.; Sun, F.; et al. Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov. 2022, 8, 29. [Google Scholar] [CrossRef]
- Ng, S.W.; Norwitz, S.G.; Taylor, H.S.; Norwitz, E.R. Endometriosis: The Role of Iron Overload and Ferroptosis. Reprod. Sci. 2020, 27, 1383–1390. [Google Scholar] [CrossRef]
- Li, B.; Duan, H.; Wang, S.; Li, Y. Ferroptosis resistance mechanisms in endometriosis for diagnostic model establishment. Reprod. Biomed. Online 2021, 43, 127–138. [Google Scholar] [CrossRef]
- Hao, S.; Liang, B.; Huang, Q.; Dong, S.; Wu, Z.; He, W.; Shi, M. Metabolic networks in ferroptosis. Oncol. Lett. 2018, 15, 5405–5411. [Google Scholar] [CrossRef]
- Mancias, J.D.; Wang, X.; Gygi, S.P.; Harper, J.W.; Kimmelman, A.C. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature 2014, 509, 105–109. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Guo, X.; Liu, F.; Deng, J.; Dai, P.; Qin, Y.; Li, Z.; Wang, B.; Fan, A.; Wang, Z.; Zhao, Y. Electron-Accepting Micelles Deplete Reduced Nicotinamide Adenine Dinucleotide Phosphate and Impair Two Antioxidant Cascades for Ferroptosis-Induced Tumor Eradication. ACS Nano 2020, 14, 14715–14730. [Google Scholar] [CrossRef]
- Yu, H.; Guo, P.; Xie, X.; Wang, Y.; Chen, G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J. Cell. Mol. Med. 2017, 21, 648–657. [Google Scholar] [CrossRef]
- Wan, Y.; Song, Y.; Chen, J.; Kong, J.; Gu, C.; Huang, J.; Zuo, L. Upregulated Fibulin-1 Increased Endometrial Stromal Cell Viability and Migration by Repressing EFEMP1-Dependent Ferroptosis in Endometriosis. Biomed. Res. Int. 2022, 2022, 4809415. [Google Scholar] [CrossRef]
- Holmila, R.; Sklias, A.; Muller, D.C.; Degli Esposti, D.; Guilloreau, P.; Mckay, J.; Sangrajrang, S.; Srivatanakul, P.; Hainaut, P.; Merle, P.; et al. Targeted deep sequencing of plasma circulating cell-free DNA reveals Vimentin and Fibulin 1 as potential epigenetic biomarkers for hepatocellular carcinoma. PLoS ONE. 2017, 12, e0174265. [Google Scholar] [CrossRef]
- Taylor, H.S.; Alderman Iii, M.; D’Hooghe, T.M.; Fazleabas, A.T.; Duleba, A.J. Effect of simvastatin on baboon endometriosis. Biol. Reprod. 2017, 97, 32–38. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; López-Navarro, E.; Candenas, L.; Pinto, F.; Ortega, F.J.; Sabater-Masdeu, M.; Fernández-Sánchez, M.; Blasco, V.; Romero-Ruiz, A.; Fontán, M.; et al. Ferroportin mRNA is down-regulated in granulosa and cervical cells from infertile women. Fertil. Steril. 2017, 107, 236–242. [Google Scholar] [CrossRef]
- Chen, X.; Zhou, Y.; Wu, D.; Shu, C.; Wu, R.; Li, S.; Huang, Q.; Shu, J. Iron overload compromises preimplantation mouse embryo development. Reprod. Toxicol. 2021, 105, 156–165. [Google Scholar] [CrossRef]
- Clower, L.; Fleshman, T.; Geldenhuys, W.J.; Santanam, N. Targeting Oxidative Stress Involved in Endometriosis and Its Pain. Biomolecules 2022, 12, 1055. [Google Scholar] [CrossRef]
- Yi, Z.H.; Li, S.Q.; Ke, J.Y.; Wang, Y.; Zhao, M.Z.; Li, J.; Li, M.Q.; Zhu, Z.L. Baicalein Relieves Ferroptosis-Mediated Phagocytosis Inhibition of Macrophages in Ovarian Endometriosis. Curr. Issues Mol. Biol. 2022, 44, 6189–6204. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.M., Jr.; Walker, S.M. Effects of iron overload on the immune system. Ann. Clin. Lab. Sci. 2000, 30, 354–365. [Google Scholar] [PubMed]
- Zhang, C.; Liu, X.; Jin, S.; Chen, Y.; Guo, R. Ferroptosis in cancer therapy: A novel approach to reversing drug resistance. Mol. Cancer 2022, 21, 47. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell. 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, X.; Yang, M.; Dong, X. Recent progress in ferroptosis inducers for cancer therapy. Adv. Mater. 2019, 31, e1904197. [Google Scholar] [CrossRef]
- Yu, M.; Gai, C.; Li, Z.; Ding, D.; Zheng, J.; Zhang, W.; Lv, S.; Li, W. Targeted exosome-encapsulated erastin induced ferroptosis in triple negative breast cancer cells. Cancer Sci. 2019, 110, 3173–3182. [Google Scholar] [CrossRef]
- Shi, Q.; Liu, R.; Chen, L. Ferroptosis inhibitor ferrostatin-1 alleviates homocysteine-induced ovarian granulosa cell injury by regulating TET activity and DNA methylation. Mol. Med. Rep. 2022, 25, 130. [Google Scholar] [CrossRef]
- Mukheef, M.A.; Ali, R.A.; Alheidery, H.H.A. Follicular fluid 8-Hydroxy-2-Deoxyguanosine (8-OHdG) as biomarker for oxidative stress in intracytoplasmic sperm injection. J. Med. Investig. 2022, 69, 112–116. [Google Scholar] [CrossRef]
- Hambright, W.S.; Fonseca, R.S.; Chen, L.; Na, R.; Ran, Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017, 12, 8–17. [Google Scholar] [CrossRef]
- Santanam, N.; Zoneraich, N.; Parthasarathy, S. Myeloperoxidase as a Potential Target in Women with Endometriosis Undergoing IVF. Reprod. Sci. 2017, 24, 619–626. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobayashi, H.; Yoshimoto, C.; Matsubara, S.; Shigetomi, H.; Imanaka, S. Current Understanding of and Future Directions for Endometriosis-Related Infertility Research with a Focus on Ferroptosis. Diagnostics 2023, 13, 1926. https://doi.org/10.3390/diagnostics13111926
Kobayashi H, Yoshimoto C, Matsubara S, Shigetomi H, Imanaka S. Current Understanding of and Future Directions for Endometriosis-Related Infertility Research with a Focus on Ferroptosis. Diagnostics. 2023; 13(11):1926. https://doi.org/10.3390/diagnostics13111926
Chicago/Turabian StyleKobayashi, Hiroshi, Chiharu Yoshimoto, Sho Matsubara, Hiroshi Shigetomi, and Shogo Imanaka. 2023. "Current Understanding of and Future Directions for Endometriosis-Related Infertility Research with a Focus on Ferroptosis" Diagnostics 13, no. 11: 1926. https://doi.org/10.3390/diagnostics13111926
APA StyleKobayashi, H., Yoshimoto, C., Matsubara, S., Shigetomi, H., & Imanaka, S. (2023). Current Understanding of and Future Directions for Endometriosis-Related Infertility Research with a Focus on Ferroptosis. Diagnostics, 13(11), 1926. https://doi.org/10.3390/diagnostics13111926





