DCDC2-Related Ciliopathy: Report of Six Polish Patients, Novel DCDC2 Variant, and Literature Review of Reported Cases
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
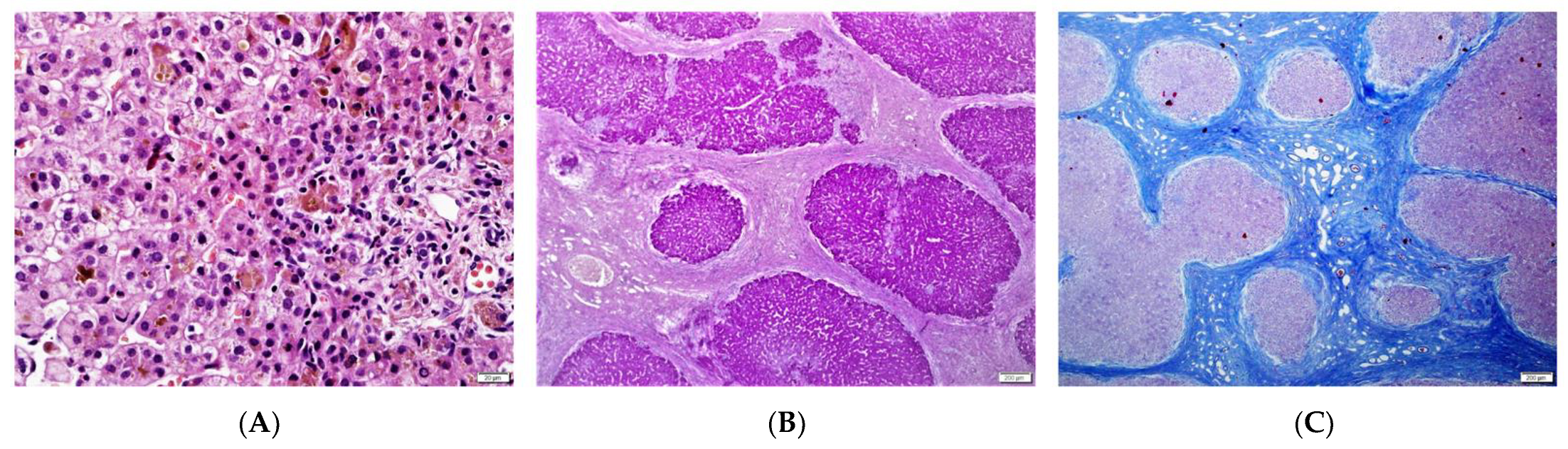
2.2. Liver Biopsy
2.3. Molecular Analysis
2.4. Literature Search
3. Results
3.1. Patients’ Presentation
3.2. Literature Review
4. Discussion
5. Conclusions
- The main clinical presentation of DCDC2-related ciliopathy is liver disease in the form of neonatal sclerosing cholangitis.
- The predominance of early and severe liver disease associated with no or mildly expressed kidney involvement is observed in DCDC2-related ciliopathy.
- Our findings expand the molecular spectrum of pathogenic DCDC2 variants, provide a more accurate picture of the phenotypic expression associated with molecular changes in this gene and confirm a loss of functional behaviour as the mechanism of disease.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rock, N.; McLin, V. Liver involvement in children with ciliopathies. Clin. Res. Hepatol. Gastroenterol. 2014, 38, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Gunay-Aygun, M. Liver and kidney disease in ciliopathies. Am. J. Med. Genet. C Semin. Med. Genet. 2009, 151C, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lee, J.M.; Ahn, Y.H.; Kang, H.G.; Ha, I.I.; Lee, J.H.; Park, Y.S.; Kim, N.K.; Park, W.Y.; Cheong, H.I. Hepatorenal fibrocystic diseases in children. Pediatr. Nephrol. 2016, 31, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wicher, D.; Obrycki, Ł.; Jankowska, I. Autosomal Recessive Polycystic Kidney Disease-The Clinical Aspects and Diagnostic Challenges. J. Pediatr. Genet. 2021, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stokman, M.; Lilien, M.; Knoers, N. Nephronophthisis-Related Ciliopathies 2016 [updated 2023 Mar 2]. In GeneReviews®; Adam, M.P., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Bean, L.J.H., Gripp, K.W., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Lipiński, P.; Ciara, E.; Jurkiewicz, D.; Pollak, A.; Wypchło, M.; Płoski, R.; Cielecka-Kuszyk, J.; Socha, P.; Pawłowska, J.; Jankowska, I. Targeted Next-Generation Sequencing in Diagnostic Approach to Monogenic Cholestatic Liver Disorders-Single-Center Experience. Front. Pediatr. 2020, 8, 414. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, H.E.; Shaheen, R.; Ewida, N.; Bubshait, D.K.; Alkuraya, H.; Almardawi, E.; Howaidi, A.; Sabr, Y.; Abdalla, E.M.; Alfaifi, A.Y.; et al. The morbid genome of ciliopathies: An update. Genet. Med. 2020, 22, 1051–1060. [Google Scholar] [CrossRef] [PubMed]
- Schueler, M.; Braun, D.A.; Chandrasekar, G.; Gee, H.Y.; Klasson, T.D.; Halbritter, J.; Bieder, A.; Porath, J.D.; Airik, R.; Zhou, W.; et al. DCDC2 mutations cause a renal-hepatic ciliopathy by disrupting Wnt signaling. Am. J. Hum. Genet. 2015, 96, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Girard, M.; Bizet, A.A.; Lachaux, A.; Gonzales, E.; Filhol, E.; Collardeau-Frachon, S.; Jeanpierre, C.; Henry, C.; Fabre, M.; Viremouneix, L.; et al. DCDC2 Mutations Cause Neonatal Sclerosing Cholangitis. Hum. Mutat. 2016, 37, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Grammatikopoulos, T.; Sambrotta, M.; Strautnieks, S.; Foskett, P.; Knisely, A.S.; Wagner, B.; Deheragoda, M.; Starling, C.; Mieli-Vergani, G.; Smith, J.; et al. Mutations in DCDC2 (doublecortin domain containing protein 2) in neonatal sclerosing cholangitis. J. Hepatol. 2016, 65, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Lu, Y.; Qiu, Y.L.; Wang, J.S. Neonatal sclerosing cholangitis caused by DCDC2 variations in two siblings and literature review. Zhonghua Er Ke Za Zhi 2018, 56, 623–627. [Google Scholar] [PubMed]
- Slater, B.; Bekheirnia, N.; Angelo, J.; Bi, W.; Braun, M.C.; Bekheirnia, M.R. Nephronophthisis due to a novel DCDC2 variant in a patient from African-Caribbean descent: A case report. Am. J. Med. Genet. A 2020, 182, 527–531. [Google Scholar] [CrossRef] [PubMed]
- Vogel, G.F.; Maurer, E.; Entenmann, A.; Straub, S.; Knisely, A.S.; Janecke, A.R.; Müller, T. Co-existence of ABCB11 and DCDC2 disease: Infantile cholestasis requires both next-generation sequencing and clinical-histopathologic correlation. Eur. J. Hum. Genet. 2020, 28, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhang, J.; Li, X.; Zheng, D.; Yu, X.; Liu, Y.; Lan, F.; Wang, Z. Biallelic mutations in DCDC2 cause neonatal sclerosing cholangitis in a Chinese family. Clin. Res. Hepatol. Gastroenterol. 2020, 44, e103–e108. [Google Scholar] [CrossRef] [PubMed]
- Syryn, H.; Hoorens, A.; Grammatikopoulos, T.; Deheragoda, M.; Symoens, S.; Vande Velde, S.; Van Biervliet, S.; Van Winckel, M.; Verloo, P.; Callewaert, B.; et al. Two cases of DCDC2-related neonatal sclerosing cholangitis with developmental delay and literature review. Clin. Genet. 2021, 100, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Teker Düztaş, D.; Sarı, S.; Eğritaş Gürkan, Ö.; Kayhan, G.; Dalgıç, A.; Dalgıç, B. Two Cases With Neonatal Cholestasis and Renal Disorders Due to DCDC2 Mutation. Exp. Clin. Transplant. 2022, 20, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Fang, Y.; Wang, J.S.; Wang, Y.Z.; Zhang, Y.; Abuduxikuer, K.; Chen, L. Neonatal sclerosing cholangitis with novel mutations in DCDC2 (doublecortin domain-containing protein 2) in Chinese children. Front. Pediatr. 2023, 11, 1094895. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.omim.org/entry/605755 (accessed on 1 March 2023).
- Amedee-Manesme, O.; Bernard, O.; Brunelle, F.; Hadchouel, M.; Polonovski, C.; Baudon, J.J.; Beguet, P.; Alagille, D. Sclerosing cholangitis with neonatal onset. J. Pediatr. 1987, 111, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Hadj-Rabia, S.; Baala, L.; Vabres, P.; Hamel-Teillac, D.; Jacquemin, E.; Fabre, M.; Lyonnet, S.; De Prost, Y.; Munnich, A.; Hadchouel, M.; et al. Claudin-1 gene mutations in neonatal sclerosing cholangitis associated with ichthyosis: A tight junction disease. Gastroenterology 2004, 127, 1386–1390. [Google Scholar] [CrossRef] [PubMed]
- Baala, L.; Hadj-Rabia, S.; Hamel-Teillac, D.; Hadchouel, M.; Prost, C.; Leal, S.M.; Jacquemin, E.; Sefiani, A.; De Prost, Y.; Courtois, G.; et al. Homozygosity mapping of a locus for a novel syndromic ichthyosis to chromosome 3q27-q28. J. Invest. Dermatol. 2002, 119, 70–76. [Google Scholar] [CrossRef] [PubMed]
| Patient/ Gender | Origin | Age at First Presentation | Presenting Signs and Symptoms | Intrahepatic Cholangiopathy on MRCP | Liver Phenotype, Including Histology | LTx/ Age at LTx | Follow-Up | Other | Genotype |
|---|---|---|---|---|---|---|---|---|---|
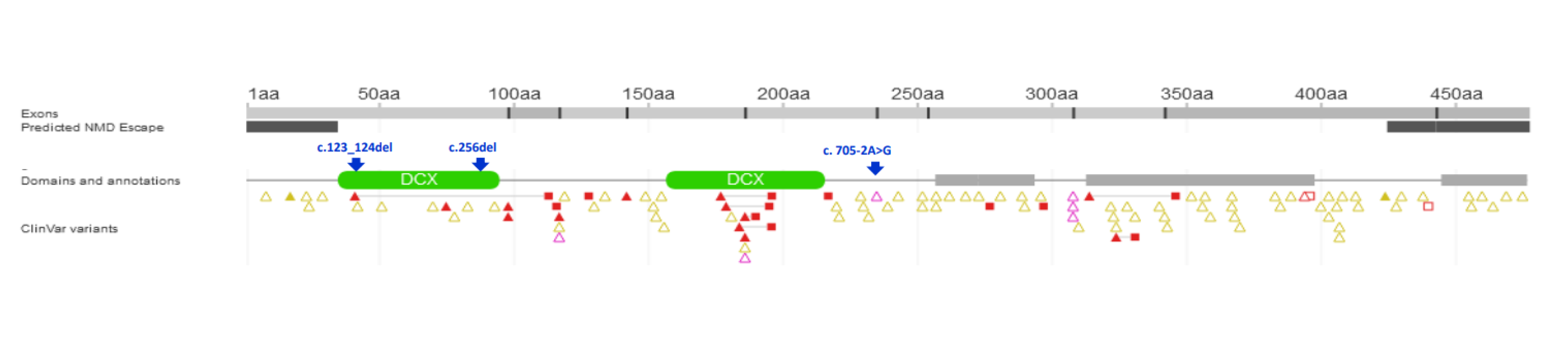
| 1/F | Caucasian | 25th day of life | Jaundice, acholic stools, elevated GGT | No | 2 m—liver biopsy: severe cholestasis, porto-portal fibrosis, moderate giant cell transformation of hepatocytes, no proliferation of bile ductules; 9 m—1st episode of oesophageal varices bleeding; 3.5 y—liver biopsy: mild cholestasis without biliary plugs, ductopenia, severe liver fibrosis; 5 y—2 episodes of oesophageal varices bleeding; 6 y—LTx with splenectomy, liver cirrhosis in the hepatic explant. | Yes/6 y | 8 y | Normal kidney function | c.[123_124del];[256del], p.(Ser42Glnfs*72)/ p.(Tyr86Thrfs*17) compound heterozygote |
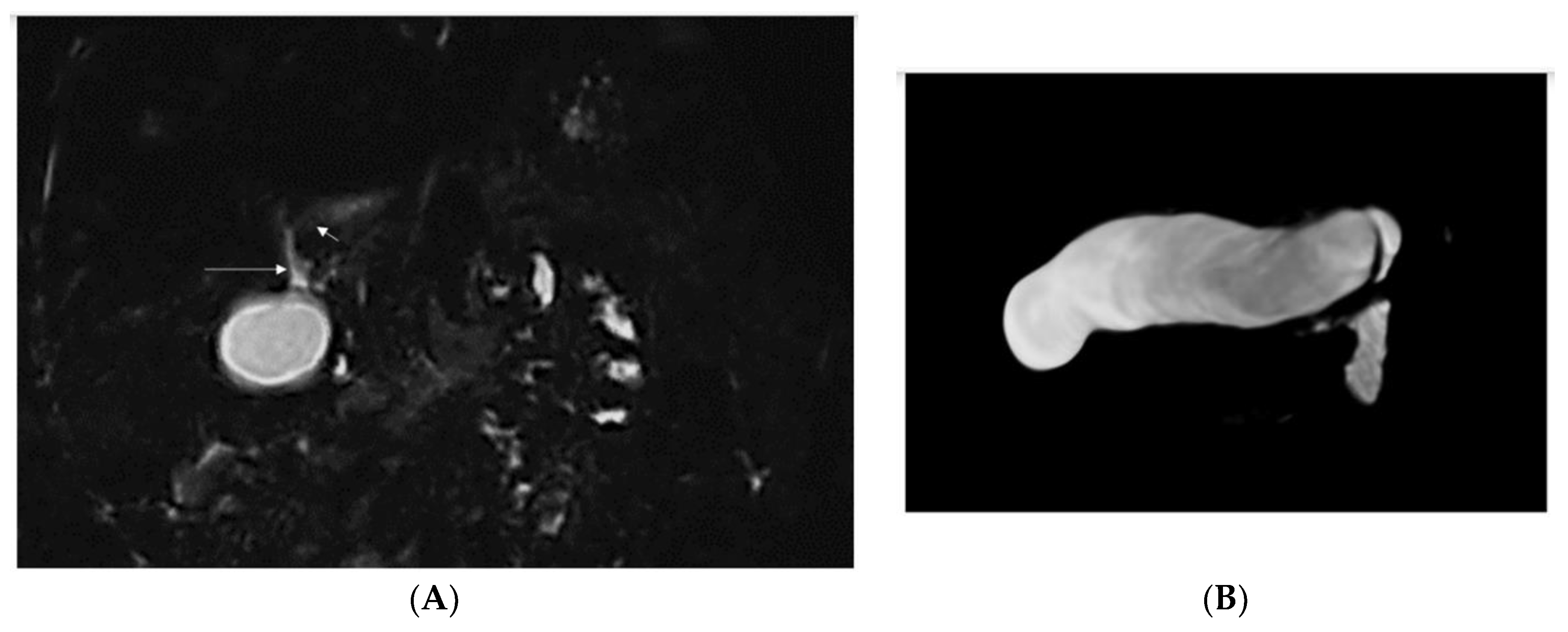
| 2/F Sister of Pt 1 | Caucasian | 2nd day of life | Jaundice, acholic stools, elevated GGT | Yes (8 mo) See Figure 1 | No liver biopsy | No | 9 m | right vesico-ureteral reflux II degree, persistent urachus | c.[123_124del];[256del], p.(Ser42Glnfs*72)/ p.(Tyr86Thrfs*17) compound heterozygote |
| 3/F | Caucasian | 2 m | Jaundice, elevated GGT | n.a. | 2 m—liver biopsy: mild cholestasis, portal fibrosis, focal ductular proliferation; 4 y—1st episode of oesophageal varices bleeding, ascites. | No | 12.5 y | Normal kidney function | c.[123_124del];[705-2A>G] p.(Ser42Glnfs*72)/ p.? compound heterozygote |
| 4/M Brother of Pt 3 and 5 | Caucasian | 2 m | Jaundice, elevated GGT | n.a. | 4 m—liver biopsy: moderate cholestasis, portal fibrosis, focal ductular proliferation; 12 m—oesophageal varices in gastroscopy. | No | 10 y | Normal kidney function | c.[123_124del];[705-2A>G] p.(Ser42Glnfs*72)/ p.? compound heterozygote |
| 5/M Brother of Pt 3 and 4 | Caucasian | 2 m | Jaundice, elevated GGT | n.a. | No liver biopsy | No | 5 y | Normal kidney function | c.[123_124del];[705-2A>G] p.(Ser42Glnfs*72)/ p.? compound heterozygote |
| 6/M | Caucasian | 3 m | Jaundice, elevated GGT | No | 5 m—liver biopsy—severe cholestasis with bile plugs, ductular proliferation; 2 y—oesophageal varices in gastroscopy; 2.5 y—liver biopsy: moderate cholestasis, severe fibrosis, ductopenia; 13 y—abdominal MR—liver cirrhosis, FNH. | No | 24 y | Normal kidney function | c.[123_124del];[ 123_124del] p.(Ser42Glnfs*72)/ p.(Ser42Glnfs*72) homozygote |
| Patient Age at Liver Biopsy | Fibrosis | Cholestasis | Ductular Changes | Inflammation | Giant Cell Transformation |
|---|---|---|---|---|---|
| Patient 1 | |||||
| 2 m | porto-portal fibrosis | ductal, acinar bile plugs, severe cholestasis | no | mild portal | moderate |
| 4.5 y | severe fibrosis | mild, hepatocellular | ductopenia | no | No |
| hepatic explant (6 y) | micronodular cirrhosis | mild cholestasis | diffuse ductular proliferation | mild portal | No |
| Patient 3 | |||||
| 2 m | portal fibrosis | mild, hepatocellular | focal ductular proliferation | mild portal, ductitis | No |
| Patient 4 | |||||
| 4 m | portal fibrosis | moderate hepatocellular | focal ductular proliferation | mild portal, ductitis | no |
| Patient 6 | |||||
| 5 m | periportal, bridging fibrosis | ductal, acinar bile plugs, severe cholestasis | ductular proliferation, focal DPM | mild portal | no |
| 2.5 y | severe fibrosis | ductular bile plugs, moderate hepatocellular | ductopenia, ductular proliferation | no | no |
| Patient/ Gender | Origin/ Consanguinity | Age at First Presentation | Presenting Signs and Symptoms | Intrahepatic Cholangiopathy on ERCP/MRCP | Liver Phenotype, Including Histology | LTx/ Age at LTx | Follow-Up | Other | Genetic Result for NM_016356.5(DCDC2) RefSeq | References |
|---|---|---|---|---|---|---|---|---|---|---|
| 1/n.a. | UK/Yes | n.a. | n.a. | n.a. | Hepatosplenomegaly, extensive fibrosis (11 mo) with destruction of bile ducts, bile focal duct proliferation with cholestasis | No | Died at 16 y from GI bleeding | Increased echogenicity, severe interstitial fibrosis, tubular dilation with prominent epithelial luminal budding, ESRD at 14 y | c.649A>T p.(Lys217*)/ c.649A>T p.(Lys217*) | Schueler et al., 2015 [8] |
| 2/n.a. | Czech/No | n.a. | n.a. | n.a. | Hepatosplenomegaly, ductal plate malformation, hepatic fibrosis, scant cholestasis | Yes/2 y | current age 9 y | No renal involvement | c.123_124del, p.(Ser42Glnfs*72)/ c.349-2A>G, p.(Val117Leufs*54) | |
| 3/F | Asian/Yes | 20 wk | Jaundice, acholic stools, GGT 247 IU/L | Yes | n.a. | No/ listed | Died at 16 y | n.a. | c.649A>T, p.(Lys217*)/ c.649A>T, p.(Lys217*) | Grammatikopoulos et al., 2016 [10] |
| 4/F | Caucasian/No | 21 wk | Jaundice, ascites, splenomegaly, GI bleeding; GGT 447 IU/L | Yes | 8 m—porto-portal bridging fibrosis, ductular reaction with ductal bile plugs. Hepatectomy specimen at 10 y—biliary cirrhosis, peripheral ductopenia. | Yes/10 y | 12 y | n.a. | c.890T>A, p.(Leu297*)/ c.890T>A, p.(Leu297*) | |
| 5/M | Arabic/Yes | 6 wk | Jaundice, GI bleeding; GGT 711 IU/L | n.a. | 8 wk—ductal plate malformation, cholestasis. Hepatectomy at 14 y—biliary cirrhosis, peripheral ductopenia. | Yes/14 y | 16 y | n.a. | c.757insG, p.(Ser253Argfs*4)/ c.757insG, p.(Ser253Argfs*4) | |
| 6/F | Caucasian/No | 4 wk | Jaundice, splenomegaly; GGT 210 IU/L | Yes | Hepatectomy at 15 y—porto-portal bridging fibrosis, peripheral ductopenia, ectasia and cystic dilatation of perihilar bile ducts. | Yes/15 y | Died at 17 y | n.a. | c.529dup, p.(Ile177Asnfs*20)/ c.890T>A, p.(Leu297*) | |
| 7/M | Caucasian/No | 6 wk | Jaundice, splenomegaly; GGT 962 IU/L | Yes | 9 wk—porto-portal bridging fibrosis, cholestasis, ductular proliferation with cholangiopathic features. 6 y—mild fibrosis, cholestasis, focal interlobular bile duct loss and cholangiopathic features in remaining bile ducts. | No | 6 y | n.a. | c.123_124del, p.(Ser42Glnfs*72)/ c.890T>A, p.(Leu297*) | |
| 8/M | Caucasian/No | 7 wk | Jaundice, splenomegaly; GGT 365 IU/L | Yes | 4 m—porto-portal bridging fibrosis, cholestasis, ductular proliferation and ductal bile plugs. Hepatectomy at 15 y—biliary cirrhosis, peripheral ductopaenia, ectasia and cystic dilatation of perihilar bile ducts. | Yes/15 y | 18 y | n.a. | c.123_124del, p.(Ser42Glnfs*72)/ c.123_124del, p.(Ser42Glnfs*72) | |
| 9/F | Caucasian/No | 1 wk | Jaundice; GGT 196 IU/L | Yes | 10 wk—ductal plate malformation, ductal bile plugs. 9 y—mild portal fibrosis, cholestasis, interlobular portal tract ductopaenia. Hepatectomy at 14 y—biliary cirrhosis with peripheral ductopaenia, ectasia and cystic dilatation of perihilar bile ducts. | Yes/14 y | 24 y | n.a. | c.529dup, p.(Ile177Asnfs*20)/ c.890T>A, p.(Leu297*) | |
| 10/M | n.a./Yes | n.a. | Jaundice, acholic stools | Yes | Liver histology for all 4 patients typical for NSC with early portal fibrosis, bile duct proliferation and tortuous bile ducts surrounded by fibrosis | Yes/14 y | 14 y | Renal malformation—left vesico-ureteral reflux with left ureteral duplication, without ureteral dilatation. Small left kidney with right kidney hypertrophy compensation. Mild intellectual disability. Renal insufficiency after liver transplantation. | c.51G>C, p.(Lys17Asn)/ c.51G>C, p.(Lys17Asn) | Girard et al., 2016 [9] |
| 11/M Sibling of 10 | n.a./Yes | n.a. | Yes/25 y | 9 y | Renal insufficiency after liver transplantation. | c.51G>C, p.(Lys17Asn)/ c.51G>C, p.(Lys17Asn) | ||||
| 12/F | n.a./Yes | n.a. | Yes/6 y | n.a. | No renal disease (imaging and function) at the age of 6 y. | c.426_557del; p.(Phe142_Arg186del)/ c.426_557del; p.(Phe142_Arg186del) | ||||
| 13/M Sibling of 12 | n.a./Yes | n.a. | Yes/3.5 y | n.a. | 4 mo—hyperechogenic left kidney at ultrasound and hypophosphatemia; normal renal function at 3 y. | c.426_557del; p.(Phe142_Arg186del)/ c.426_557del; p.(Phe142_Arg186del) | ||||
| 14/M | Chinese/n.a. | 2 mo | Jaundice; GGT 161-1 092 IU/L | Yes | n.a. | No | Diagnosis at 3 y 2 mo | bilateral hydronephrosis at 3 y 2 mo | c.529dup, p.(Ile177Asnfs*20)/ c.529dup, p.(Ile177Asnfs*20) | Li et al., 2018 [11] |
| 15/M | Chinese/n.a. | 9 mo | jaundice | Yes | n.a. | No | Diagnosis at 9 y 9 mo | hydrocephalus and left internal carotid artery aneurysms with vascular malformations diagnosed at 9 y 9 mo | c.529dup, p.(Ile177Asnfs*20)/ c.529dup, p.(Ile177Asnfs*20) | |
| 16/F | African-Caribbean/Yes | n.a. | n.a. | Yes | 13 y—13 years, 8 months with liver fibrosis, chronic liver failure 13 y—liver fibrosis, chronic liver failure; Hepatectomy—diffuse micronodular biliary cirrhosis, ductular proliferation | Yes (KLTx)/13 y | Nephronophthisis; Atrophic echogenic kidneys with decreased corticomedullary differentiation; Unilateral sensorineural deafness; brain imaging abnormalities | c.383C>G, p.(Ser128*)/ c.383C>G, p.(Ser128*) | Slater et al., 2019 [12] | |
| 17/M | Chinese/No | 1 wk | Jaundice; GGT 247 IU/L | n.a. | early portal fibrosis and bile duct proliferation; 3 y—cirrhosis | Yes/23 y | n.a. | Abnormal creatinine, no renal biopsy | c.705-2A>G, p.?/ c.923-283_ 1023+141del, p.? | Lin et al., 2020 [14] |
| 18/M Sibling of 17 | Chinese/No | 1 wk | Jaundice; GGT 102 IU/L | n.a. | n.a. | Yes/12 y | 18 y | Abnormal creatinine at 18 y | c.705-2A>G, p.?/ c.923-283_1023+141del, p.? | |
| 19/M Sibling of 17 and 18 | Chinese/No | 1 wk | Jaundice | n.a. | Clinical suspicion of biliary atresia; cirrhosis and liver failure at 8 m | No | Died at 8 m | n.a. | c.705-2A>G, p.?/ c.923-283_1023+141del, p.? | |
| 20/M | Caucasian/Yes | 1 wk | Jaundice, high GGT | No | Explanted liver—cirrhosis, hepatocellular and canalicular cholestasis, giant-cell change of hepatocytes | Yes/8 mo | 2 y | n.a. | c.294-2A>G, p.?/ c.294-2A>G, p.? | Vogel et al., 2020 [13] |
| 21/M | Turkish/Yes | 2 wk | Jaundice, acholic stools | Yes | Liver biopsy—bilirubin stasis, cholangiolytic changes, and septal fibrosis. | No | 3 y 7 m | 2 y—bilateral nephronophthisis; Psychomotor delay | c.367_368del, p.(Ser123Glnfs*9)/ c.367_368del, p.(Ser123Glnfs*9) | Syryn et al., 2021 [15] |
| 22/M | Syria/Yes | 3 mo | Jaundice, hepatosplenomegaly | Yes | Liver biopsy—biliary cirrhosis; Portal hypertension with GI bleeding | Yes/2y 10 mo | n.a. | Normal renal function; Psychomotor delay, microcephaly | c.73G>A p.(Gly25Arg)/ c.73G>A p.(Gly25Arg)/ | |
| 23/M | Turkish/Yes | 1 mo | Jaundice | n.a. | Liver biopsy (5 y)—congenital hepatic fibrosis | Yes (KLtx)/ 14.5 y | n.a. | Burkitt lymphoma at 11 y with renal failure development | n.a. | Duztas et al., 2022 [16] |
| 24/F | Turkish/Yes | 1 mo | Jaundice | n.a. | Liver biopsy (6 y)—cholestatic liver cirrhosis, ductopenia | Yes/6 y | n.a. | 6 y—enlargement of the right kidney with a cystic mass | c.656C>G, p.(Pro2219Arg)/ c.656C>G, p.(Pro2219Arg)/ | |
| 25/F | Chinese/No | 1 wk | Jaundice, elevated GGT | Yes | Giant cell changes of hepatocytes, bile plugs in hepatocytes and capillary bile ducts; ductular proliferation, cholestatic cirrhosis | No | n.a. | n.a. | c.1024-1G>T, p.?/ c.544G>A, p.(Gly182Arg) | Wei et al., 2023 [17] |
| 26/F | Chinese/No | 1 wk | Jaundice, elevated GGT | Yes | Cholestasis, liver fibrosis (stage 3), ductular proliferation, giant cell changes of hepatocytes | No | n.a. | n.a. | c.1024-1G>T, p.?/ c.544G>A, p.(Gly182Arg) | |
| 27/M | Chinese/No | 1 wk | Jaundice, elevated GGT | Yes | No liver biopsy | No | n.a. | n.a. | c.529dup, p.(Ile177Asnfs*20)/ c.529dup, p.(Ile177Asnfs*20) | |
| 28/M | Chinese/No | 1 wk | Jaundice, elevated GGT | No | No liver biopsy | No | n.a. | n.a. | c.529dup, p.(Ile177Asnfs*20)/ c.529dup, p.(Ile177Asnfs*20) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lipiński, P.; Ciara, E.; Jurkiewicz, D.; Mekrouda, M.; Cielecka-Kuszyk, J.; Jurkiewicz, E.; Płoski, R.; Pawłowska, J.; Jankowska, I. DCDC2-Related Ciliopathy: Report of Six Polish Patients, Novel DCDC2 Variant, and Literature Review of Reported Cases. Diagnostics 2023, 13, 1917. https://doi.org/10.3390/diagnostics13111917
Lipiński P, Ciara E, Jurkiewicz D, Mekrouda M, Cielecka-Kuszyk J, Jurkiewicz E, Płoski R, Pawłowska J, Jankowska I. DCDC2-Related Ciliopathy: Report of Six Polish Patients, Novel DCDC2 Variant, and Literature Review of Reported Cases. Diagnostics. 2023; 13(11):1917. https://doi.org/10.3390/diagnostics13111917
Chicago/Turabian StyleLipiński, Patryk, Elżbieta Ciara, Dorota Jurkiewicz, Magda Mekrouda, Joanna Cielecka-Kuszyk, Elżbieta Jurkiewicz, Rafał Płoski, Joanna Pawłowska, and Irena Jankowska. 2023. "DCDC2-Related Ciliopathy: Report of Six Polish Patients, Novel DCDC2 Variant, and Literature Review of Reported Cases" Diagnostics 13, no. 11: 1917. https://doi.org/10.3390/diagnostics13111917
APA StyleLipiński, P., Ciara, E., Jurkiewicz, D., Mekrouda, M., Cielecka-Kuszyk, J., Jurkiewicz, E., Płoski, R., Pawłowska, J., & Jankowska, I. (2023). DCDC2-Related Ciliopathy: Report of Six Polish Patients, Novel DCDC2 Variant, and Literature Review of Reported Cases. Diagnostics, 13(11), 1917. https://doi.org/10.3390/diagnostics13111917






