Fat Hounsfield Unit Reference Interval Derived through an Indirect Method
Abstract
:1. Introduction
2. Methods
2.1. Participants
- Upper abdomen—through the left adrenal gland
- Upper abdomen—through superior mesenteric artery ostium
- Lower abdomen—through the umbilicus
- Lower abdomen—through the anterior superior iliac spine
2.2. Statistical Analysis
- Lower RI margin = α*2.5 + β
- Upper RI margin = α*97.5 + β.
3. Results and Discussion
3.1. Previous Studies
3.2. Limitations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vaamonde, J.G.; Álvarez-Món, M.A. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 19 December 2022).
- Micklesfield, L.K.; Goedecke, J.H.; Punyanitya, M.; Wilson, K.E.; Kelly, T.L. Dual-Energy X-Ray Performs as Well as Clinical Computed Tomography for the Measurement of Visceral Fat. Obesity (Silver Spring) 2012, 20, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Godfrey, N. Hounsfield—Nobel Lecture—NobelPrize.Org. Available online: https://www.nobelprize.org/prizes/medicine/1979/hounsfield/lecture/ (accessed on 19 December 2022).
- Cross-Sectional Imaging Made Easy: 9780443071874: Medicine & Health Science Books @ Amazon.Com. Available online: https://www.amazon.com/Cross-Sectional-Imaging-Made-Simon-Jackson/dp/044307187X (accessed on 19 December 2022).
- NIST: X-ray Mass Attenuation Coefficients—Table 4. Available online: https://physics.nist.gov/PhysRefData/XrayMassCoef/tab4.html (accessed on 19 December 2022).
- Ceriotti, F. Prerequisites for Use of Common Reference Intervals. Clin. Biochem. Rev. 2007, 28, 115. [Google Scholar] [PubMed]
- Sjöström, L.; KVIST, H. Regional Body Fat Measurements with CT-Scan and Evaluation of Anthropometric Predictions. Acta Med. Scand. Suppl. 1988, 723, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Enzi, G.; Gasparo, M.; Raimondo Biondetti, P.; Fiore, D.; Semisa, M.; Zurlo, F. Subcutaneous and Visceral Fat Distribution According to Sex, Age, and Overweight, Evaluated by Computed Tomography. Am. J. Clin. Nutr. 1986, 44, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Islam, A.H.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal Fat: Standardized Technique for Measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Katayev, A.; Balciza, C.; Seccombe, D.W. Establishing Reference Intervals for Clinical Laboratory Test Results: Is There a Better Way? Am. J. Clin. Pathol. 2010, 133, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Hounsfield, G.N. Computerized Transverse Axial Scanning (Tomography). 1. Description of System. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef] [PubMed]
- Pop, R.M.; Pop, M.; Dogaru, G.; Bacarea, V.C. A Web-Based Nutritional Assessment Tool. Stud. Inform. Control 2013, 22, 307–314. [Google Scholar] [CrossRef]
- Yu, X.; Huang, Y.H.; Feng, Y.Z.; Cheng, Z.Y.; Wang, C.C.; Cai, X.R. Association of Body Composition with Postoperative Complications after Laparoscopic Sleeve Gastrectomy and Roux-En-Y Gastric Bypass. Eur. J. Radiol. 2023, 162, 110768. [Google Scholar] [CrossRef] [PubMed]
- Yi, W.; Kim, K.; Im, M.; Ryang, S.; Kim, E.H.; Kim, M.; Jeon, Y.K.; Kim, S.S.; Kim, B.H.; Pak, K.; et al. Association between Visceral Adipose Tissue Volume, Measured Using Computed Tomography, and Cardio-Metabolic Risk Factors. Sci. Rep. 2022, 12, 387. [Google Scholar] [CrossRef] [PubMed]
- Derstine, B.A.; Holcombe, S.A.; Ross, B.E.; Wang, N.C.; Wang, S.C.; Su, G.L. Healthy US Population Reference Values for CT Visceral Fat Measurements and the Impact of IV Contrast, HU Range, and Spinal Levels. Sci. Rep. 2022, 12, 2374. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Massaro, J.; Fox, C.S.; Moselewski, F.; O’Donnell, C.J.; Hoffmann, U. Comparison of Anthropometric, Area- and Volume-Based Assessment of Abdominal Subcutaneous and Visceral Adipose Tissue Volumes Using Multi-Detector Computed Tomography. Int. J. Obes. 2007, 31, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.D.; Kanaley, J.A.; Reed, J.E.; Sheedy, P.F. Measurement of Abdominal and Visceral Fat with Computed Tomography and Dual-Energy x-Ray Absorptiometry. Am. J. Clin. Nutr. 1995, 61, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, M.; Besutti, G.; Ottone, M.; Canovi, S.; Bonelli, E.; Venturelli, F.; Farì, R.; Damato, A.; Bonelli, C.; Pinto, C.; et al. Abdominal Fat Characteristics and Mortality in Rectal Cancer: A Retrospective Study. Nutrients 2023, 15, 374. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.; Park, H.W.; Kim, G. Associations Between Trunk Muscle/Fat Composition, Narrowing Lumbar Disc Space, and Low Back Pain in Middle-Aged Farmers: A Cross-Sectional Study. Ann. Rehabil. Med. 2022, 46, 122. [Google Scholar] [CrossRef] [PubMed]
- Barbalho, E.R.; da Rocha, I.M.G.; de Medeiros, G.O.C.; Friedman, R.; Fayh, A.P.T. Agreement between Software Programmes of Body Composition Analyses on Abdominal Computed Tomography Scans of Obese Adults. Arch. Endocrinol. Metab. 2019, 64, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.J.; Lee, K.H.; Choe, Y.H.; Lee, K.S. Acute Aortic Diseases: Evaluation with Computed Tomography and Magnetic Resonance Imaging. In Emergency Chest Radiology; Springer: Singapore, 2021; pp. 63–88. [Google Scholar]
- Lee, Y.S.; Hong, N.; Witanto, J.N.; Choi, Y.R.; Park, J.; Decazes, P.; Eude, F.; Kim, C.O.; Chang Kim, H.; Goo, J.M.; et al. Deep Neural Network for Automatic Volumetric Segmentation of Whole-Body CT Images for Body Composition Assessment. Clin. Nutr. 2021, 40, 5038–5046. [Google Scholar] [CrossRef] [PubMed]
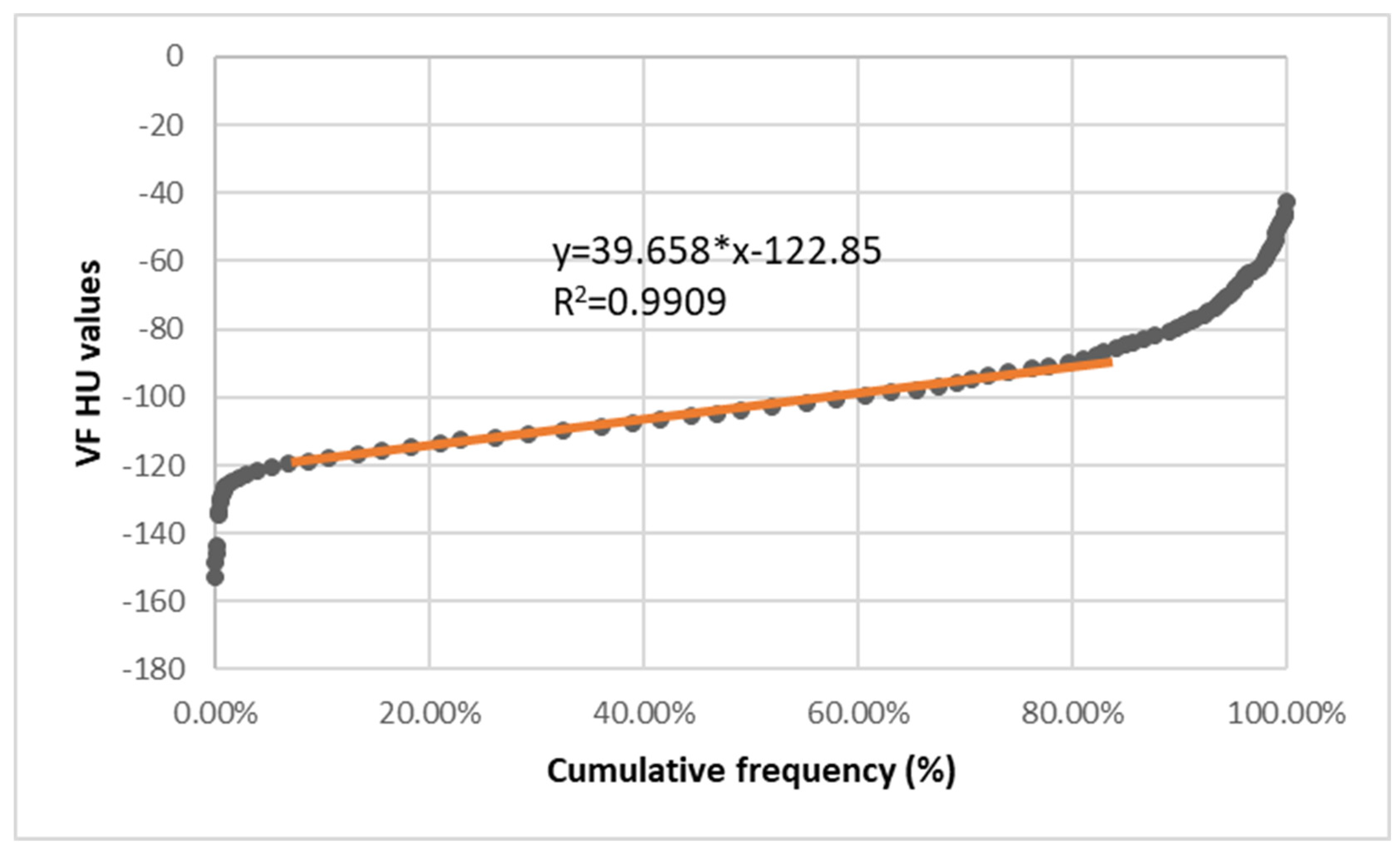

| Equation | R2 | RI | |
|---|---|---|---|
| Total Abdominal Fat | y = 35.37*x − 123.48 | >0.99 | −122.59:−88.98 |
| Subcutaneous Fat | y = 31.28*x − 123.71 | >0.99 | −122.98:−93.21 |
| Visceral Fat | y = 39.65*x − 122.85 | >0.99 | −121.86:−84.18 |
| Previous Reports | Reported/Used RI | Absolute Difference (Upper/Lower) |
|---|---|---|
| Yu, 2023 [13] | −150:−50 | 27:−39 |
| Yi, 2022 [14] | −195:−45 | 72:−44 |
| Brian, 2022 [15] | −205:−51 | 82:−38 |
| Maurovich, 2007 [16] | −195:−45 | 72:−44 |
| Jensen, 2001 [17] | −149+/−12:−68+/−7 | 26:−21 |
| Kvist, 1998 [7]; also used by Pellegrini, 2022 [18]; Baek, 2022 [19]; Barbalho, 2022 [20]; Jung, 2021 [21]; Lee, 2021 [22] | −190:−30 | −67:59 |
| Enzi, 1986 [8] | −250:−50 | −127:39 |
| Hounsfield, 1979 [3] | −90:−70 | 33:19:00 |
| Hounsfield, 1973 [11] | −100 (−10% of water) | 23:−11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pop, M.; Mărușteri, M. Fat Hounsfield Unit Reference Interval Derived through an Indirect Method. Diagnostics 2023, 13, 1913. https://doi.org/10.3390/diagnostics13111913
Pop M, Mărușteri M. Fat Hounsfield Unit Reference Interval Derived through an Indirect Method. Diagnostics. 2023; 13(11):1913. https://doi.org/10.3390/diagnostics13111913
Chicago/Turabian StylePop, Marian, and Marius Mărușteri. 2023. "Fat Hounsfield Unit Reference Interval Derived through an Indirect Method" Diagnostics 13, no. 11: 1913. https://doi.org/10.3390/diagnostics13111913
APA StylePop, M., & Mărușteri, M. (2023). Fat Hounsfield Unit Reference Interval Derived through an Indirect Method. Diagnostics, 13(11), 1913. https://doi.org/10.3390/diagnostics13111913





