Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture
Abstract
1. Introduction
- Dual-Energy X-ray Absorptiometry (DXA) measures bone mineral density (BMD) and is universally recognized as the gold standard for the diagnosis of osteoporosis [2]. Unfortunately, DXA has certain limitations; a 2015 study determined that 90% of DXA exams experienced errors (including errors while setting the location information for the patient, during patient positioning, and errors occurring during the analysis) [3]. Moreover, some artifacts can alter DXA results; for example, aortic calcification can lead to an overestimation of bone mineral density [4]; vertebroplasty, especially when involving two or more lumbar vertebrae, limits the ability to obtain an adequate assessment of BMD using DXA [5]; and osteoarthritis (OA), which represents the most common cause of artifacts when using DXA, especially in older patients [6]. In fact, the presence of structural abnormalities caused by OA (sclerosis, osteophytosis) artificially increases BMD measurements for the lumbar spine when using DXA [6].
- Quantitative ultrasonography (QUS) measures the transmission speed and attenuation of waves at the level of the heel, patella, and phalanges of the hands; however, QUS has seen little use because it does not measure bone status at axial skeletal sites and does not allow a diagnostic classification of osteoporosis.
- Quantitative Computed Tomography (QCT) accurately measures the relationship between cortical and trabecular bone portions; this exam represents the most specific method for assessing bone status but is also the most complex and is not always available. High-Resolution Peripheral Quantitative Computed Tomography (HR-pQCT) is used to assess bone density in the tibia and radius bone architecture; however, there is an unclear correlation between HR-pQCT and non-vertebral fractures and higher exposure to ionizing radiation, and cost do not allow this tool to be used in clinical practice [7,8].
- Magnetic Resonance Imaging (MRI) determines information about bone microarchitecture. It is a non-ionizing technique that is not currently usable in clinical practice because it is expensive, not readily available, and takes a long time to carry out.
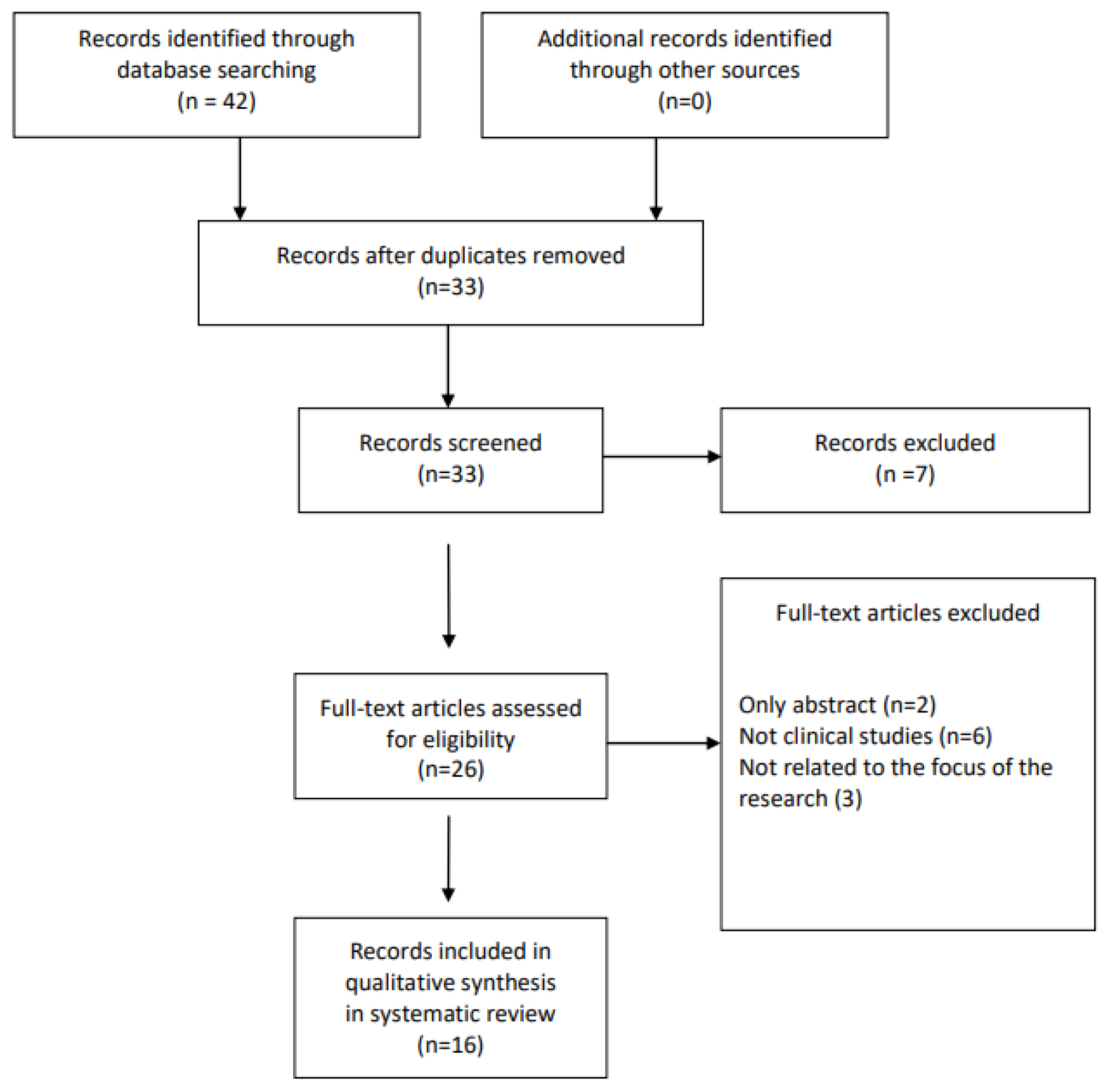
2. Materials and Methods
3. Results
3.1. Main Characteristics of the Studies on Validation of the REMS Technique
3.2. Main Characteristics of the Studies on the Use of REMS Technology in Real-Life Clinical Practice
3.3. Future Perspectives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peck, W.A. Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [Google Scholar]
- Kanis, J.A. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: Synopsis of a WHO report. Osteoporos. Int. 1994, 4, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Messina, C.; Bandirali, M.; Sconfienza, L.M.; D’Alonzo, N.K.; Di Leo, G.; Papini, G.D.E.; Ulivieri, F.M.; Sardanelli, F. Prevalence and type of errors in dual-energy X-ray absorptiometry. Eur. Radiol. 2015, 25, 1504–1511. [Google Scholar] [CrossRef] [PubMed]
- Frye, M.A.; Melton, L.J.; Bryant, S.C.; Fitzpatrick, L.A.; Wahner, H.W.; Schwartz, R.S.; Riggs, B.L. Osteoporosis and calcification of the aorta. Bone Miner. 1992, 19, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Martineau, P.; Bazarjani, S.; Zuckier, L.S. Artifacts and Incidental Findings Encountered on Dual Energy X-ray absortiometry: Atlas and Analysis. Semin. Nucl. Med. 2015, 45, 458–469. [Google Scholar] [CrossRef]
- Stewart, A.; Black, A.J. Bone mineral density in osteoarthritis. Curr. Opin. Rheumatol. 2000, 12, 464–477. [Google Scholar] [CrossRef]
- Diez-Perez, A.; Brandi, M.L.; Al-Daghri, N.; Branco, J.C.; Bruyère, O.; Cavalli, L.; Cooper, C.; Cortet, B.; Dawson-Hughes, B.; Dimai, H.P.; et al. Radiofrequency echographic multi-spectrometry for the in-vivo assessment of bone strength: State of the art-outcomes of an expert consensus meeting organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO). Aging Clin. Exp. Res. 2019, 31, 1375–1389. [Google Scholar] [CrossRef]
- Cheung, A.M.; Adachi, J.D.; Hanley, D.A.; Kendler, D.L.; Davison, K.S.; Josse, R.; Brown, J.P.; Ste-Marie, L.G.; Kremer, R.; Erlandson, M.C.; et al. High-resolution peripheral quantitative computed tomography for the assessment of bone strength and structure: A review by the Canadian Bone Strength Working Group. Curr. Osteoporos. Rep. 2013, 11, 136–146. [Google Scholar] [CrossRef]
- Conversano, F.; Franchini, R.; Greco, A.; Soloperto, G.; Chiriacò, F.; Casciaro, E.; Aventaggiato, M.; Renna, M.D.; Pisani, P.; Di Paola, M.; et al. A NovelUltrasoundMethodology for Estimating Spine MineralDensity. Ultrasound Med. Biol. 2015, 41, 281–300. [Google Scholar] [CrossRef]
- Iwaszkiewicz, C.; Leszczyński, P. Bone densitometry by radiofrequency echographic multi-spectrometry (REMS) in the diagnosis of osteoporosis. Forum Reumatol. 2019, 5, 81–88. [Google Scholar] [CrossRef]
- Di Paola, M.; Gatti, D.; Viapiana, O.; Cianferotti, L.; Cavalli, L.; Caffarelli, C.; Conversano, F.; Quarta, E.; Pisani, P.; Girasole, G.; et al. Radiofrequencyechographicmultispectrometrycompared with dual X-ray absorptiometry for osteoporosisdiagnosis on lumbar spine and femoralneck. Osteoporos. Int. 2019, 30, 391–402. [Google Scholar] [CrossRef]
- Cortet, B.; Dennison, E.; Diez-Perez, A.; Locquet, M.; Muratore, M.; Nogués, X.; Crespo, D.O.; Quarta, E.; Brandi, M.L. RadiofrequencyEchographic multi spectrometry (REMS) for the diagnosis of osteoporosis in a European multicenter clinical context. Bone 2021, 143, 115786. [Google Scholar] [CrossRef]
- Adami, G.; Arioli, G.; Bianchi, G.; Brandi, M.L.; Caffarelli, C.; Cianferotti, L.; Gatti, D.; Girasole, G.; Gonnelli, S.; Manfredini, M.; et al. Radiofrequencyechographic multi spectrometry for the prediction of incidentfragilityfractures: A 5-year follow-up study. Bone 2020, 134, 115297. [Google Scholar] [CrossRef] [PubMed]
- Amorim, D.M.R.; Sakane, E.N.; Maeda, S.S.; LazarettiCastro, M. New technology REMS for bone evaluation compared to DXA in adult women for the osteoporosis diagnosis: A real-life experience. Arch. Osteoporos. 2021, 16, 175. [Google Scholar] [CrossRef] [PubMed]
- Nowakowska-Płaza, A.; Wroński, J.; Płaza, M.; Sudoł-Szopińska, I.; Głuszko, P. Diagnostic agreement between radiofrequency echographicmultispectrometry and dual-energy X-ray absorptiometry in the assessment of osteoporosis in a Polish group of patients. Pol. Arch. Intern. Med. 2021, 131, 840–847. [Google Scholar] [CrossRef] [PubMed]
- Kirilova, E.; Kirilov, N.; Popov, I.; Vladeva, S. Bone mineral density of lumbar spine and femoral neck assessed by novel echographic approach-Radiofrequency Echographic Multi Spectrometry (REMS). Clin. Cases Miner. Bone Metab. 2019, 16, 14–17. [Google Scholar]
- Sergio, R.O.; Nayelli, R.G.E. Evaluation of the bone mineral density in the Mex ican female population using the Radiofrequency Echographic Multi Spectrometry (REMS). Arch. Osteoporos. 2022, 17, 43. [Google Scholar] [CrossRef] [PubMed]
- Lalli, P.; Mautino, C.; Busso, C.; Bardesono, F.; Di Monaco, M.; Lippi, L.; Invernizzi, M.; Minetto, M.A. Reproducibility and Accuracy of the Radiofrequency Echographic Multi-Spectrometry for Femoral Mineral Density Estimation and Discriminative Power of the Femoral Fragility Score in Patients with Primary and Disuse-Related Osteoporosis. J. Clin. Med. 2022, 11, 3761. [Google Scholar] [CrossRef]
- Pisani, P.; Conversano, F.; Muratore, M.; Adami, G.; Brandi, M.L.; Caffarelli, C.; Casciaro, E.; Di Paola, M.; Franchini, R.; Gatti, D.; et al. Fragility Score: A REMS-based indicator for the prediction of incident fragility fractures at 5 years. Aging Clin. Exp. Res. 2023, 35, 763–773. [Google Scholar] [CrossRef]
- Greco, A.; Pisani, P.; Conversano, F.; Soloperto, G.; Renna, M.D.; Muratore, M.; Casciaro, S. Ultrasound Fragility Score: An innovative approach for the assessment of bone fragility. Measurement 2017, 101, 236–242. [Google Scholar] [CrossRef]
- Caffarelli, C.; TomaiPitinca, M.D.; Al Refaie, A.; Ceccarelli, E.; Gonnelli, S. Ability of radiofrequency echographic multispectrometry to identify osteoporosis status in elderly women with type 2 diabetes. Aging Clin. Exp. Res. 2022, 34, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Rolla, M.; Halupczok-Żyła, J.; Jawiarczyk-Przybyłowska, A.; Bolanowski, M. Bone densitometry by radiofrequency echographic multi-spectrometry (REMS) in acromegaly patients. Endokrynol. Pol. 2020, 71, 524–531. [Google Scholar] [CrossRef] [PubMed]
- Bojincă, V.C.; Popescu, C.C.; Decianu, R.D.; Dobrescu, A.; Bălănescu, Ș.M.; Bălănescu, A.R.; Bojincă, M. A novel quantitative method for estimating bone mineral density using B-mode ultrasound and radiofrequency signals-a pilot study on patients with rheumatoid arthritis. Exp. Ther. Med. 2019, 18, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Tomai Pitinca, M.D.; Fortini, P.; Gonnelli, S.; Caffarelli, C. Could Radiofrequency Echographic Multi-Spectrometry (REMS) Overcome the Limitations of BMD by DXA Related to Artifacts? A Series of 3 Cases. J. Ultrasound Med. 2021, 40, 2773–2777. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.Y.; Center, J.R.; Eisman, J.A.; Nguyen, T.V. Bone mineral density and association of osteoarthritis with fracture risk. Osteoarthr. Cartil. 2014, 22, 1251–1258. [Google Scholar] [CrossRef]
- Castaño-Betancourt, M.; Oei, L.; Rivadeneira, F.; de Schepper, E.; Hofman, A.; Bierma-Zeinstra, S.; Pols, H.; Uitterlinden, A.; Van Meurs, J. Association of lumbar disc degeneration with osteoporotic fractures: The Rotterdam Study and meta-analysis from systematic review. Bone 2013, 57, 284–289. [Google Scholar] [CrossRef]
- Caffarelli, C.; Tomai Pitinca, M.D.; Al Refaie, A.; De Vita, M.; Catapano, S.; Gonnelli, S. Could radiofrequency echographic multispectrometry (REMS) overcome the overestimation in BMD by dual-energy X-ray absorptiometry (DXA) at the lumbar spine? BMC Musculoskelet. Disord. 2022, 23, 469. [Google Scholar] [CrossRef]
- Pimentel, A.; Ureña-Torres, P.; Zillikens, M.C.; Bover, J.; Cohen-Solal, M. Fractures in patients with CKD–diagnosis, treatment, and prevention: A review by members of the European Calcified Tissue Society and the European Renal Association of Nephrology Dialysis and Transplantation. Kidney Int. 2017, 92, 1343–1355. [Google Scholar] [CrossRef]
- Jadoul, M.; Albert, J.M.; Akiba, T.; Akizawa, T.; Arab, L.; Bragg-Gresham, J.L.; Mason, N.; Prutz, K.G.; Young, E.W.; Pisoni, R.L. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2006, 70, 1358–1366. [Google Scholar] [CrossRef]
- Fassio, A.; Andreola, S.; Gatti, D.; Bianco, B.; Gatti, M.; Gambaro, G.; Rossini, M.; Viapiana, O.; Negrelli, R.; Adami, G. Radiofrequency echographic multi-spectrometry and DXA for the evaluation of bone mineral density in a peritoneal dialysis setting. Aging Clin. Exp. Res. 2023, 35, 185–192. [Google Scholar] [CrossRef]
- Caffarelli, C.; Al Refaie, A.; De Vita, M.; Tomai Pitinca, M.D.; Goracci, A.; Fagiolini, A.; Gonnelli, S. Radiofrequency echographic multispectrometry (REMS): An innovative technique for the assessment of bone status in young women with anorexia nervosa. Eat. Weight. Disord.—Stud. Anorex. Bulim. Obes. 2022, 27, 3207–3213. [Google Scholar] [CrossRef] [PubMed]
- Degennaro, V.A.; Brandi, M.L.; Cagninelli, G.; Lombardi, F.A.; Pisani, P.; Ghi, T. First assessment of bone mineral density in healthy pregnant women by means of Radiofrequency Echographic Multi Spectrometry (REMS) technology. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 263, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Møller, U.K.; Við Streym, S.; Mosekilde, L.; Rejnmark, L. Changes in bone mineral density and body composition during pregnancy and postpartum. A controlled cohort study. Osteoporos. Int. 2012, 23, 1213–1223. [Google Scholar] [CrossRef] [PubMed]
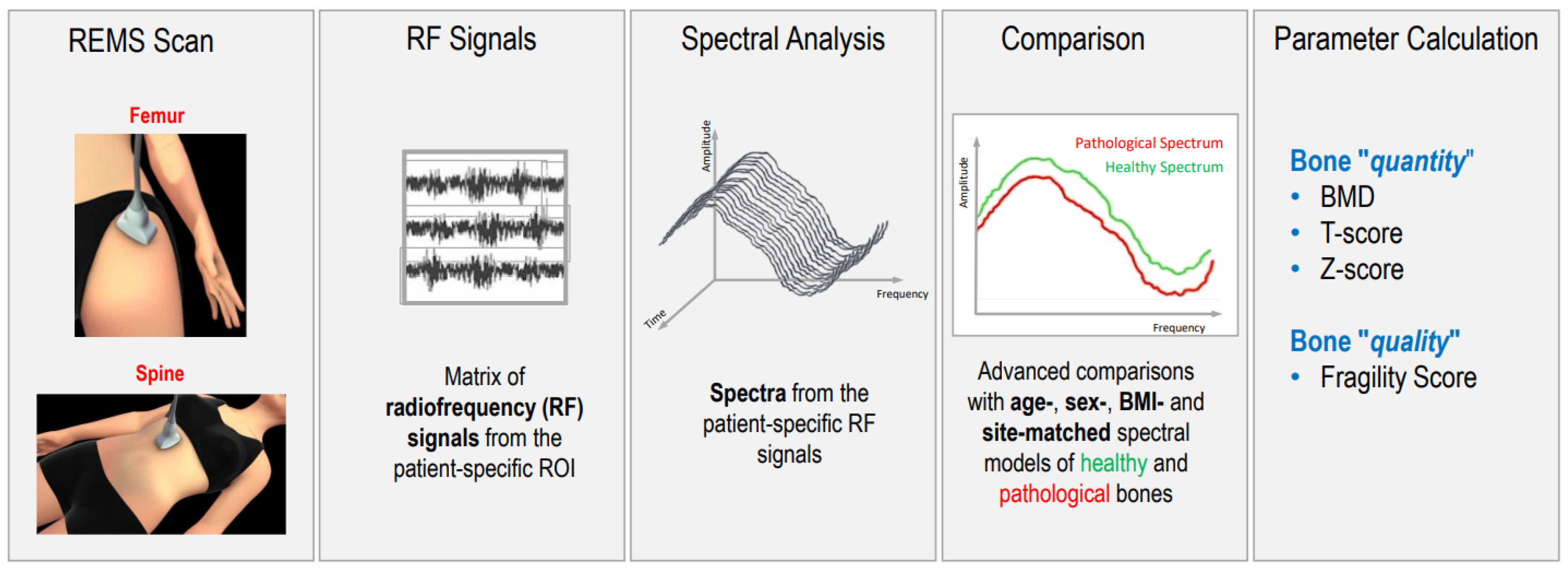
| Study/Year | Study Characteristics | Population | Outcomes | Results | Conclusions |
|---|---|---|---|---|---|
| Di Paola M (2019) Italy [11] | Multicenter cross-sectional observational | 1914 PMO ♀ (51–70 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN | Sensitivity DXA vs. REMS LS = 91.7% FN = 91.5% Specificity DXA vs. REMS LS = 92.0% FN = 91.8% Diagnostic Concordance DXA vs. REMS LS = 88.8% (k = 0.824, p < 0.001) FN = 88.2% (k = 0.794, p < 0.001) Correlation DXA vs. REMS LS = (r = 0.94, p < 0.001) FN = (r = 0.93, p < 0.001) | REMS approach had a good level of accuracy and precision in osteoporosis diagnosis |
| Kirilova E (2019) Bulgaria [16] | Cross-sectional observational | 25 premenopausal ♀: (24–50 years) 140 PMO ♀ (38–86 years) | REMS-LS; REMS-FN; REMS-TH | REMS-LS, REMS-FN, REMS-TH measurements of PMO group were significantly lower than those of premenopausal OP | REMS identify lower BMD between PMO and premenopausal OP |
| Adami G. (2020) Italy [13] | Longitudinal observational (5 years) | 1370 ♀ (30–90 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN; Incidence of fragility fractures | Fracture incidence was 14.0% For a T-score cut-off −2.5 identified Fx REMS-LS = sensitivity of 65.1% and specificity of 57.7% DXA-LS = sensitivity of 57.1% and a specificity of 56.3% REMS-FN = sensitivity 40.2% and specificity 79.9% DXA-FN = sensitivity 42.3% and specificity 79.3%. | REMS T-score resulted an effective predictor of fragility fractures |
| Cortet B (2021) Europe [12] | Multicenter cross-sectional observational | 4307 ♀ (30–90 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN; | Sensitivity DXA vs. REMS LS = 90.9% FN = 90.4% Specificity DXA vs. REMS LS = 95.1% FN = 95.5% ability to recognize fractured patients DXA and REMS The AUCs of the ROC curve: LS = 0.603 and 0.640 (p = 0.0002) FN = 0.631 for DXA and 0.683 for REMS (p < 0.001). | Diagnostic effectiveness of REMS technology was reconfirmed in a larger and younger population |
| Nowakowska-Płaza A (2021) Poland [15] | Cross-sectional observational | 98 ♀ and 18 ♂ (40–90 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN; | diagnostic agreement DXA vs. REMS LS = 82.8% FN = 84.8% | Significant diagnostic agreement between DXA and REMS |
| Amorim DMR (2021) Brazil [14] | Cross-sectional observational | 343 ♀ (30–80 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN; | Correlation DXA vs. REMS LS = (r = 0.75, p < 0.001) FN = (r = 0.78, p < 0.001) The AUCs of the ROC curve using DXA T-score as reference: LS = 0.94; FN = 0.97 | REMS comparing with DXA had high accuracy for the diagnosis of osteoporosis |
| Sergio RO (2022) Mexico [17] | Cross-sectional observational | 455 ♀ (40–87 years) | REMS-LS; REMS-FN; | diagnostic agreement between REMS-LS and REMS-FN = 73%. Good correlation between LS, FN by REMS Prevalence of OP ↑ with age and ↓ BMI | Age and BMI variations correlate with the prevalence of osteoporosis. |
| Lalli P (2022) Italy [18] | Cross-sectional observational | 140 primary OP: 120 ♀, 20 ♂ (64–81 years) 35 disuse-related OP: 14 ♀; 20 ♂ (49–65.3 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN; | Diagnostic concordance DXA and REMS Primary OP = 63% (Cohen’s kappa = 0.31) Disuse-relate OP = 13% (Cohen’s kappa = −0.04) Correlation FS and FRAX major Fx = (R = 0.65, p = 0.0001) Correlation FS and hip Fx (R = 0.62, p = 0.0001) in primary OP no Fx | REMS showed excellent test–retest reproducibility diagnostic concordance between DXA and REMS was minimal |
| Pisani P (2023) Italy [19] | Longitudinal observational (5 years) | 1289 ♀ (54–66 years) Fx: 181 (63–74 years) No Fx: 1108 (54–64 years) 515 ♂ (48.3–73.0 years) Fx: 67 (57.3–78 years) No Fx: 448 (47–71 years) | DXA-LS; DXA-FN; REMS-LS; REMS-FN; FS Incident Fx | For prediction of generic fracture FS provided AUC = 0.811 for ♀ and AUC = 0.780 for ♂, which resulted in AUC = 0.715 and AUC = 0.758 adjusted for age and BMI For prediction of hip fracture, the corresponding values were AUC = 0.780 for ♀ and AUC = 0.809 for ♂, which became AUC = 0.735 and AUC = 0.758 adjusted for age and BMI | FS displayed a superior performance in fracture prediction, representing a valuable diagnostic tool to accurately detect a short-term fracture risk |
| Author/Year, Country | Study | Population | Assessments | Results | Conclusions |
|---|---|---|---|---|---|
| Bojincă VC (2019) Romania [23] | Cross-sectional observational | RA: 106 ♀ (65 ± 8 years) controls: 119 ♀ (64 ± 13 years) | REMS-LS; REMS-FN dx; REMS-FN sn | RA patients ↓ BMD at all sites RA had higher prevalence of osteoporosis | REMS is able to replicate the results of the established DXA measurements |
| Rolla M (2020) Poland [22] | Cross-sectional observational | AG: 25 ♀, 8 ♂ (59.1 ± 9.8 years) CG: 17 ♀, 7 ♂ (age-matched) | DXA-LS; DXA-FN; REMS-LS; REMS-FN | REMS BMD-LS, T-score LS and Z-score LS and BMD-FN, T-score FN, Z-score FN are in agreement with DXA measurements in AG and CG. | REMS may be considered a potential method in assessment of bone status in acromegaly |
| Caffarelli C (2021) Italy [21] | Cross-sectional observational | TDM2: 90 ♀ (70.5 ± 7.6 years) controls: 90 ♀ (69.2 ± 7.5 years) | DXA-LS; DXA-FN; DXA-TH REMS-LS; REMS-FN; REMS-TH | REMS BMD ↓ T2DM than in non T2DM REMS classified as “osteoporotic” more T2DM respect to those classified by DXA (47.0% vs. 28.0%, respectively) | REMS technology may represent a useful approach to enhance the diagnosis of osteoporosis in patients with T2DM |
| Degennaro VA (2021) Italy [32] | Cross-sectional case—control observational | pregnant: 78 ♀ (32.9 ± 5.0 years) controls: 78 ♀ (32.9 ± 5.2 years) | REMS-FN | REMS-FN BMD ↓ (8.1%) in pregnant women than in controls | It is the first study that demonstrate decreased BMD in pregnancy thanks to REMS |
| Caffarelli C (2022) Italy [27] | Cross-sectional observational | OA: 113 ♀ (63.2 ± 11.3 years) Vertebral Fx: 43 ♀ (73.6 ± 18.5 years) | DXA-LS; DXA-FN; DXA-TH REMS-LS; REMS-FN; REMS-TH | REMS BMD T-scores LS, FN and TH were significantly lower than DXA BMD T-score LS (p < 0.01) FN and TH (p < 0.05). In OA group REMS classified as “osteoporotic” more subjects respect to those classified by DXA (35.1% vs. 9.3%, respectively). In Vertebral Fx group REMS classified as “osteoporotic” more subjects respect to those classified by DXA (58.7% vs. 23.3%, respectively). | REMS appears to be able to overcome the most common artifacts, such as OA and vertebral Fxe of the lumbar spine, which affect the value of BMD by DXA. |
| Caffarelli C (2022) Italy [31] | Cross-sectional observational | AN: 47 ♀ (31.7 ± 10.3 years) controls: 30 ♀ (32.9 ± 9.5 years) | DXA-LS; DXA-FN; DXA-TH REMS-LS; REMS-FN; REMS-TH | Correlation DXA vs. REMS LS = (r = 0.64, p < 0.01) FN = (r = 0.86, p < 0.01) TH = (r = 0.84, p < 0.01) Good agreement REMS between DXA by Bland–Altman analysis AN with Fx have lower values of both BMD-LS and BMD-TH by DXA and by REMS with respect to AN without Fx | REMS represent an important tool for the evaluation AN in young women, especially during the fertile age and in case of pregnancy and breastfeeding. |
| Fassio A (2023) Italy [30] | Cross-sectional observational | 41 (29♂; 12♀) (61.1 ± 13.7 years) | DXA-LS-AP; DXA-LS-LL; DXA-FN; DXA-TH REMS-LS; REMS-FN; REMS-TH | No significant differences between BMD T-scores FN and TH measured by DXA or REMS BMD-LS-AP by DXA was higher (−0.49 ± 1.98) respect to BMD-LS-LL (−1.66 ± 0.99) by DXA and BMD-LS (−2.00 ± 1.94) by REMS | promising agreement, in a real-life PD setting, between the DXA and REMS BMD values and in the consequent fracture risk assessment. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Refaie, A.; Baldassini, L.; Mondillo, C.; Giglio, E.; De Vita, M.; Tomai Pitinca, M.D.; Gonnelli, S.; Caffarelli, C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture. Diagnostics 2023, 13, 1666. https://doi.org/10.3390/diagnostics13101666
Al Refaie A, Baldassini L, Mondillo C, Giglio E, De Vita M, Tomai Pitinca MD, Gonnelli S, Caffarelli C. Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture. Diagnostics. 2023; 13(10):1666. https://doi.org/10.3390/diagnostics13101666
Chicago/Turabian StyleAl Refaie, Antonella, Leonardo Baldassini, Caterina Mondillo, Elisa Giglio, Michela De Vita, Maria Dea Tomai Pitinca, Stefano Gonnelli, and Carla Caffarelli. 2023. "Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture" Diagnostics 13, no. 10: 1666. https://doi.org/10.3390/diagnostics13101666
APA StyleAl Refaie, A., Baldassini, L., Mondillo, C., Giglio, E., De Vita, M., Tomai Pitinca, M. D., Gonnelli, S., & Caffarelli, C. (2023). Radiofrequency Echographic Multi Spectrometry (R.E.M.S.): New Frontiers for Ultrasound Use in the Assessment of Bone Status—A Current Picture. Diagnostics, 13(10), 1666. https://doi.org/10.3390/diagnostics13101666





