Differences in Striatal Metabolism in [18F]FDG PET in Parkinson’s Disease and Atypical Parkinsonism
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Image Acquisition
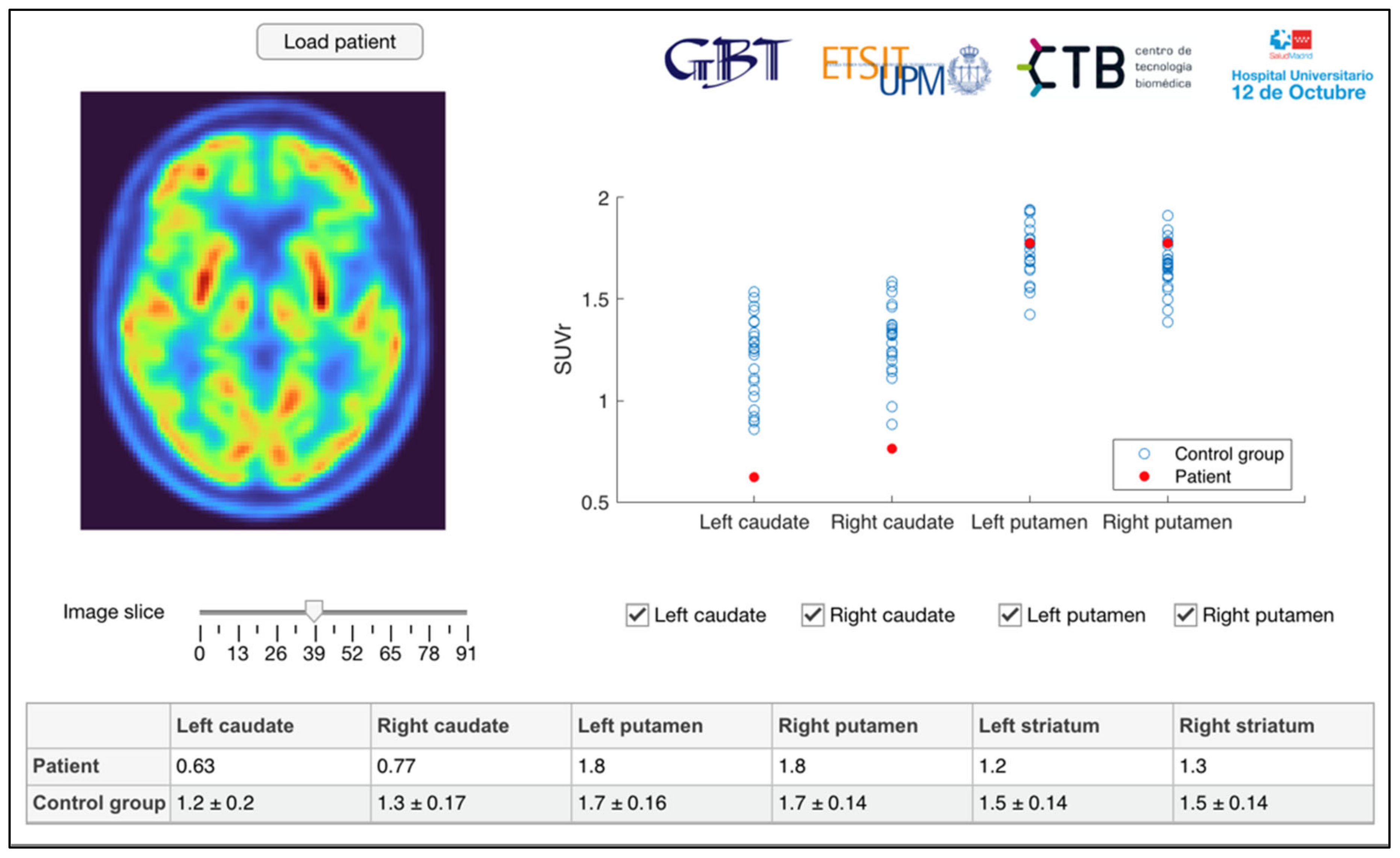
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Study Cohort
3.2. Comparison of PD, Atypical Parkinsonism and Control
3.3. Comparison of Atypical Parkinsonisms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elahi, F.M.; Miller, B.L. A Clinicopathological Approach to the Diagnosis of Dementia. Nat. Rev. Neurol. 2017, 13, 457–476. [Google Scholar] [CrossRef] [PubMed]
- Erkkinen, M.G.; Kim, M.O.; Geschwind, M.D. Clinical Neurology and Epidemiology of the Major Neurodegenerative Diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.B.; O’Callaghan, J.P. Biomarkers of Parkinson’s Disease: Present and Future. Metabolism 2015, 64, S40–S46. [Google Scholar] [CrossRef]
- Levin, J.; Kurz, A.; Arzberger, T.; Giese, A.; Höglinger, G.U. The Differential Diagnosis and Treatment of Atypical Parkinsonism. Dtsch. Arztebl. Int. 2016, 113, 61. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.C.; Poston, K.L.; Eckert, T.; Feigin, A.; Frucht, S.; Gudesblatt, M.; Dhawan, V.; Lesser, M.; Vonsattel, J.P.; Fahn, S.; et al. Differential Diagnosis of Parkinsonism: A Metabolic Imaging Study Using Pattern Analysis. Lancet Neurol. 2010, 9, 149–158. [Google Scholar] [CrossRef]
- Morbelli, S.; Esposito, G.; Arbizu, J.; Barthel, H.; Boellaard, R.; Bohnen, N.I.; Brooks, D.J.; Darcourt, J.; Dickson, J.C.; Douglas, D.; et al. EANM Practice Guideline/SNMMI Procedure Standard for Dopaminergic Imaging in Parkinsonian Syndromes 1.0. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1885–1912. [Google Scholar] [CrossRef]
- Benamer, H.T.S.; Patterson, J.; Grosset, D.G.; Booij, J.; de Bruin, K.; van Royen, E.; Speelman, J.D.; Horstink, M.H.I.M.; Sips, H.J.W.A.; Dierckx, R.A.; et al. Accurate Differentiation of Parkinsonism and Essential Tremor Using Visual Assessment of [123I]-FP-CIT SPECT Imaging: The [123I]-FP-CIT Study Group. Mov. Disord. 2000, 15, 503–510. [Google Scholar] [CrossRef]
- Scherfler, C.; Schwarz, J.; Antonini, A.; Grosset, D.; Valldeoriola, F.; Marek, K.; Oertel, W.; Tolosa, E.; Lees, A.J.; Poewe, W. Role of DAT-SPECT in the Diagnostic Work up of Parkinsonism. Mov. Disord. 2007, 22, 1229–1238. [Google Scholar] [CrossRef] [PubMed]
- Schreckenberger, M.; Hägele, S.; Siessmeier, T.; Buchholz, H.G.; Armbrust-Henrich, H.; Rösch, F.; Gründer, G.; Bartenstein, P.; Vogt, T. The Dopamine D2 Receptor Ligand 18F-Desmethoxyfallypride: An Appropriate Fluorinated PET Tracer for the Differential Diagnosis of Parkinsonism. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 1128–1135. [Google Scholar] [CrossRef] [PubMed]
- Nobili, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Agosta, F.; Nestor, P.; Walker, Z.; Boccardi, M.; Festari, C.; Altomare, D.; et al. European Association of Nuclear Medicine and European Academy of Neurology Recommendations for the Use of Brain 18F-Fluorodeoxyglucose Positron Emission Tomography in Neurodegenerative Cognitive Impairment and Dementia: Delphi Consensus. Eur. J. Neurol. 2018, 25, 1201–1217. [Google Scholar] [CrossRef]
- Brown, R.K.J.; Bohnen, N.I.; Wong, K.K.; Minoshima, S.; Frey, K.A. Brain PET in Suspected Dementia: Patterns of Altered FDG Metabolism. Radiographics 2014, 34, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Seiffert, A.P.; Gómez-Grande, A.; Villarejo-Galende, A.; Gómez, E.J.; Sánchez-González, P. Methodologies for the Quantification and Classification of PET Neuroimaging of Patients with Neurodegenerative Diseases for the Clinical Decision Support. In Proceedings of the XXXIX Congreso Anual de la Sociedad Española de Ingeniería Biomédica, Online, 25–26 November 2021; pp. 79–82. [Google Scholar]
- Eidelberg, D.; Moeller, J.R.; Dhawan, V.; Spetsieris, P.; Takikawa, S.; Ishikawa, T.; Chaly, T.; Robeson, W.; Margouleff, D.; Przedborski, S.; et al. The Metabolic Topography of Parkinsonism. J. Cereb. Blood Flow Metab. 1994, 14, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Meles, S.K.; Renken, R.J.; Pagani, M.; Teune, L.K.; Arnaldi, D.; Morbelli, S.; Nobili, F.; van Laar, T.; Obeso, J.A.; Rodríguez-Oroz, M.C.; et al. Abnormal Pattern of Brain Glucose Metabolism in Parkinson’s Disease: Replication in Three European Cohorts. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 437–450. [Google Scholar] [CrossRef]
- Eckert, T.; Barnes, A.; Dhawan, V.; Frucht, S.; Gordon, M.F.; Feigin, A.S.; Eidelberg, D. FDG PET in the Differential Diagnosis of Parkinsonian Disorders. Neuroimage 2005, 26, 912–921. [Google Scholar] [CrossRef]
- Eckert, T.; Tang, C.; Ma, Y.; Brown, N.; Lin, T.; Frucht, S.; Feigin, A.; Eidelberg, D. Abnormal Metabolic Networks in Atypical Parkinsonism. Mov. Disord. 2008, 23, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Teune, L.K.; Bartels, A.L.; de Jong, B.M.; Willemsen, A.T.M.; Eshuis, S.A.; de Vries, J.J.; van Oostrom, J.C.H.; Leenders, K.L. Typical Cerebral Metabolic Patterns in Neurodegenerative Brain Diseases. Mov. Disord. 2010, 25, 2395–2404. [Google Scholar] [CrossRef] [PubMed]
- Niccolini, F.; Politis, M. A Systematic Review of Lessons Learned from PET Molecular Imaging Research in Atypical Parkinsonism. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 2244–2254. [Google Scholar] [CrossRef]
- Walker, Z.; Gandolfo, F.; Orini, S.; Garibotto, V.; Agosta, F.; Arbizu, J.; Bouwman, F.; Drzezga, A.; Nestor, P.; Boccardi, M.; et al. Clinical Utility of FDG PET in Parkinson’s Disease and Atypical Parkinsonism Associated with Dementia. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1534–1545. [Google Scholar] [CrossRef]
- Buchert, R.; Buhmann, C.; Apostolova, I.; Meyer, P.T.; Gallinat, J. Nuclear Imaging in the Diagnosis of Clinically Uncertain Parkinsonian Syndromes. Dtsch. Arztebl. Int. 2019, 116, 747–754. [Google Scholar] [CrossRef]
- Verger, A.; Grimaldi, S.; Ribeiro, M.J.; Frismand, S.; Guedj, E. Single Photon Emission Computed Tomography/Positron Emission Tomography Molecular Imaging for Parkinsonism: A Fast-Developing Field. Ann. Neurol. 2021, 90, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Presotto, L.; Iaccarino, L.; Sala, A.; Vanoli, E.G.; Muscio, C.; Nigri, A.; Bruzzone, M.G.; Tagliavini, F.; Gianolli, L.; Perani, D.; et al. Low-Dose CT for the Spatial Normalization of PET Images: A Validation Procedure for Amyloid-PET Semi-Quantification. NeuroImage Clin. 2018, 20, 153–160. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- GE Healthcare DaTQUANT. Available online: https://www.gehealthcare.com/products/nuclear-imaging-agents/datquant (accessed on 13 September 2022).
- Garcia-Garcia, D.; Clavero, P.; Salas, C.G.; Lamet, I.; Arbizu, J.; Gonzalez-Redondo, R.; Obeso, J.A.; Rodriguez-Oroz, M.C. Posterior Parietooccipital Hypometabolism May Differentiate Mild Cognitive Impairment from Dementia in Parkinson’s Disease. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1767–1777. [Google Scholar] [CrossRef] [PubMed]
- Arbizu, J.; Luquin, M.R.; Abella, J.; de la Fuente-Fernández, R.; Fernandez-Torrón, R.; García-Solís, D.; Garrastachu, P.; Jiménez-Hoyuela, J.M.; Llaneza, M.; Lomeña, F.; et al. Functional Neuroimaging in the Diagnosis of Patients with Parkinsonism: Update and Recommendations for Clinical Use. Rev. Esp. Med. Nucl. Imagen Mol. 2014, 33, 215–226. [Google Scholar] [CrossRef]
- Hellwig, S.; Amtage, F.; Kreft, A.; Buchert, R.; Winz, O.H.; Vach, W.; Spehl, T.S.; Rijntjes, M.; Hellwig, B.; Weiller, C.; et al. [18F]FDG-PET Is Superior to [123I]IBZM-SPECT for the Differential Diagnosis of Parkinsonism. Neurology 2012, 79, 1314–1322. [Google Scholar] [CrossRef]
- Matías-Guiu, J.A.; García-Ramos, R. Primary Progressive Aphasia: From Syndrome to Disease. Neurol. (Engl. Ed.) 2013, 28, 366–374. [Google Scholar] [CrossRef]
- Albrecht, F.; Ballarini, T.; Neumann, J.; Schroeter, M.L. FDG-PET Hypometabolism Is More Sensitive than MRI Atrophy in Parkinson’s Disease: A Whole-Brain Multimodal Imaging Meta-Analysis. NeuroImage Clin. 2019, 21, 101594. [Google Scholar] [CrossRef]
- Berding, G.; Odin, P.; Brooks, D.J.; Nikkhah, G.; Matthies, C.; Peschel, T.; Shing, M.; Kolbe, H.; van den Hoff, J.; Fricke, H.; et al. Resting Regional Cerebral Glucose Metabolism in Advanced Parkinson’s Disease Studied in the off and on Conditions with [18F]FDG-PET. Mov. Disord. 2001, 16, 1014–1022. [Google Scholar] [CrossRef]
- Feigin, A.; Fukuda, M.; Dhawan, V.; Przedborski, S.; Jackson-Lewis, V.; Mentis, M.J.; Moeller, J.R.; Eidelberg, D. Metabolic Correlates of Levodopa Response in Parkinson’s Disease. Neurology 2001, 57, 2083–2088. [Google Scholar] [CrossRef]
- Ko, J.H.; Lerner, R.P.; Eidelberg, D. Effects of Levodopa on Regional Cerebral Metabolism and Blood Flow. Mov. Disord. 2015, 30, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, K.; Onishi, A.; Fujiwara, Y.; Oda, K.; Ishiwata, K.; Ishii, K. Longitudinal Effects of Aging on 18F-FDG Distribution in Cognitively Normal Elderly Individuals. Sci. Rep. 2018, 8, 11557. [Google Scholar] [CrossRef] [PubMed]
- van Aalst, J.; Devrome, M.; Van Weehaeghe, D.; Rezaei, A.; Radwan, A.; Schramm, G.; Ceccarini, J.; Sunaert, S.; Koole, M.; Van Laere, K. Regional Glucose Metabolic Decreases with Ageing Are Associated with Microstructural White Matter Changes: A Simultaneous PET/MR Study. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 664–680. [Google Scholar] [CrossRef] [PubMed]
| Diagnosis | N | Age (Years ± SD) | Sex (m/f) |
|---|---|---|---|
| PD | 3 | 74.33 ± 11.72 | 1/2 |
| Atypical parkinsonism | 29 | 73.28 ± 8.45 | 17/12 |
| PSP | 10 | 71.80 ± 7.94 | 9/1 |
| CBD | 6 | 74.00 ± 7.40 | 5/1 |
| MSA | 2 | 69.50 ± 10.61 | 0/2 |
| DLB | 7 | 75.43 ± 9.20 | 2/5 |
| NC | 4 | 74.00 ± 12.25 | 1/3 |
| Control group | 25 | 63.84 ± 8.23 | 11/14 |
| Region | PD | Atypical Parkinsonism | Control Group | p-Value | |
|---|---|---|---|---|---|
| Caudate | L | 1.29 ± 0.03 | 0.89 ± 0.23 | 1.20 ± 0.20 | <0.001 |
| R | 1.32 ± 0.03 | 0.98 ± 0.28 | 1.29 ± 0.17 | <0.001 | |
| Putamen | L | 1.84 ± 0.05 | 1.60 ± 0.14 | 1.75 ± 0.16 | 0.001 |
| R | 1.81 ± 0.05 | 1.56 ± 0.17 | 1.68 ± 0.14 | 0.003 | |
| Striatum | L | 1.57 ± 0.02 | 1.25 ± 0.13 | 1.48 ± 0.14 | <0.001 |
| R | 1.57 ± 0.02 | 1.28 ± 0.16 | 1.49 ± 0.13 | <0.001 | |
| Whole | 1.57 ± 0.01 | 1.27 ± 0.14 | 1.48 ± 0.13 | <0.001 | |
| Region | PD—Atypical Parkinsonism | PD—Control Group | Atypical Parkinsonism—Control Group | |
|---|---|---|---|---|
| Caudate | L | 0.011 | 1.000 | <0.001 |
| R | 0.059 | 1.000 | <0.001 | |
| Putamen | L | 0.034 | 0.983 | 0.002 |
| R | 0.025 | 0.475 | 0.017 | |
| Striatum | L | 0.001 | 0.802 | <0.001 |
| R | 0.005 | 1.000 | <0.001 | |
| Whole | 0.001 | 0.874 | <0.001 | |
| Region | PSP | CBD | MSA | DLB | NC | p-Value | |
|---|---|---|---|---|---|---|---|
| Caudate | L | 0.92 ± 0.14 | 0.94 ± 0.23 | 1.26 ± 0.27 | 0.80 ± 0.28 | 0.68 ± 0.10 | 0.034 |
| R | 1.05 ± 0.19 | 1.04 ± 0.24 | 1.44 ± 0.06 | 0.90 ± 0.26 | 0.64 ± 0.19 | 0.010 | |
| Putamen | L | 1.56 ± 0.45 | 1.67 ± 0.17 | 1.66 ± 0.09 | 1.59 ± 0.14 | 1.59 ± 0.15 | 0.690 |
| R | 1.52 ± 0.13 | 1.63 ± 0.18 | 1.61 ± 0.30 | 1.58 ± 0.17 | 1.49 ± 0.20 | 0.713 | |
| Striatum | L | 1.25 ± 0.08 | 1.31 ± 0.16 | 1.46 ± 0.09 | 1.21 ± 0.12 | 1.14 ± 0.10 | 0.027 |
| R | 1.29 ± 0.12 | 1.35 ± 0.16 | 1.53 ± 0.13 | 1.25 ± 0.10 | 1.08 ± 0.18 | 0.008 | |
| Whole | 1.27 ± 0.09 | 1.33 ± 0.16 | 1.50 ± 0.02 | 1.23 ± 0.11 | 1.11 ± 0.13 | 0.035 | |
| Atypical Parkinsonism | Caudate | Putamen | Striatum |
|---|---|---|---|
| PSP | 0.263 | <0.001 | 0.197 |
| CBD | 0.003 | 0.007 | 0.002 |
| MSA | <0.001 | <0.001 | <0.001 |
| DLB | 0.001 | 0.001 | 0.004 |
| NC | 0.592 | 0.044 | 0.190 |
| Atypical Parkinsonism | Caudate–Putamen | |
|---|---|---|
| L | R | |
| PSP | 0.278 | 0.735 |
| CBD | 0.531 | 0.634 |
| MSA | 0.180 | 0.655 |
| DLB | 0.018 | 0.018 |
| NC | 0.068 | 0.261 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seiffert, A.P.; Gómez-Grande, A.; Alonso-Gómez, L.; Méndez-Guerrero, A.; Villarejo-Galende, A.; Gómez, E.J.; Sánchez-González, P. Differences in Striatal Metabolism in [18F]FDG PET in Parkinson’s Disease and Atypical Parkinsonism. Diagnostics 2023, 13, 6. https://doi.org/10.3390/diagnostics13010006
Seiffert AP, Gómez-Grande A, Alonso-Gómez L, Méndez-Guerrero A, Villarejo-Galende A, Gómez EJ, Sánchez-González P. Differences in Striatal Metabolism in [18F]FDG PET in Parkinson’s Disease and Atypical Parkinsonism. Diagnostics. 2023; 13(1):6. https://doi.org/10.3390/diagnostics13010006
Chicago/Turabian StyleSeiffert, Alexander P., Adolfo Gómez-Grande, Laura Alonso-Gómez, Antonio Méndez-Guerrero, Alberto Villarejo-Galende, Enrique J. Gómez, and Patricia Sánchez-González. 2023. "Differences in Striatal Metabolism in [18F]FDG PET in Parkinson’s Disease and Atypical Parkinsonism" Diagnostics 13, no. 1: 6. https://doi.org/10.3390/diagnostics13010006
APA StyleSeiffert, A. P., Gómez-Grande, A., Alonso-Gómez, L., Méndez-Guerrero, A., Villarejo-Galende, A., Gómez, E. J., & Sánchez-González, P. (2023). Differences in Striatal Metabolism in [18F]FDG PET in Parkinson’s Disease and Atypical Parkinsonism. Diagnostics, 13(1), 6. https://doi.org/10.3390/diagnostics13010006





