A Pain in the Neck: Lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma—Systematic Review of the Literature, Meta-Analysis and Case Series
Abstract
1. Introduction
2. Materials and Methods
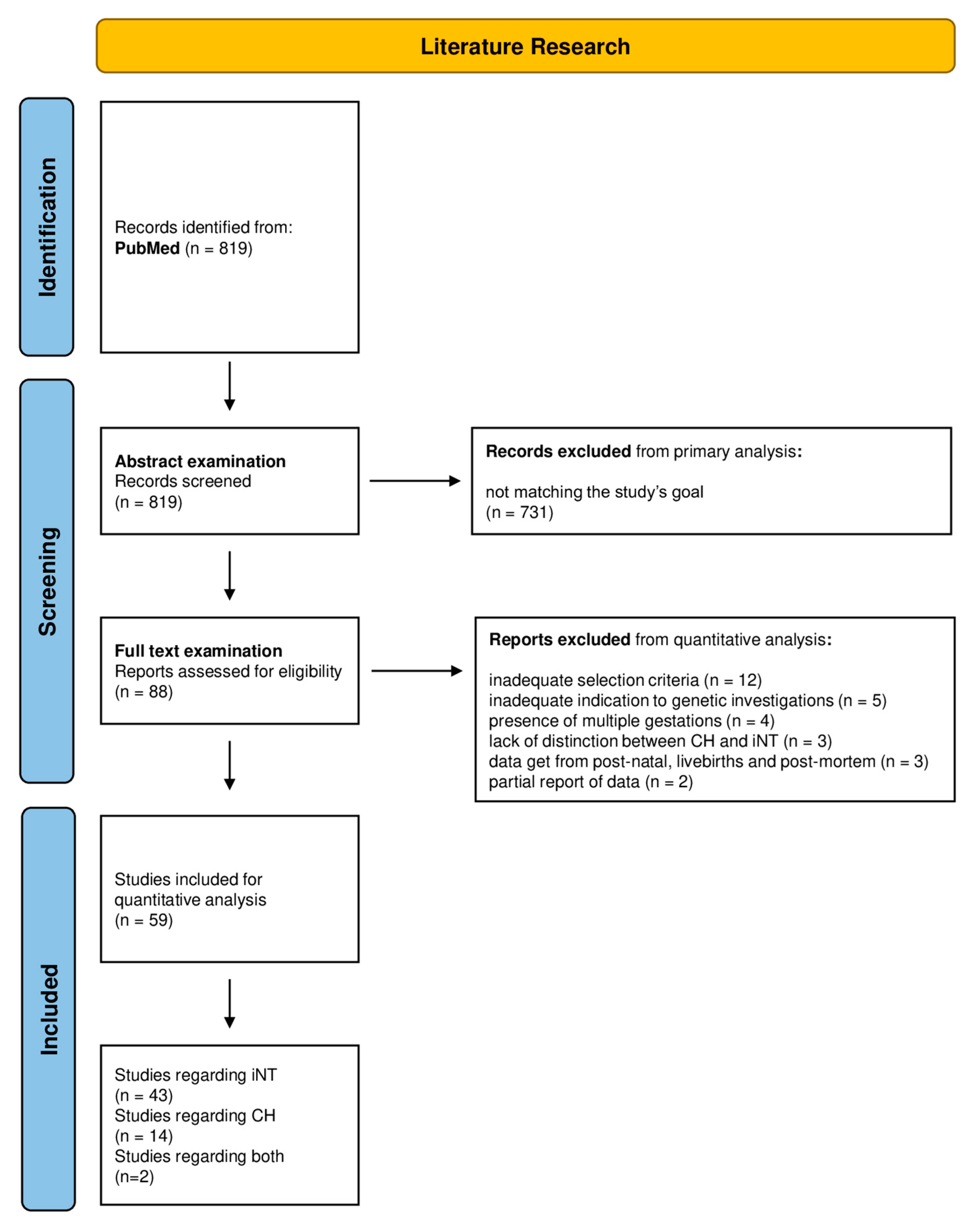
2.1. Systematic Review of the Literature and Meta-Analysis
2.2. Fetal Cohort
3. Results
3.1. Systematic Review of the Literature
3.2. Meta-Analysis
3.3. Present Fetal Cohort
3.3.1. Cystic Hygroma
3.3.2. Increased Nuchal Translucency
4. Discussion
4.1. Physiopathology of Nuchal Fluid Collections
4.2. Meta-Analysis on Genetic Testing and Present Fetal Cohort Considerations
4.3. Long-Term Follow-Up after Pregnancies with Nuchal Fluid Collections
4.4. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Apkon, M. Pathophysiology of hydrops fetalis. Semin. Perinatol. 1995, 19, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Molina, F.S.; Avgidou, K.; Kagan, K.O.; Poggi, S.; Nicolaides, K.H. Cystic hygromas, nuchal edema, and nuchal translucency at 11–14 weeks of gestation. Obstet. Gynecol. 2006, 107, 678–683. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Evans, M.I.; Krantz, D.A.; Hallahan, T.W.; Sherwin, J. Impact of nuchal translucency credentialing by the FMF, the NTQR or both on screening distributions and performance. Ultrasound Obstet. Gynecol. 2012, 39, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Sahota, D.S.; Chen, M.; Leung, T.Y.; Chan, L.W.; Fung, T.Y.; Ting, Y.H.; Lau, T.K. Assessment of sonographer nuchal translucency measurement performance central tendency and dispersion. J. Matern. Fetal Neonatal Med. 2011, 24, 812–816. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Xu, J.; Hu, S.-Q.; Chen, M.; Ma, R.-M.; Lau, T.K.; Zhang, L.; Xiao, X.; Qian, Y.; Guo, Z. Distribution and normal reference range of fetal nuchal translucency thickness in Kunming pregnant women in the first trimester. Zhonghua Fu Chan Ke Za Zhi 2012, 47, 514–517. [Google Scholar] [PubMed]
- Mack, L.M.; Lee, W.; Mastrobattista, J.M.; Belfort, M.A.; Van den Veyver, I.B.; Shamshirsaz, A.A.; Ruano, R.; Sanz Cortes, M.; Espinoza, A.; Thiam Diouf, A.; et al. Are First Trimester Nuchal Septations Independent Risk Factors for Chromosomal Anomalies? J. Ultrasound Med. 2017, 36, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Alamillo, C.M.; Fiddler, M.; Pergament, E. Increased nuchal translucency in the presence of normal chromosomes: What’s next? Curr. Opin. Obstet. Gynecol. 2012, 24, 102–108. [Google Scholar] [CrossRef]
- Wright, D.; Kagan, K.O.; Molina, F.S.; Gazzoni, A.; Nicolaides, K.H. A mixture model of nuchal translucency thickness in screening for chromosomal defects. Ultrasound Obstet. Gynecol. 2008, 31, 376–383. [Google Scholar] [CrossRef]
- Pandya, P.P.; Kondylios, A.; Hilbert, L.; Snijders, R.; Nicolaides, K. Chromosomal defects and outcome in 1015 fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 1995, 5, 15–19. [Google Scholar] [CrossRef]
- Snijders, R.; Noble, P.; Sebire, N.; Souka, A.; Nicolaides, K. UK multicentre project on assessment of risk of trisomy 21 by maternal age and fetal nuchal-translucency thickness at 10–14 weeks of gestation. Lancet 1998, 352, 343–346. [Google Scholar] [CrossRef]
- Gedikbasi, A.; Oztarhan, K.; Aslan, G.; Demirali, O.; Akyol, A.; Sargin, M.A.; Ceylan, Y. Multidisciplinary approach in cystic hygroma: Prenatal diagnosis, outcome and postnatal follow-up. Pediatr. Int. 2009, 51, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.; Durfee, S.M.; Russell, M.A.; Heard, A.J.; Iyer, C.; Alammari, R.; Coletta, J.; Craigo, S.D.; Fuchs, K.M.; D’Alton, M.; et al. First-trimester cystic hygroma: Relationship of nuchal translucency thickness and outcomes. Obstet. Gynecol. 2012, 120, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Zhou, H.; Zhao, Q.; Lu, Y.; Meng, Y. Application of expanded noninvasive prenatal test in prenatal diagnosis of fetuses with increased nuchal translucency. J. Matern. Fetal. Neonatal. Med. 2021, 35, 6213–6218. [Google Scholar] [CrossRef] [PubMed]
- Sinajon, P.; Chitayat, D.; Roifman, M.; Wasim, S.; Carmona, S.; Ryan, G.; Noor, A.; Kolomietz, E.; Chong, K. Microarray and RASopathy-disorder testing in fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 2020, 55, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Di Giosaffatte, N.; Pinna, V.; Daniele, P.; Corno, S.; D’Ambrosio, V.; Andreucci, E.; Marozza, A.; Sirchia, F.; Tortora, G.; et al. When to test fetuses for RASopathies? Proposition from a systematic analysis of 352 multicenter cases and a postnatal cohort. Genet. Med. 2021, 23, 1116–1124. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef]
- Richards, S.; Aziz, N.; Bale, S.; Bick, D.; Das, S.; Gastier-Foster, J.; Grody, W.W.; Hegde, M.; Lyon, E.; Spector, E.; et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet. Med. 2015, 17, 405–424. [Google Scholar] [CrossRef]
- Riggs, E.R.; Andersen, E.F.; Cherry, A.M.; Kantarci, S.; Kearney, H.; Patel, A.; Raca, G.; Ritter, D.I.; South, S.T.; Thorland, E.C.; et al. Technical standards for the interpretation and reporting of constitutional copy-number variants: A joint consensus recommendation of the American College of Medical Genetics and Genomics (ACMG) and the Clinical Genome Resource (ClinGen). Genet. Med. 2020, 22, 245–257. [Google Scholar] [CrossRef]
- Petersen, O.; Smith, E.; Van Opstal, D.; Polak, M.; Knapen, M.F.C.M.; Diderich, K.E.M.; Bilardo, C.M.; Arends, L.R.; Vogel, I.; Srebniak, M.I. Nuchal translucency of 3.0–3.4 mm an indication for NIPT or microarray? Cohort analysis and literature review. Acta Obstet. Gynecol. Scand. 2020, 99, 765–774. [Google Scholar] [CrossRef]
- Yang, X.; Li, R.; Fu, F.; Zhang, Y.; Li, D.; Liao, C. Submicroscopic chromosomal abnormalities in fetuses with increased nuchal translucency and normal karyotype. J. Matern. Fetal Neonatal Med. 2017, 30, 194–198. [Google Scholar] [CrossRef]
- Lichtenbelt, K.D.; Diemel, B.D.M.; Koster, M.P.H.; Manten, G.T.R.; Siljee, J.; Schuring-Blom, G.H.; Page-Christiaens, G.C.M.L. Detection of fetal chromosomal anomalies: Does nuchal translucency measurement have added value in the era of non-invasive prenatal testing? Prenat. Diagn. 2015, 35, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Fu, F.; Li, R.; Liu, Z.; Liao, C. Prenatal diagnosis and pregnancy outcome analysis of thickened nuchal fold in the second trimester. Medicine 2018, 97, e13334. [Google Scholar] [CrossRef] [PubMed]
- Grande, M.; Solernou, R.; Ferrer, L.; Borobio, V.; Jimenez, J.M.; Bennasar, M.; Soler, A.; Borrell, A. Is nuchal translucency a useful aneuploidy marker in fetuses with crown-rump length of 28–44 mm? Ultrasound Obstet. Gynecol. 2014, 43, 520–524. [Google Scholar] [CrossRef] [PubMed]
- Arigita, M.; Grande, M.; Mula, R.; Borobio, V.; Sanchez, A.; Soler, A.; Borrell, A. Nuchal translucency thickness in the prediction of unbalanced translocations. Prenat. Diagn. 2014, 34, 982–985. [Google Scholar] [CrossRef]
- Özcan H, Ç.; Uğur, M.G.; Balat, Ö.; Sucu, S.; Bayramoğlu Tepe, N.; Öztürk, E.; Kömürcü Karuserci, Ö.; Kazaz, T.G. Analysis of cystic hygroma diagnosed in the prenatal period: 5-years’ experience at a tertiary hospital in Southeastern Turkey. J. Matern. Fetal Neonatal Med. 2019, 32, 1800–1805. [Google Scholar] [CrossRef]
- Cheng, P.-J.; Chang, S.-D.; Shaw, S.-W.; Soong, Y.-K. Nuchal translucency thickness in fetuses with chromosomal translocation at 11–12 weeks of gestation. Obstet. Gynecol. 2005, 105, 1058–1062. [Google Scholar] [CrossRef]
- Chitty, L.S.; O Kagan, K.; Molina, F.S.; Waters, J.J.; Nicolaides, K.H. Fetal nuchal translucency scan and early prenatal diagnosis of chromosomal abnormalities by rapid aneuploidy screening: Observational study. BMJ 2006, 332, 452–455. [Google Scholar] [CrossRef]
- Christiansen, M.; Ekelund, C.K.; Petersen, O.B.; Hyett, J.; Eastwood, N.; Ball, S.; Tabor, A.; Vogel, I. Nuchal translucency distributions for different chromosomal anomalies in a large unselected population cohort. Prenat. Diagn. 2016, 36, 49–55. [Google Scholar] [CrossRef]
- Kievskaya, J.; Shilova, N.; Kanivets, I.; Kudryavtseva, E.; Pyankov, D.; Korostelev, S. SNP-Based Chromosomal Microarray Analysis for Detecting DNA Copy Number Variations in Fetuses with a Thickened Nuchal Fold. Sovrem. Tekhnologii V Meditsine 2021, 13, 72–76. [Google Scholar] [CrossRef]
- Has, R.; Kalelioglu, I.; Ermis, H.; Ibrahimoglu, L.; Yuksel, A.; Yildirim, A.; Basaran, S. Screening for fetal chromosomal abnormalities with nuchal translucency measurement in the first trimester. Fetal Diagn. Ther. 2006, 21, 355–359. [Google Scholar] [CrossRef]
- Hellmuth, S.G.; Pedersen, L.H.; Miltoft, C.B.; Petersen, O.; Kjaergaard, S.; Ekelund, C.K.; Tabor, A. Increased nuchal translucency thickness and risk of neurodevelopmental disorders. Ultrasound Obstet. Gynecol. 2017, 49, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Schou, K.V.; Kirchhoff, M.; Nygaard, U.; Jørgensen, C.; Sundberg, K. Increased nuchal translucency with normal karyotype: A follow-up study of 100 cases supplemented with CGH and MLPA analyses. Ultrasound Obstet. Gynecol. 2009, 34, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Noia, G.; Maltese, P.E.; Zampino, G.; D’Errico, M.; Cammalleri, V.; Convertini, P.; Marceddu, G.; Mueller, M.; Guerri, G.; Bertelli, M. Cystic Hygroma: A Preliminary Genetic Study and a Short Review from the Literature. Lymphat. Res. Biol. 2019, 17, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.M.; Chasen, S.T.; Norton, M.E. Testing for Noonan syndrome after increased nuchal translucency. Prenat. Diagn. 2017, 37, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.; Pynaker, C.; Bonacquisto, L.; Lindquist, A.; Poulton, A.; Kluckow, E.; Hutchinson, B.; Norris, F.; Pertile, M.D.; Gugasyan, L.; et al. Reexamining the optimal nuchal translucency cutoff for diagnostic testing in the cell-free DNA and microarray era: Results from the Victorian Perinatal Record Linkage study. Am. J. Obstet. Gynecol. 2021, 225, 527.e1–527.e12. [Google Scholar] [CrossRef] [PubMed]
- Axt-Fliedner, R.; Hartge, D.; Chiriac, A.; Krapp, M.; Berg, C.; Geipel, A.; Germer, U.; Gembruch, U. Long-term outcome for children born after a first-trimester measurement of increased nuchal translucency with a normal karyotype: A retrospective analysis. Ultraschall Medizin 2009, 30, 558–563. [Google Scholar] [CrossRef]
- Goncé, A.; Borrell, A.; Meler, E.; Arigita, M.; Martínez, J.M.; Botet, F.; Sánchez, A.; Gratacós, E. Prevalence and perinatal outcome of dichorionic and monochorionic twins with nuchal translucency above the 99(th) percentile and normal karyotype. Ultrasound Obstet. Gynecol. 2010, 35, 14–18. [Google Scholar] [CrossRef]
- Miranda, J.; Paz YMiño, F.; Borobio, V.; Badenas, C.; Rodriguez-Revenga, L.; Pauta, M.; Borrell, A. Should cell-free DNA testing be used in pregnancy with increased fetal nuchal translucency? Ultrasound Obstet. Gynecol. 2020, 55, 645–651. [Google Scholar] [CrossRef]
- Şahin Uysal, N.; Gülümser, Ç.; Çelik, Z.Y.; Yanık, F.B. Increased nuchal translucency and pregnancy outcomes: Experience of Başkent University Ankara Hospital. Turk. J. Obstet. Gynecol. 2019, 16, 100–106. [Google Scholar] [CrossRef]
- Yoshida, S.; Miura, K.; Yamasaki, K.; Miura, S.; Shimada, T.; Tanigawa, T.; Yoshida, A.; Nakayama, D.; Masuzaki, H. Does increased nuchal translucency indicate a fetal abnormality? A retrospective study to clarify the clinical significance of nuchal translucency in Japan. J. Hum. Genet. 2008, 53, 688. [Google Scholar] [CrossRef]
- Cicatiello, R.; Pignataro, P.; Izzo, A.; Mollo, N.; Pezone, L.; Maruotti, G.M.; Sarno, L.; Sglavo, G.; Conti, A.; Genesio, R.; et al. Chromosomal Microarray Analysis versus Karyotyping in Fetuses with Increased Nuchal Translucency. Med. Sci. 2019, 7, 40. [Google Scholar] [CrossRef] [PubMed]
- Zalel, Y.; Zemet, R.; Kivilevitch, Z. The added value of detailed early anomaly scan in fetuses with increased nuchal translucency. Prenat. Diagn. 2017, 37, 235–243. [Google Scholar] [CrossRef] [PubMed]
- BBehera, S.; Bawa, M.; Kanojia, R.P.; Saha, P.K.; Singh, T.; Samujh, R. Outcome of antenatally diagnosed cystic hygroma—Lessons learnt. Int. J. Pediatr. Otorhinolaryngol. 2020, 138, 110227. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Poon, L.C.; Akolekar, R.; Choy, K.W.; Leung, T.Y.; Nicolaides, K.H. Is high fetal nuchal translucency associated with submicroscopic chromosomal abnormalities on array CGH? Ultrasound Obstet. Gynecol. 2014, 43, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Baer, R.J.; Norton, M.E.; Shaw, G.M.; Flessel, M.C.; Goldman, S.; Currier, R.J.; Jelliffe-Pawlowski, L.L. Risk of selected structural abnormalities in infants after increased nuchal translucency measurement. Am. J. Obstet. Gynecol. 2014, 211, 675.e1–675.e19. [Google Scholar] [CrossRef] [PubMed]
- Westin, M.; Saltvedt, S.; Bergman, G.; Almström, H.; Grunewald, C.; Valentin, L. Is measurement of nuchal translucency thickness a useful screening tool for heart defects? A study of 16,383 fetuses. Ultrasound Obstet. Gynecol. 2006, 27, 632–639. [Google Scholar] [CrossRef]
- Zhang, Z.; Hu, T.; Wang, J.; Li, Q.; Wang, H.; Liu, S. Prenatal Diagnostic Value of Chromosomal Microarray in Fetuses with Nuchal Translucency Greater than 2.5 mm. BioMed Res. Int. 2019, 2019, 6504159. [Google Scholar] [CrossRef]
- Zhao, X.-R.; Gao, L.; Wu, Y.; Wang, Y.-L. Application of chromosomal microarray in fetuses with increased nuchal translucency. J. Matern. -Fetal Neonatal Med. 2020, 33, 1749–1754. [Google Scholar] [CrossRef]
- Yang, X.; Huang, L.Y.; Pan, M.; Xu, L.L.; Zhen, L.; Han, J.; Li, D.Z. Exome sequencing improves genetic diagnosis of fetal increased nuchal translucency. Prenat. Diagn. 2020, 40, 1426–1431. [Google Scholar] [CrossRef]
- Su, L.; Huang, H.; An, G.; Cai, M.; Wu, X.; Li, Y.; Xie, X.; Lin, Y.; Wang, M.; Xu, L. Clinical application of chromosomal microarray analysis in fetuses with increased nuchal translucency and normal karyotype. Mol. Genet. Genom. Med. 2019, 7, e811. [Google Scholar] [CrossRef]
- Stuurman, K.E.; van der Mespel-Brouwer, M.H.; Engels, M.A.J.; Elting, M.W.; Bhola, S.L.; Meijers-Heijboer, H. Isolated Increased Nuchal Translucency in First Trimester Ultrasound Scan: Diagnostic Yield of Prenatal Microarray and Outcome of Pregnancy. Front. Med. 2021, 8, 737936. [Google Scholar] [CrossRef]
- Srebniak, M.I.; de Wit, M.C.; Diderich, K.E.; Govaerts, L.C.; Joosten, M.; Knapen, M.F.; Bos, M.J.; Looye-Bruinsma, G.A.; Koningen, M.; Go, A.T.; et al. Enlarged NT (≥3.5 mm) in the first trimester—Not all chromosome aberrations can be detected by NIPT. Mol. Cytogenet. 2016, 9, 69. [Google Scholar] [CrossRef][Green Version]
- Xue, S.; Yan, H.; Chen, J.; Li, N.; Wang, J.; Liu, Y.; Zhang, H.; Li, S.; Zhang, W.; Chen, D.; et al. Genetic Examination for Fetuses with Increased Fetal Nuchal Translucency by Genomic Technology. Cytogenet. Genome Res. 2020, 160, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sagi-Dain, L.; Singer, A.; Ben Shachar, S.; Ben Yehoshua, S.J.; Feingold-Zadok, M.; Greenbaum, L.; Maya, I. Risk of Clinically Significant Chromosomal Microarray Analysis Findings in Fetuses With Nuchal Translucency From 3.0 mm Through 3.4 mm. Obstet. Gynecol. 2021, 137, 126–131. [Google Scholar] [CrossRef]
- Pan, M.; Han, J.; Zhen, L.; Yang, X.; Li, R.; Liao, C.; Li, D.-Z. Prenatal diagnosis of fetuses with increased nuchal translucency using an approach based on quantitative fluorescent polymerase chain reaction and genomic microarray. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 197, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Long, N.H.; Cuong, T.D.; Anh, N.T. Relation Between Increased Fetal Nuchal Translucency Thickness and Chromosomal Defects in Northern Vietnam. Cureus 2021, 13, e18446. [Google Scholar]
- Mellis, R.; Eberhardt, R.; Hamilton, S.; McMullan, D.; Kilby, M.D.; Maher, E.; Hurles, M.; Giordano, J.; Aggarwal, V.; Goldstein, D.; et al. Fetal exome sequencing for isolated increased nuchal translucency: Should we be doing it? BJOG Int. J. Obstet. Gynaecol. 2022, 129, 52–61. [Google Scholar] [CrossRef]
- Maya, I.; Yacobson, S.; Kahana, S.; Yeshaya, J.; Tenne, T.; Agmon-Fishman, I.; Cohen-Vig, L.; Shohat, M.; Basel-Vanagaite, L.; Sharony, R. Cut-off value of nuchal translucency as indication for chromosomal microarray analysis. Ultrasound Obstet. Gynecol. 2017, 50, 332–335. [Google Scholar] [CrossRef]
- Lund, I.C.B.; Christensen, R.; Petersen, O.; Vogel, I.; Vestergaard, E.M. Chromosomal microarray in fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 2015, 45, 95–100. [Google Scholar] [CrossRef]
- Lan, L.; Wu, H.; She, L.; Zhang, B.; He, Y.; Luo, D.; Wang, H.; Zheng, Z. Analysis of copy number variation by sequencing in fetuses with nuchal translucency thickening. J. Clin. Lab. Anal. 2020, 34, e23347. [Google Scholar] [CrossRef]
- Wang, C.; Tang, J.; Tong, K.; Huang, D.; Tu, H.; Zhu, J. Chromosomal microarray analysis versus noninvasive prenatal testing in fetuses with increased nuchal translucency. J. Hum. Genet. 2022, 67, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Zoppi, M.A.; Ibba, R.M.; Floris, M.; Manca, F.; Axiana, C.; Monni, G. Changes in nuchal translucency thickness in normal and abnormal karyotype fetuses. BJOG Int. J. Obstet. Gynaecol. 2003, 110, 584–588. [Google Scholar] [CrossRef]
- Holzer, I.; Husslein, P.W.; Bettelheim, D.; Scheidl, J.; Kiss, H.; Farr, A. Value of increased nuchal translucency in the era of noninvasive prenatal testing with cell-free DNA. Int. J. Gynaecol. Obstet. 2019, 145, 319–323. [Google Scholar] [CrossRef] [PubMed]
- Iuculano, A.; Pagani, G.; Stagnati, V.; Floris, M.; Ibba, R.M.; Monni, G. Pregnancy outcome and long-term follow-up of fetuses with isolated increased NT: A retrospective cohort study. J. Perinat. Med. 2016, 44, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Socolov, D.; Socolov, R.; Gorduza, V.E.; Butureanu, T.; Stanculescu, R.; Carauleanu, A.; Pavaleanu, I. Increased nuchal translucency in fetuses with a normal karyotype-diagnosis and management: An observational study. Medicine 2017, 96, e7521. [Google Scholar] [CrossRef] [PubMed]
- Abotorabi, S.; Moeini, N.; Moghbelinejad, S. High Frequency of Fetal Loss in Fetuses With Normal Karyotype and Nuchal Translucency ≥ 3 Among the Iranian Pregnant Women. J. Fam. Reprod. Health 2020, 14, 81–87. [Google Scholar] [CrossRef]
- Alexioy, E.; Trakakis, E.; Kassanos, D.; Farmakidis, G.; Kondylios, A.; Laggas, D.; Salamalekis, E.; Florentin, L.; Kanavakis, E.; Basios, G.; et al. Predictive value of increased nuchal translucency as a screening test for the detection of fetal chromosomal abnormalities. J. Matern. -Fetal Neonatal Med. 2009, 22, 857–862. [Google Scholar] [CrossRef]
- Ayras, O.; Tikkanen, M.; Eronen, M.; Paavonen, J.; Stefanovic, V. Increased nuchal translucency and pregnancy outcome: A retrospective study of 1063 consecutive singleton pregnancies in a single referral institution. Prenat. Diagn. 2013, 33, 856–862. [Google Scholar] [CrossRef]
- Bardi, F.; Bosschieter, P.; Verheij, J.; Go, A.; Haak, M.; Bekker, M.; Sikkel, E.; Coumans, A.; Pajkrt, E.; Bilardo, C. Is there still a role for nuchal translucency measurement in the changing paradigm of first trimester screening? Prenat. Diagn. 2020, 40, 197–205. [Google Scholar] [CrossRef]
- Bilardo, C.M.; Müller, M.A.; Pajkrt, E.; Clur, S.A.; van Zalen, M.M.; Bijlsma, E.K. Increased nuchal translucency thickness and normal karyotype: Time for parental reassurance. Ultrasound Obstet. Gynecol. 2007, 30, 11–18. [Google Scholar] [CrossRef]
- Choy, K.W.; Wang, H.; Shi, M.; Chen, J.; Yang, Z.; Zhang, R.; Yan, H.; Wang, Y.; Chen, S.; Chau, M.H.K.; et al. Prenatal Diagnosis of Fetuses With Increased Nuchal Translucency by Genome Sequencing Analysis. Front. Genet. 2019, 10, 761. [Google Scholar] [CrossRef] [PubMed]
- Egloff, M.; Hervé, B.; Quibel, T.; Jaillard, S.; Le Bouar, G.; Uguen, K.; Saliou, A.-H.; Valduga, M.; Perdriolle, E.; Coutton, C.; et al. Diagnostic yield of chromosomal microarray analysis in fetuses with isolated increased nuchal translucency: A French multicenter study. Ultrasound Obstet. Gynecol. 2018, 52, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Gouas, L.; Kémény, S.; Beaufrère, A.-M.; Eymard-Pierre, E.; Pebrel-Richard, C.; Tchirkov, A.; Lemery, D.; Laurichesse-Delmas, H.; Vago, P.; Goumy, C. Prenatal Screening of 21 Microdeletion/Microduplication Syndromes and Subtelomeric Imbalances by MLPA in Fetuses with Increased Nuchal Translucency and Normal Karyotype. Cytogenet. Genome Res. 2015, 146, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, J.; Zhang, G.; Jiao, H.; Zhu, J.; Li, Z.; Chen, C.; Zhang, X.; Huang, H.; Wang, J. A Chinese multicenter retrospective study of isolated increased nuchal translucency associated chromosome anomaly and prenatal diagnostic suggestions. Sci. Rep. 2021, 11, 5596. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.-Y.; Pan, M.; Han, J.; Zhen, L.; Yang, X.; Li, D.-Z. What would be missed in the first trimester if nuchal translucency measurement is replaced by cell free DNA foetal aneuploidy screening? J. Obstet. Gynaecol. 2018, 38, 498–501. [Google Scholar] [CrossRef] [PubMed]
- Hui, A.S.; Chau, M.H.K.; Chan, Y.M.; Cao, Y.; Kwan, A.H.; Zhu, X.; Kwok, Y.K.; Chen, Z.; Lao, T.T.; Choy, K.W.; et al. The role of chromosomal microarray analysis among fetuses with normal karyotype and single system anomaly or nonspecific sonographic findings. Acta Obstet. Gynecol. Scand. 2021, 100, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Kagan, K.O.; Avgidou, K.; Molina, F.S.; Gajewska, K.; Nicolaides, K.H. Relation between increased fetal nuchal translucency thickness and chromosomal defects. Obstet. Gynecol. 2006, 107, 6–10. [Google Scholar] [CrossRef]
- Leung, T.Y.; Yeung, K.C.A.; Leung, W.C.; Leung, K.Y.; Lo, T.K.; To, W.W.K.; Lau, W.L.; Chan, L.W.; Sahota, D.; Choy, R.K.W. Prenatal diagnosis of pathogenic genomic imbalance in fetuses with increased nuchal translucency but normal karyotyping using chromosomal microarray. Hong Kong Med. J. 2019, 25, 30–32. [Google Scholar]
- Nakamura, Y.; Fujita, S.; Yamada, K.; Song, M.; Tajima, A.; Matsumoto, J.; Kihira, C.; Kurata, Y.; Arakawa, H.; Tamura, C. Outcome of the fetuses with severely increased nuchal translucency thickness in the first trimester. J. Obstet. Gynaecol. Res. 2020, 46, 1977–1981. [Google Scholar] [CrossRef]
- Lithner, C.U.; Kublickas, M.; Ek, S. Pregnancy outcome for fetuses with increased nuchal translucency but normal karyotype. J. Med. Screen. 2016, 23, 1–6. [Google Scholar] [CrossRef]
- Tekesin, I. The diagnostic value of a detailed first trimester anomaly scan in fetuses with increased nuchal translucency thickness. J. Perinat. Med. 2019, 47, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Tekesin, I. Pregnancy outcome in foetuses with increased nuchal translucency—10-years’ experience in a prenatal medical practice. J. Obstet. Gynaecol. 2020, 40, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebpour, A.; Rafiee, N.B.; Ghaffari, S.; Jamal, A. Increased nuchal translucency and pregnancy outcome. Iran. J. Public Health 2012, 41, 92–97. [Google Scholar] [PubMed]
- Shakoor, S.; Dileep, D.; Tirmizi, S.; Rashid, S.; Amin, Y.; Munim, S. Increased nuchal translucency and adverse pregnancy outcomes. J. Matern. -Fetal Neonatal Med. 2017, 30, 1760–1763. [Google Scholar] [CrossRef]
- Senat, M.V.; Bussières, L.; Couderc, S.; Roume, J.; Rozenberg, P.; Bouyer, J.; Ville, Y. Long-term outcome of children born after a first-trimester measurement of nuchal translucency at the 99th percentile or greater with normal karyotype: A prospective study. Am. J. Obstet. Gynecol. 2007, 196, 53.e1–53.e536. [Google Scholar] [CrossRef][Green Version]
- Senat, M.-V.; Bussières, L.; Couderc, S.; Roume, J.; Rozenberg, P.; Bouyer, J.; Ville, Y. Pregnancy outcome in fetuses with increased nuchal translucency and normal karyotype. Prenat. Diagn. 2002, 22, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Scott, F.; Evans, J.; McLennan, A. Perinatal outcome in fetuses with extremely large nuchal translucency measurement. Aust. New Zealand J. Obstet. Gynaecol. 2009, 49, 254–257. [Google Scholar] [CrossRef]
- Müller, M.A.; Pajkrt, E.; Bleker, O.P.; Bonsel, G.J.; Bilardo, C.M. Disappearance of enlarged nuchal translucency before 14 weeks’ gestation: Relationship with chromosomal abnormalities and pregnancy outcome. Ultrasound Obstet. Gynecol. 2004, 24, 169–174. [Google Scholar] [CrossRef]
- Orgul, G.; Ozyuncu, O.; Oktem, A.; Beksac, M.S. Management and outcomes of cystic hygromas: Experience of a tertiary center. J. Ultrasound 2017, 20, 127–131. [Google Scholar] [CrossRef]
- Graesslin, O.; Derniaux, E.; Alanio, E.; Gaillard, D.; Vitry, F.; Quéreux, C.; Ducarme, G. Characteristics and outcome of fetal cystic hygroma diagnosed in the first trimester. Acta Obstet. Gynecol. Scand. 2007, 86, 1442–1446. [Google Scholar] [CrossRef]
- Malone, F.D.; Ball, R.H.; Nyberg, D.A.; Comstock, C.H.; Saade, G.R.; Berkowitz, R.L.; Gross, S.J.; Dugoff, L.; Craigo, S.D.; Timor-Tritsch, I.E.; et al. First-trimester septated cystic hygroma: Prevalence, natural history, and pediatric outcome. Obstet. Gynecol. 2005, 106, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Schreurs, L.; Lannoo, L.; De Catte, L.; Van Schoubroeck, D.; Devriendt, K.; Richter, J. First trimester cystic hygroma colli: Retrospective analysis in a tertiary center. Eur. J. Obstet. Gynecol. Reprod. Biol. 2018, 231, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Lee, C.P.; Lin, S.M.; Lam, Y.H.; Tang, R.Y.K.; Tse, H.Y.; Tang, M.H.Y. Cystic hygroma detected in the first trimester scan in Hong Kong. J. Matern. -Fetal Neonatal Med. 2014, 27, 342–345. [Google Scholar] [CrossRef] [PubMed]
- Yakıştıran, B.; Altınboğa, O.; Canpolat, E.; Çakar, E.; Çelen, Ş.; Çağlar, A.T.; Üstün, Y.E. Analysis of cystic hygroma diagnosed in the first trimester: Single-center experience. J. Turk. Ger. Gynecol. Assoc. 2020, 21, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ganapathy, R.; Guven, M.; Sethna, F.; Vivekananda, U.; Thilaganathan, B. Natural history and outcome of prenatally diagnosed cystic hygroma. Prenat. Diagn. 2004, 24, 965–968. [Google Scholar] [CrossRef]
- Kharrat, R.; Yamamoto, M.; Roume, J.; Couderc, S.; Vialard, F.; Hillion, Y.; Ville, Y. Karyotype and outcome of fetuses diagnosed with cystic hygroma in the first trimester in relation to nuchal translucency thickness. Prenat. Diagn. 2006, 26, 369–372. [Google Scholar] [CrossRef]
- Tanriverdi, H.; Ertan, A.; Hendrik, H.; Remberger, K.; Schmidt, W. Outcome of cystic hygroma in fetuses with normal karyotypes depends on associated findings. Eur. J. Obstet. Gynecol. Reprod. Biol. 2005, 118, 40–46. [Google Scholar] [CrossRef]
- Scholl, J.; Chasen, S.T. First trimester cystic hygroma: Does early detection matter? Prenat. Diagn. 2016, 36, 432–436. [Google Scholar] [CrossRef]
- Sanhal, C.; Mendilcioglu, I.; Özekinci, M.; Yakut, S.; Merdun, Z.; Şimşek, M.; Lüleci, G. Prenatal management, pregnancy and pediatric outcomes in fetuses with septated cystic hygroma. Braz. J. Med. Biol. Res. 2014, 47, 799–803. [Google Scholar] [CrossRef]
- Noia, G.; Pellegrino, M.; Masini, L.; Visconti, D.; Manzoni, C.; Chiaradia, G.; Caruso, A. Fetal cystic hygroma: The importance of natural history. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 407–413. [Google Scholar] [CrossRef]
- Beke, A.; Joó, J.G.; Csaba, A.; Lázár, L.; Bán, Z.; Papp, C.; Tóth-Pál, E.; Papp, Z. Incidence of chromosomal abnormalities in the presence of fetal subcutaneous oedema, such as nuchal oedema, cystic hygroma and non-immune hydrops. Fetal Diagn. Ther. 2009, 25, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Esmer, A.C.; Kalelioglu, I.; Keyif, B.; Ozsurmeli, M.; Yüksel, A.; Has, R. Significance of septa in first trimester increased nuchal translucency thickness. J. Med. Ultrason. 2014, 41, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Westin, M.; Saltvedt, S.; Almström, H.; Grunewald, C.; Valentin, L. By how much does increased nuchal translucency increase the risk of adverse pregnancy outcome in chromosomally normal fetuses? A study of 16,260 fetuses derived from an unselected pregnant population. Ultrasound Obstet. Gynecol. 2007, 29, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Motta, M.; Giancotti, A.; Mastromoro, G.; Chandramouli, B.; Pinna, V.; Pantaleoni, F.; Di Giosaffatte, N.; Petrini, S.; Mazza, T.; D’Ambrosio, V.; et al. Clinical and functional characterization of a novel RASopathy-causing SHOC2 mutation associated with prenatal-onset hypertrophic cardiomyopathy. Hum. Mutat. 2009, 40, 1046–1056. [Google Scholar]
- Pajkrt, E.; De Graaf, I.M.; Mol, B.W.; Van Lith, J.M.; Bleker, O.P.; Bilardo, C.M. Weekly nuchal translucency measurements in normal fetuses. Obstet. Gynecol. 1998, 91, 208–211. [Google Scholar] [CrossRef]
- Pajkrt, E.; Bleker, O.P.; Bilardo, C.M.; Van Lith, J.M.; Mol, B.W. Nuchal translucency measurement in normal fetuses. Obstet. Gynecol. 1995, 86, 994–997. [Google Scholar] [CrossRef]
- Pandya, P.P.; Snijders, R.; Johnson, S.; Nicolaides, K. Natural history of trisomy 21 fetuses with increased nuchal translucency thickness. Ultrasound Obstet. Gynecol. 1995, 5, 381–383. [Google Scholar] [CrossRef]
- van Zalen-Sprock, R.M.; van Vugt, J.M.; van Geijn, H.P. First-trimester diagnosis of cystic hygroma--course and outcome. Am. J. Obstet. Gynecol. 1992, 167, 94–98. [Google Scholar] [CrossRef]
- Chervenak, F.A.; Isaacson, G.; Blakemore, K.J.; Breg, W.R.; Hobbins, J.C.; Berkowitz, R.L.; Tortora, M.; Mayden, K.; Mahoney, M.J. Fetal cystic hygroma. Cause and natural history. N. Engl. J. Med. 1983, 309, 822–825. [Google Scholar] [CrossRef]
- Gustavii, B.; Edvall, H. First-trimester diagnosis of cystic nuchal hygroma. Acta Obstet. Gynecol. Scand. 1984, 63, 377–378. [Google Scholar] [CrossRef]
- Mastromoro, G.; Hashemian, N.K.; Guadagnolo, D.; Giuffrida, M.G.; Torres, B.; Bernardini, L.; Ventriglia, F.; Piacentini, G.; Pizzuti, A. Chromosomal Microarray Analysis in Fetuses Detected with Isolated Cardiovascular Malformation: A Multicenter Study, Systematic Review of the Literature and Meta-Analysis. Diagnostics 2022, 12, 1328. [Google Scholar] [CrossRef] [PubMed]
- Moscoso, G. Fetal nuchal translucency: A need to understand the physiological basis. Ultrasound Obstet. Gynecol. 1995, 5, 6–8. [Google Scholar] [CrossRef] [PubMed]
- Hyett, J.A.; Brizot, M.L.; Von Kaisenberg, C.S.; McKie, A.T.; Farzaneh, F.; Nicolaides, K.H. Cardiac gene expression of atrial natriuretic peptide and brain natriuretic peptide in trisomic fetuses. Obstet. Gynecol. 1996, 87, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, A.; Kreiselmaier, P.; Krapp, M.; Smrcek, J.; Axt-Fliedner, R. Umbilical cord diameter at 11–14 weeks of gestation: Relationship to nuchal translucency, ductus venous blood flow and chromosomal defects. Fetal Diagn. Ther. 2006, 21, 390–395. [Google Scholar] [CrossRef]
- Ghezzi, F.; Raio, L.; Di Naro, E.; Franchi, M.; Buttarelli, M.; Schneider, H. First-trimester umbilical cord diameter: A novel marker of fetal aneuploidy. Ultrasound Obstet. Gynecol. 2002, 19, 235–239. [Google Scholar] [CrossRef]
- El Daouk, M.; Brustman, L.; Langer, O.; Lysikiewicz, A. Male-to-female gender ratio in fetuses with increased nuchal translucency. J. Matern. -Fetal Neonatal Med. 2012, 25, 2613–2615. [Google Scholar] [CrossRef]
- Timmerman, E.; Pajkrt, E.; Bilardo, C.M. Male gender as a favorable prognostic factor in pregnancies with enlarged nuchal translucency. Ultrasound Obstet. Gynecol. 2009, 34, 373–378. [Google Scholar] [CrossRef]
- Gbrumfield, C.; Dwenstrom, K.; Odavis, R.; Owen, J.; Cosper, P. Second-trimester cystic hygroma: Prognosis of septated and nonseptated lesions. Obstet. Gynecol. 1996, 88, 979–982. [Google Scholar] [CrossRef]
- Bernstein, H.S.; Filly, R.A.; Goldberg, J.D.; Golbus, M.S. Prognosis of fetuses with a cystic hygroma. Prenat. Diagn. 1991, 11, 349–355. [Google Scholar] [CrossRef]
- Maymon, R.; Tercanli, S.; Dreazen, E.; Sartorius, G.; Holzgreve, W.; Herman, A. Comparison of pregnancy outcome of euploid fetuses with increased nuchal translucency (NT) expressed in NT MoM or delta-NT. Ultrasound Obstet. Gynecol. 2004, 23, 477–481. [Google Scholar] [CrossRef]
- Rizzo, G.; Muscatello, A.; Angelini, E.; Capponi, A. Abnormal cardiac function in fetuses with increased nuchal translucency. Ultrasound Obstet. Gynecol. 2003, 21, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Souka, A.P.; von Kaisenberg, C.S.; Hyett, J.A.; Sonek, J.D.; Nicolaides, K.H. Increased nuchal translucency with normal karyotype. Am. J. Obstet. Gynecol. 2005, 192, 1005–1021. [Google Scholar] [CrossRef] [PubMed]
- Bohlandt, S.; von Kaisenberg, C.; Wewetzer, K.; Christ, B.; Nicolaides, K.; Brand-Saberi, B. Hyaluronan in the nuchal skin of chromosomally abnormal fetuses. Hum. Reprod. 2000, 15, 1155–1158. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hyett, J.; Perdu, M.; Sharland, G.; Snijders, R.; Nicolaides, K. Using fetal nuchal translucency to screen for major congenital cardiac defects at 10–14 weeks of gestation: Population based cohort study. BMJ 1999, 318, 81–85. [Google Scholar] [CrossRef]
- Sebire, N.; Souka, A.; Skentou, H.; Geerts, L.; Nicolaides, K. Early prediction of severe twin-to-twin transfusion syndrome. Hum. Reprod. 2000, 15, 2008–2010. [Google Scholar] [CrossRef]
- Sebire, N.J.; D’Ercole, C.; Hughes, K.; Carvalho, M.; Nicolaides, K. Increased nuchal translucency thickness at 10–14 weeks of gestation as a predictor of severe twin-to-twin transfusion syndrome. Ultrasound Obstet. Gynecol. 1997, 10, 86–89. [Google Scholar] [CrossRef]
- Shi, X.; Liu, Q.; He, W.; Liu, Y.; Rao, T.; Fang, L.; Wu, J. Prenatal and perinatal outcomes of twin pregnancy discordant for one fetus with nuchal translucency above the 95th percentile. J. Matern. -Fetal Neonatal Med. 2022, 35, 3152–3157. [Google Scholar] [CrossRef]
- Krispin, E.; Kushnir, A.; Shemer, A.; Rienstein, S.; Berkenstadt, M.; Yinon, Y.; Weisz, B. Abnormal nuchal translucency followed by normal microarray analysis is associated with placental pathology-related complications. Prenat. Diagn. 2021, 41, 855–860. [Google Scholar] [CrossRef]
- Quaresima, P.; Homfray, T.; Greco, E. Obstetric complications in pregnancies with life-limiting malformations. Curr. Opin. Obstet. Gynecol. 2019, 31, 375–387. [Google Scholar] [CrossRef]
- Buffin, R.; Fichez, A.; Decullier, E.; Roux, A.; Bin, S.; Combourieu, D.; Diez, B.P.; Huissoud, C.; Picaud, J.; Basson, E.; et al. Neurodevelopmental outcome at 2 years of corrected age in fetuses with increased nuchal translucency thickness and normal karyotype compared with matched controls. Ultrasound Obstet. Gynecol. 2021, 57, 790–797. [Google Scholar] [CrossRef]
- Guadagnolo, D.; Mastromoro, G.; Di Palma, F.; Pizzuti, A.; Marchionni, E. Prenatal Exome Sequencing: Background, Current Practice and Future Perspectives-A Systematic Review. Diagnostics 2021, 11, 224. [Google Scholar] [CrossRef] [PubMed]
- Mastromoro, G.; Guadagnolo, D.; Khaleghi Hashemian, N.; Marchionni, E.; Traversa, A.; Pizzuti, A. Molecular Approaches in Fetal Malformations, Dynamic Anomalies and Soft Markers: Diagnostic Rates and Challenges-Systematic Review of the Literature and Meta-Analysis. Diagnostics 2022, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.N.; Lianoglou, B.R.; Adami, R.R.; Pluym, I.D.; Holliman, K.; Duffy, J.; Downum, S.L.; Patel, S.; Faubel, A.; Boe, N.M.; et al. Exome Sequencing for Prenatal Diagnosis in Nonimmune Hydrops Fetalis. N. Engl. J. Med. 2020, 383, 1746–1756. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, D.-Z. Further genetic testing in prenatal cases of nonimmune hydrops fetalis with a normal array: A targeted panel or exome? Am. J. Obstet. Gynecol. 2022, 226, 276–277. [Google Scholar] [CrossRef] [PubMed]
- Mone, F.; Eberhardt, R.Y.; Hurles, M.E.; Mcmullan, D.J.; Maher, E.R.; Lord, J.; Chitty, L.S.; Dempsey, E.; Homfray, T.; Giordano, J.L.; et al. Fetal hydrops and the Incremental yield of Next-generation sequencing over standard prenatal Diagnostic testing (FIND) study: Prospective cohort study and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 58, 509–518. [Google Scholar] [CrossRef]
- Quinn, A.M.; Valcarcel, B.N.; Makhamreh, M.M.; Al-Kouatly, H.B.; Berger, S.I. A systematic review of monogenic etiologies of nonimmune hydrops fetalis. Genet. Med. 2021, 23, 3–12. [Google Scholar] [CrossRef]
- Norton, M.E.; Ziffle, J.V.; Lianoglou, B.R.; Hodoglugil, U.; Devine, W.P.; Sparks, T.N. Exome sequencing vs targeted gene panels for the evaluation of nonimmune hydrops fetalis. Am. J. Obstet. Gynecol. 2022, 226, 128.e1–128.e11. [Google Scholar] [CrossRef]
- Al-Kouatly, H.B.; Makhamreh, M.M.; Rice, S.M.; Smith, K.; Harman, C.; Quinn, A.; Valcarcel, B.N.; Firman, B.; Liu, R.; Hegde, M.; et al. High diagnosis rate for nonimmune hydrops fetalis with prenatal clinical exome from the Hydrops-Yielding Diagnostic Results of Prenatal Sequencing (HYDROPS) Study. Genet. Med. 2021, 23, 1325–1333. [Google Scholar] [CrossRef]
| Nuchal Translucency | ||||||
|---|---|---|---|---|---|---|
| Reference | Dimension (mm) | Association | Yield (Incremental) | |||
| Karyotype | CMA | RASopathy Panel | ES | |||
| [47] | 2.5–2.9 | unspecified | 8/134 (5.97%) | 3/126 (2.38%) | . | . |
| 3.0–3.4 | unspecified | 8/146 (5.48%) | 2/138 (1.45%) | . | . | |
| 3.5–4.4 | unspecified | 23/140 (16.43%) | 7/117 (5.98%) | . | . | |
| 4.5–5.4 | unspecified | 7/32 (21.88%) | 0/25 (0.00%) | . | . | |
| 5.5–6.4 | unspecified | 7/13 (53.85%) | 1/6 (16.67%) | . | . | |
| ≥6.5 | unspecified | 18/34 (52.94%) | 2/16 (12.50%) | . | . | |
| [48] | 3.0–3.4 | unspecified | 15/110 (13.64%) | 3/60 (5.00%) | . | . |
| 3.5–4.4 | unspecified | 28/83 (33.74%) | 3/37 (8.11%) | . | . | |
| 4.5–5.4 | unspecified | 18/40 (45.00%) | 1/40 (2.50%) | . | . | |
| 5.5–6.4 | unspecified | 12/30 (40.00%) | . | . | . | |
| 6.5–7.4 | unspecified | 9/21 (42.86%) | . | . | . | |
| ≥7.5 | unspecified | 21/35 (60.00%) | . | . | . | |
| ≥4.5 | unspecified | 60/126 (47.62%) | 1/40 (2.50%) | . | . | |
| [49] | ≥3.5 | apparently isolated | . | . | 2/73 (2.74%) | 2/71 (2.82%) |
| [50] | ≥2.5 | unspecified | 18/192 (9.38%) | . | . | . |
| 2.5–3.4 | apparently isolated | . | 2/119 (1.68%) | . | . | |
| 2.5–3.4 | associated | . | 0/15 (0.00%) | . | . | |
| ≥3.5 | apparently isolated | . | 1/43 (2.33%) | . | . | |
| ≥3.5 | associated | . | 2/12 (16.67%) | . | . | |
| [51] | ≥3.5 | apparently isolated | . | 1/39 (2.56%) | . | . |
| [52] | ≥3.5 | apparently isolated | 134/362 (37.02%) | 1/229 (0.44%) | . | . |
| [14] | ≥3.5 | unspecified | 123/226 (54.42%) | 2/103 (1.94%) | 3/103 (2.74%) | . |
| [53] | 95ct–3.4 | apparently isolated | 18/114 (15.79%) | 2/96 (2.01%) | . | . |
| 3.5–4.4 | apparently isolated | 30/150 (20.00%) | 2/120 (1.67%) | . | . | |
| 4.5–5.4 | apparently isolated | 16/55 (30.09%) | 2/39 (5.13%) | . | . | |
| ≥5.5 | apparently isolated | 29/55 (52.73%) | 1/26 (3.85%) | 1/22 (4.55%) | 2/21 (9.52%) | |
| [54] | 3.0–3.4 | apparently isolated | 20/619 (32.31%) | 9/599 (1.50%) | . | . |
| [55] | ≥3.5 | unspecified | 57/175 (32.57%) | 3/118 (2.54%) | . | . |
| [56] | 2.5–3.4 | unspecified | 245/1372 (17.86%) | . | . | . |
| 3.5–4.4 | unspecified | 182/866 (21.02%) | . | . | . | |
| 4.5–5.4 | unspecified | 77/282 (27.30%) | . | . | . | |
| 5.5–6.4 | unspecified | 29/109 (26.61%) | . | . | . | |
| ≥6.5 | unspecified | 27/91 (29.67%) | . | . | . | |
| [57] | ≥3.5 | apparently isolated | . | . | 0/111 (0.00%) | 2/111 (1.80%) |
| ≥3.5 | associated | . | . | 7/91 (7.69%) | 17/84 (20.24%) | |
| [58] | 3.0–3.4 | apparently isolated | 8/170 (4.70%) | 3/162 (1.85%) | . | . |
| ≥3.5 | apparently isolated | 16/138 (42.11%) | 3/122 (2.46%) | . | . | |
| [50] | 3.5–4.4 | apparently isolated | 16/76 (21.05%) | 3/60 (5.00%) | . | . |
| ≥4.5 | apparently isolated | 27/56 (48.21%) | 5/29 (17.24%) | . | . | |
| [60] | 2.5–3.4 | unspecified | 7/57 (12.28%) | 1/49 (2.94%) | . | . |
| 3.5–4.4 | unspecified | 10/39 (25.64%) | 1/29 (3.45%) | . | . | |
| 4.5–5.4 | unspecified | 7/19 (36.84%) | 0/12 (0.00%) | . | . | |
| ≥5.5 | unspecified | 13/24 (54.17%) | 0/11 (0.00%) | . | . | |
| [61] | 2.5–2.9 | apparently isolated | 10/86 (11.63%) | 1/76 (1.32%) | . | . |
| 3.0–3.4 | apparently isolated | 11/73 (15.06%) | 2/62 (3.23%) | . | . | |
| 3.5–4.4 | apparently isolated | 9/50 (18.00%) | 2/41 (4.88%) | . | . | |
| 4.5–5.4 | apparently isolated | 10/21 (47.62%) | 0/11 (0.00%) | . | . | |
| ≥5.5 | apparently isolated | 7/11 (63.64%) | 0/4 (0.00%) | . | . | |
| [62] | ≥95ct | unspecified | 66/287 (23.00%) | . | . | . |
| [63] | ≥3.5 | unspecified | 179/242 (73.96%) | . | . | . |
| [64] | ≥95ct | unspecified | 119/541 (22.00%) | . | . | . |
| [65] | ≥3.5 | unspecified | 16/71 (22.54%) | . | . | . |
| [66] | ≥3.0 | unspecified | 6/105 (5.71%) | . | . | . |
| [67] | ≥2.5 | unspecified | 18/122 (14.75%) | . | . | . |
| [68] | ≥3 | unspecified | 224/1058 (21.17%) | . | . | . |
| 3.0–3.4 | unspecified | 65/676 (9.62%) | . | . | . | |
| 3.5–4.4 | unspecified | 51/208 (24.52%) | . | . | . | |
| 4.5–5.4 | unspecified | 29/67 (43.28%) | . | . | . | |
| 5.5–6.4 | unspecified | 26/37 (70.27%) | . | . | . | |
| ≥6.5 | unspecified | 53/70 (75.71%) | . | . | . | |
| [69] | 95ct–3.4 | unspecified | 124/894 (13.87%) | 8/770 (1.04%) | . | . |
| ≥3.5 | unspecified | 436/1007 (43.30%) | 30/571 (5.25%) | . | . | |
| 3.5–4.9 | unspecified | 138/492 (28.05%) | 16/354 (4.52%) | . | . | |
| 5.0–6.4 | unspecified | 113/199 (56.78%) | 7/86 (8.14%) | . | . | |
| 6.5–7.9 | unspecified | 93/155 (60.00%) | 5/62 (8.06%) | . | . | |
| ≥8.0 | unspecified | 92/162 (56.79%) | 2/70 (2.86%) | . | . | |
| [101] | ≥3 | unspecified | 10/120 (8.33%) | . | . | . |
| [70] | 95ct–3.4 | unspecified | 40/263 (15.21%) | . | . | . |
| 3.5–4.4 | unspecified | 32/169 (18.93%) | . | . | . | |
| 4.5–5.4 | unspecified | 37/79 (46.84%) | . | . | . | |
| 5.5–6.4 | unspecified | 33/52 (63.46%) | . | . | . | |
| ≥6.5 | unspecified | 55/85 (64.71%) | . | . | . | |
| [71] | ≥3.5 | apparently isolated | . | 5/34 (14.70%) | . | . |
| ≥3.5 | associated | . | 3/16 (18.75%) | 1/13 (7.69%) | 5/12 (41.67%) | |
| [102] | ≥3.0 | unspecified | 8/46 (17.39%) | . | . | . |
| 3.0–3.9 | unspecified | 4/35 (11.43%) | . | . | . | |
| 4.0–4.9 | unspecified | 2/7 (28.57%) | . | . | . | |
| 5.0–5.9 | unspecified | 1/3 (33.33%) | . | . | . | |
| ≥6.0 | unspecified | 1/1 (100.00%) | . | . | . | |
| [72] | 3.5–4.4 | unspecified | . | 4/343 (1.17%) | . | . |
| 4.5–5.4 | unspecified | . | 3/124 (2.42%) | . | . | |
| 5.5–6.4 | unspecified | . | 4/73 (5.48%) | . | . | |
| ≥6.5 | unspecified | . | 0/59 (0.00%) | . | . | |
| [73] | ≥99ct | unspecified | 94/221 (42.53%) | 1/106 (0.94%) | . | . |
| [74] | ≥3.0 | apparently isolated | 21/108 (19.44%) | 9/87 (10.34%) | . | . |
| 3.0–3.9 | apparently isolated | 12/81 (14.81%) | 6/69 (8.70%) | . | . | |
| ≥4.0 | apparently isolated | 9/27 (33.33%) | 3/18 (16.67%) | . | . | |
| [75] | ≥3.0 | unspecified | 172/775 (22.19%) | 4/256 (1.56%) | . | . |
| [76] | ≥3.5 | apparently isolated | . | 1/172 (0.58%) | . | . |
| [77] | 95ct–3.4 | unspecified | 507/7109 (7.13%) | . | . | . |
| 3.5–4.4 | unspecified | 423/2101 (20.13%) | . | . | . | |
| 4.5–5.4 | unspecified | 321/707 (45.40%) | . | . | . | |
| 5.5–6.4 | unspecified | 219/437 (50.11%) | . | . | . | |
| 6.5–7.4 | unspecified | 218/309 (70.55%) | . | . | . | |
| 7.5–8.4 | unspecified | 148/209 (70.81%) | . | . | . | |
| 8.5–9.4 | unspecified | 126/168 (75.00%) | . | . | . | |
| 9.5–10.4 | unspecified | 74/88 (84.09%) | . | . | . | |
| 10.5–11.4 | unspecified | 45/64 (70.31%) | . | . | . | |
| ≥11.5 | unspecified | 87/123 (70.73%) | . | . | . | |
| [78] | ≥3.5 | apparently isolated | . | 3/269 (1.12%) | . | . |
| ≥3.5 | associated | . | 3/31 (9.68%) | . | . | |
| [79] | ≥6.5 | unspecified | 60/84 (71.43%) | . | . | . |
| 5.5–6.4 | unspecified | 21/37 (56.76%) | . | . | . | |
| [80] | ≥3.5 | unspecified | 164/303 (54.13%) | . | . | . |
| [81] | ≥3.5 | unspecified | 123/222 (55.41%) | . | . | . |
| ≥3.5 | apparently isolated | 32/107 (29.91%) | . | . | . | |
| ≥3.5 | associated | 91/115 (79.13%) | . | . | . | |
| [82] | ≥95ct | unspecified | 154/393 (39.19%) | . | . | . |
| 95ct–3.4 | unspecified | 31/170 (18.24%) | . | . | . | |
| 3.5–4.4 | unspecified | 29/81 (35.80%) | . | . | . | |
| 4.5–5.4 | unspecified | 23/42 (54.76%) | . | . | . | |
| 5.5–6.4 | unspecified | 15/23 (65.22%) | . | . | . | |
| ≥6.5 | unspecified | 56/77 (72.73%) | . | . | . | |
| [83] | ≥95ct | unspecified | 37/186 (19.89%) | . | . | . |
| 95ct–3.4 | unspecified | 10/92 (10.87%) | . | . | . | |
| 3.5–4.4 | unspecified | 6/50 (12.00%) | . | . | . | |
| 4.5–5.4 | unspecified | 4/12 (33.33%) | . | . | . | |
| 5.5–6.4 | unspecified | 7/15 (46.67%) | . | . | . | |
| ≥6.5 | unspecified | 10/17 (58.82%) | . | . | . | |
| [84] | 2.5–4.4 | unspecified | 29/33 (87.88%) | . | . | . |
| 4.5–6.4 | unspecified | 8/8 (100.00%) | . | . | . | |
| ≥6.5 | unspecified | 11/13 (84.62%) | . | . | . | |
| [85] | ≥99ct | unspecified | 64/248 (25.81%) | . | . | . |
| [86] | ≥4.0 | unspecified | 71/160 (44.38%) | . | . | . |
| [87] | ≥6.5 | unspecified | 89/120 (74.17%) | . | . | . |
| [88] | ≥95ct | unspecified | 44/147 (29.93%) | . | . | . |
| Cystic Hygroma | |||||
|---|---|---|---|---|---|
| Reference | Association | Yield (Incremental) | |||
| Karyotype | CMA | RASopathy Panel | ES | ||
| [89] | unspecified | 15/28 (53.50%) | . | . | . |
| [90] | apparently isolated | 25/50 (50.00%) | . | . | . |
| associated | 13/22 (59.09%) | . | . | . | |
| [91] | unspecified | 67/132 (50.76%) | . | . | . |
| [92] | unspecified | 122/185 (65.95%) | 1/40 (2.50%) | 6/15 (40.00%) | . |
| [93] | apparently isolated | 3/10 (30.00%) | . | . | . |
| associated | 13/20 (65.00%) | . | . | . | |
| [94] | unspecified | 55/85 (64.70%) | . | . | . |
| [95] | unspecified | 20/37 (54.05%) | . | . | . |
| [11] | unspecified | 18/50 (36.00%) | . | . | . |
| [101] | unspecified | 13/27 (48.15%) | . | . | . |
| [102] | unspecified | 13/30 (43.33%) | . | . | . |
| unspecified | 0/1 (0.00%) | . | . | . | |
| unspecified | 1/3 (33.33%) | . | . | . | |
| unspecified | 4/4 (100.00%) | . | . | . | |
| unspecified | 11/22 (50.00%) | . | . | . | |
| [96] | apparently isolated | 12/21 (57.14%) | . | . | . |
| associated | 13/21 (61.90%) | . | . | . | |
| [97] | apparently isolated | 5/12 (41.67%) | . | . | . |
| associated | 7/14 (50.00%) | . | . | . | |
| [12] | unspecified | 400/729 (54.87%) | . | . | . |
| [98] | unspecified | 128/194 (65.98%) | . | . | . |
| [99] | unspecified | 28/69 (40.58%) | . | . | . |
| [100] | unspecified | 45/100 (45.00%) | . | . | . |
| Nuchal Translucency | ||||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Dimension (mm) | Association | Miscarriages | Intrauterine Deaths | Live Births | Perinatal Death | Intellectual Disability | Major Childhood Morbidity |
| [52] | ≥3.5 | apparently isolated-euploid | 0/20 (0.00%) | 1/20 (5.00%) | 19/20 (95.00%) | 1/19 (5.26%) | 3/19 (15.79%) | 3/19 (15.79%) |
| [63] | ≥3.5 | unspecified-euploid | . | 5/33 (15.15%) | 28/33 (84.85%) | . | . | . |
| [64] | ≥3.5 | unspecified-euploid | 4/420 (0.95%) | . | . | . | 10/270 (3.70%) | 10/370 (2.70%) |
| [65] | ≥3.5 | unspecified-euploid | 6/52 (11.54%) | . | 46/52 (88.46%) | . | . | 6/46 (13.04%) |
| [66] | ≤3.0 | unspecified | 45/1395 (3.23%) | 19/1395 (1.36%) | 1305/1395 (93.55%) | . | . | . |
| ≥3.0 | unspecified | 22/105 (20.95%) | 15/105 (14.29%) | 33/105 (31.43%) | . | . | . | |
| 3.0–4.0 | unspecified | 10/52 (19.23%) | 5/52 (9.62%) | 28/52 (53.85%) | . | . | . | |
| 4.0–5.0 | unspecified | 10/33 (30.30%) | 7/33 (21.21%) | 5/33 (15.15%) | . | . | . | |
| 5.0–6.0 | unspecified | 2/15 (13.33%) | 3/15 (20.00%) | 0/15 (0.00%) | . | . | . | |
| ≥6.0 | unspecified | 0/5 (0.00%) | 0/5 (0.00%) | 0/5 (0.00%) | . | . | . | |
| [68] | ≥3.0 | associated-euploid | . | . | 741/834 (88.85%) | . | . | 43/741 (5.80%) |
| 3–3.4 | associated-euploid | . | . | 562/611 (91.98%) | . | . | 28/562 (4.98%) | |
| 3.5–4.4 | associated-euploid | . | . | 141/157 (89.81%) | . | . | 7/141 (4.96%) | |
| 4.5–5.4 | associated-euploid | . | . | 30/38 (78.95%) | . | . | 2/30 (6.67%) | |
| 5.5–6.4 | associated-euploid | . | . | 5/11 (45.45%) | . | . | 4/5 (80.00%) | |
| ≥6.5 | associated-euploid | . | . | 3/17 (17.65%) | . | . | 2/3 (66.76%) | |
| [102] | ≥3.0 | unspecified | . | 0/46 (0.00%) | 31/46 (67.39%) | 3/31 (9.68%) | . | 3/31 (9.68%) |
| [73] | ≥99ct | associated-euploid | . | 4/36 (11.11%) | 13/36 (36.11%) | 1/6 (16.67%) | . | 6/13 (46.15%) |
| ≥99ct | apparently isolated-euploid | 2/70 (2.86%) | 1/70 (1.73%) | 63/70 (90.00%) | . | . | 3/63 (4.76%) | |
| [103] | ≥95ct | unspecified-euploid | 23/625 (3.68%) | . | . | . | . | . |
| [80] | ≥3.5 | unspecified-euploid | 5/139 (3.60%) | 1/139 (0.72%) | 110/139 (79.14%) | . | . | 7/110 (6.36%) |
| 3.5–4.4 | unspecified-euploid | 2/86 (2.33%) | 1/86 (1.16%) | 77/86 (89.53%) | . | . | 3/77 (3.90%) | |
| 4.5–5.4 | unspecified-euploid | 0/28 (0.00%) | 0/28 (0.00%) | 20/28 (71.43%) | . | . | 2/20 (10.00%) | |
| 5.5–6.4 | unspecified-euploid | 1/12 (8.33%) | 0/12 (0.00%) | 7/12 (58.33%) | . | . | 1/7 (14.29%) | |
| ≥6.5 | unspecified-euploid | 2/13 (15.38%) | 0/13 (0.00%) | 6/13 (46.15%) | . | . | 1/6 (16.67%) | |
| [82] | ≥95ct | unspecified | . | 9/239 (3.77%) | 210/239 (87.87%) | . | . | 10/210 (4.76%) |
| 95ct–3.4 | unspecified | . | 2/139 (1.44%) | 135/139 (97.12%) | . | . | 2/135 (1.48%) | |
| 3.5–4.4 | unspecified | . | 2/52 (3.85%) | 46/52 (88.46%) | . | . | 2/46 (4.35%) | |
| 4.5–5.4 | unspecified | . | 2/19 (10.53%) | 16/19 (84.21%) | . | . | 1/16 (6.25%) | |
| 5.5–6.4 | unspecified | . | 0/8 (0.00%) | 4/8 (50.00%) | . | . | 0/4 (0.00%) | |
| ≥6.5 | unspecified | . | 3/21 (14.29%) | 9/21 (42.86%) | . | . | 5/9 (55.56%) | |
| [83] | ≥95ct | unspecified | 7/149 (4.70%) | . | 110/149 (73.83%) | . | . | . |
| 95ct–3.4 | unspecified | 1/82 (1.22%) | . | 71/82 (86.59%) | . | . | . | |
| 3.5–4.4 | unspecified | 2/43 (4.65%) | . | 35/43 (81.40%) | . | . | . | |
| 4.5–5.4 | unspecified | 0/7 (0.00%) | . | 2/7 (28.57%) | . | . | . | |
| 5.5–6.4 | unspecified | 3/8 (37.50%) | . | 1/8 (12.50%) | . | . | . | |
| ≥6.5 | unspecified | 1/19 (5.26%) | . | 1/19 (5.26%) | . | . | . | |
| [84] | 2.5–4.4 | unspecified | 4/33 (12.12%) | 0/33 (0.00%) | 20/33 (60.61%) | . | . | . |
| 4.5–6.4 | unspecified | 1/8 (12.5%) | 0/8 (0.00%) | 1/8 (12.50%) | . | . | . | |
| ≥6.5 | unspecified | 2/13 (15.38%) | 4/13 (30.77%) | 1/13 (7.69%) | . | . | . | |
| [85] | ≥99ct | unspecified-euploid | 1/179 (0.56%) | 5/179 (2.79%) | 162/179 (90.50%) | . | . | 18/162 (11.11%) |
| [86] | ≥4.0 | unspecified | . | 2/89 (2.25%) | 68/89 (76.40%) | . | 4/64 (6.25%) | 4/68 (5.88%) |
| [87] | ≥6.5 | unspecified-euploid | 6/27 (22.22%) | . | 8/27 (29.63%) | . | . | . |
| [88] | ≥95ct | unspecified-euploid | . | 10/103 (9.71%) | 87/103 (84.47%) | . | . | 10/87 (11.49%) |
| Cystic Hygroma | |||||||
|---|---|---|---|---|---|---|---|
| Reference | Association | Miscarriages | Intrauterine Deaths | Live Births | Perinatal Death | Intellectual Disability | Major Childhood Morbidity |
| [90] | unspecified-euploid | 2/34 (5.88%) | 2/34 (5.88%) | 18/34 (52.94%) | 0/18 (0.00%) | . | 2/18 (11.11%) |
| [91] | unspecified-euploid | . | 5/36 (13.89%) | 31/36 (86.11%) | 1/31 (3.23%) | 1/31 (3.23%) | 8/31 (25.81%) |
| [93] | associated-euploid | . | 1/3 (33.33%) | 2/3 (66.66%) | . | . | . |
| apparently isolated-euploid | . | . | 6/6 (100.00%) | . | . | . | |
| [102] | unspecified | . | 5/30 (16.67%) | 7/30 (23.33%) | 4/7 (57.14%) | . | 2/7 (28.57%) |
| [96] | unspecified-euploid | . | . | 7/17 (41.18%) | . | . | . |
| [97] | apparently isolated-euploid | 1/5 (20.00%) | . | 3/5 (60.00%) | . | . | 1/3 (33.33%) |
| associated-euploid | 3/7 (42.86%) | 1/7 (14.29%) | 1/7 (14.29%) | . | . | 1/1 (100.00%) | |
| [12] | unspecified | 106/295 (14.29%) | . | 180/295 (24.26%) | 9/180 (5.00%) | . | . |
| [98] | unspecified-euploid | 7/66 (10.61%) | . | 27/66 (40.91%) | . | . | . |
| [99] | unspecified | 5/41 (12.20%) | . | 12/41 (29.27%) | . | . | . |
| [100] | unspecified | . | 54/85 (63.53%) | 31/85 (36.47%) | . | . | 6/31 (19.35%) |
| Nuchal Translucency | ||||||
|---|---|---|---|---|---|---|
| Dimensions | Association | Yield (Incremental) | REF[Karyo][CMA][RAS][ES] | |||
| Karyotype | CMA | RASopathy Panel | ES | |||
| 2.5–2.9 | unspecified | 8/134 5.97% | 11/896 1.23% (1.17–1.29) | . | . | [61]; [61]; [.]; [.] |
| apparently isolated | 10/86 11.73% | 1/76 (1.32%) | . | . | [47,69]; [47]; [.]; [.] | |
| associated | . | . | . | . | [.]; [.]; [.]; [.] | |
| 2.5–3.4 | unspecified | 964/9957 9.68% (9.60–9.76) | 9/819 1.10% (1.05–1.15) | . | . | [56,60,69,77,82,83]; [60,69]; [.]; [.] |
| apparently isolated | 39/273 14.29% (14.07–14.51) | 4/215 1.86% (1.82–1.90) | . | . | [53,61]; [50,53]; [.]; [.] | |
| associated | . | 0/15 0.00% | . | . | [.]; [50]; [.]; [.] | |
| 3.0–3.4 | unspecified | 88/932 9.44% (9.18–9.70) | 5/198 2.53% (2.18–2.88) | . | . | [47,48,68]; [47,48]; [.]; [.] |
| apparently isolated | 39/862 4.52% (4.09–4.95) | 14/823 1.70% (1.64–1.76) | . | . | [54,58,61]; [54,58,61]; [.]; [.] | |
| associated | . | . | . | . | [.]; [.]; [.]; [.] | |
| ≥2.5 | unspecified | 4393/19177 22.91% (22.60–23.22) | 61/2098 2.91% (2.88–2.94) | . | . | [47,50,56,60,62,64,67,69,70,77,82,83,84,88]; [47,50,60,69]; [.]; [.] |
| apparently isolated | 140/615 22.76% (22.46–23.06) | 15/637 2.35% (2.32–2.38) | . | . | [53,61]; [50,53,61]; [.]; [.] | |
| associated | 2/134 1.49% | 2/27 (7.41%) | . | . | [50]; [50]; [.]; [.] | |
| ≥3.0 | unspecified | 616/2788 22.09% (21.74–22.44) | 23/695 3.31% (3.18–3.44) | . | . | [47,48,66,68,75,101,102]; [47,48,75]; [.]; [.] |
| apparently isolated | 82/571 14.36% (13.68–15.04) | 19/489 3.89% (3.50–4.28) | . | . | [58,61,74]; [58,61,74]; [.]; [.] | |
| associated | . | . | . | . | [.]; [.]; [.]; [.] | |
| ≥3.5 | unspecified | 3680/9204 39.98% (39.64–40.32) | 59/1581 3.73% (3.64–3.82) | 3/103 (2.91%) | . | [47,48,55,56,60,63,65,68,69,70,77,80,81,82,83]; [47,48,55,60,69,72]; [14]; [.] |
| apparently isolated | 449/1307 34.35% (33.66–35.04) | 56/1367 4.1% (3.50–4.70) | 3/108 1.44% (1.15–1.73) | 5/205 2.44% (2.26–2.62) | [14,52,53,58,59,61,81]; [14,50,51,52,53,58,59,61,71,76,78]; [49,53,57]; [49,53,57] | |
| associated | 91/115 79.13% | 8/59 13.56% (12.32–14.80) | 8/104 (7.69%) | 22/96 22.92% (19.85–25.99) | [81]; [50,71,78]; [57,71]; [57,71] | |
| Cystic Hygroma | ||||||
| Association | Yield (incremental) | REF[Karyo][CMA][RAS][ES] | ||||
| Karyotype | CMA | RASopathy panel | ES | |||
| unspecified | 927/1666 55.64% (55.18–56.10) | 1/40 2.50% | 6/15 (40.00%) | . | [11,12,89,91,92,94,95,99,100,101,102]; [92]; [92]; [.] | |
| apparently isolated | 45/93 48.39% (46.02–50.76) | . | . | . | [90,93,96,97]; [.]; [.]; [.] | |
| associated | 46/80 57.50% (55.16–59.84) | . | . | . | [90,93,96,97]; [.]; [.]; [.] | |
| ID | Sex | Growth | Soft Marker (s) | Karyotype | CMA | RASopathy Panel |
|---|---|---|---|---|---|---|
| 1 | F | - | - | 46,XX | N | N |
| 2 | F | - | - | 46,XX | N | LZTR1 c.2173_2174insA; p.(Arg725Glnfs*57) mat |
| 3 | F | - | inverted DV flow | mos 47,XX,+21[27]/46,XX [3] | . | . |
| 4 | F | - | - | 47,XX,+21 | . | . |
| 5 | F | - | - | 46,XX | N | SOS1 c.755T>C; p.(Ile252Thr) VUS pat |
| 6 | F | - | - | 46,XX | N | N |
| 7 | F | biometry <5°c | absent nasal bone | 46,XX | N | RAF1: c.770C>T; p.(Ser257Leu) dn |
| 8 | F | - | - | 45,X | . | . |
| 9 | F | - | - | 46,XX | N | N |
| 10 | F | - | - | 46,XX | N | N |
| 11 | F | - | - | 46,XX | N | N |
| 12 | F | - | - | 46,XX | N | N |
| 13 | F | - | - | 46,XX | N | PTPN11 c.179_181delTGG |
| 14 | F | - | absent nasal bone | 47,XX,+21 | . | . |
| 15 | F | - | - | 47,XX,+21,t(2;8)(q33;q22) | . | . |
| 16 | F | - | - | 47,XY,+21 | . | . |
| 17 | F | - | echoic cardiac focus | 47,XX,+21 | . | . |
| 18 | F | - | - | 45,X | . | . |
| 19 | F | - | - | 47,XX,+21 | . | . |
| 20 | F | - | - | 45,X | . | . |
| 21 | M | - | DVA | 46,XY | N | SHOC2 c.807_808delinsTT; p.(Gln269_His270delinsHisTyr) |
| 22 | M | - | hypoplastic nasal bone | 46,XY | N | SOS2 c.1709C>G; p.(Pro570Arg) VUSmat |
| 23 | M | - | - | 47,XY,+21 | n.p. | n.p. |
| 24 | M | - | - | 46,XY | N | N |
| 25 | M | - | - | 46,XY | N | N |
| 26 | M | - | - | 46,XY | 22q11.21(18894835_21464119)x1 | |
| 27 | M | - | - | 46,XY | N | N |
| 28 | M | - | DVA | 46,XY der(4)t(4;7)(4p16.3;7p22.3) | 4p16.3(71552_3872380)x1 7p22.3p22.1(42976_6870943)x3 | |
| 29 | M | - | hyperechoic bowel | 46,XY | N | N |
| 30 | M | - | - | 46,XY | N | N |
| 31 | M | - | - | 46,XY | N | SOS1 c.643T>C; p.(Tyr215His) mat |
| 32 | M | - | - | 46,XY | N | N |
| 33 | M | - | - | 46,XY | N | N |
| 34 | M | - | - | 47,XXY | . | . |
| 35 | M | - | - | 47,XY,+21 | . | . |
| 36 | M | - | - | 46,XY,del(4)(p15.2)dn | . | . |
| 37 | M | - | - | 47,XY,+21 | . | . |
| 38 | M | - | - | 47,XY,+13 | . | . |
| 39 | M | - | - | 47,XY,+21 | . | . |
| 40 | M | - | - | 47,XY,+21 | . | . |
| 41 | M | - | - | 46,XY | N | N |
| 42 | M | - | - | 46,XY | N | N |
| 43 | M | - | - | 46,XY | N | N |
| 44 | M | - | - | 46,XY | 17q23.2(60033664_60251568)x3 mat | N |
| 45 | M | - | SUA, DVA, absent nasal bone | 47,XY,+21 | . | . |
| 46 | M | - | - | 46,XY | N | N |
| 47 | M | - | absent nasal bone | 47,XY,+21 | . | . |
| ID | Sex | NT (mm) | Soft Marker (s) | Karyotype | CMA | RASopathy Panel |
|---|---|---|---|---|---|---|
| NT 2.5–2.9 mm | ||||||
| 1 | F | 2.6 | - | 47,XX,+21 | . | . |
| 2 | M | 2.6 | - | 46,XY | N | SOS1 c.643T>C (het, mat) |
| 3 | F | 2.7 | - | 47,XX,+21 | . | . |
| 4 | F | 2.8 | - | 47,XX,+21 | . | . |
| 5 | F | 2.8 | - | 46,XX | N | N |
| 6 | M | 2.9 | - | 47,XYY | . | . |
| NT 3.0–3.4 mm | ||||||
| 7 | F | 3.0 | - | 47,XX,+21 | . | . |
| 9 | F | 3.0 | - | 46,XX | N | N |
| 10 | F | 3.0 | - | 47,XX,+21 | . | . |
| 11 | M | 3.0 | - | 46,XY | 10p13p12.33(16786323_17726950)x3 mat | . |
| 12 | M | 3.0 | 47,XXY | 10q21.3(68735256_68938523)x3 pat 22q12.2(29911595_30141397)x3 mat | . | |
| 13 | M | 3.0 | hypoplastic nasal bone | 46,XY | N | N |
| 14 | M | 3.1 | - | 47,XY,+21 | . | . |
| 15 | F | 3.1 | - | 47,XX,+21 | . | . |
| 16 | F | 3.2 | - | mos 47,XX,+18[58]/46,XX [20] | . | . |
| 17 | M | 3.2 | - | 46,XY | N | N |
| 18 | M | 3.2 | - | N | N | N |
| 19 | M | 3.3 | - | 46,XY | N | N |
| 20 | M | 3.3 | - | 46,XY | N | N |
| 21 | F | 3.3 | - | 47,XX,+21 | . | . |
| 22 | M | 3.3 | - | 46,XY | N | N |
| 23 | M | 3.3 | Choroid plexus cyst, hyperechoic cardiac focus | 46,XY | N | N |
| 24 | M | 3.3 | - | 47,XY,+21 | . | . |
| 25 | F | 3.4 | - | 46,XX | 3p26.3(270649_920375)x3mat | N |
| NT 3.5–3.9 mm | ||||||
| 26 | F | 3.5 | - | 46,XX | N | N |
| 27 | M | 3.5 | - | 47,XY,+21 | . | . |
| 28 | M | 3.6 | - | 46,XY | N | N |
| 29 | F | 3.6 | - | 46,XX | N | N |
| 30 | M | 3.6 | - | N | N | N |
| 31 | M | 3.8 | - | N | N | N |
| 32 | F | 3.9 | - | 47,XX,+21 | . | . |
| 33 | F | 3.9 | - | 46,XX | N | LZTR1 c.842del (het, n/a) |
| NT ≥ 4.0 mm | ||||||
| 34 | M | 4.0 | - | 46,XY | N | N |
| 35 | M | 4.0 | - | 46,XY | N | N |
| 36 | M | 4.0 | - | 46,XY,inv(19)(p13.3q13.2)mat | N | N |
| 37 | M | 4–0 | - | 46,XY | N | N |
| 38 | F | 4.1 | - | 46,XX | N | N |
| 39 | M | 4.2 | - | 47,XY,+21 | . | . |
| 40 | M | 4.2 | - | 46,XY | N | N |
| 41 | F | 4.3 | - | N | N | N |
| 42 | F | 4.4 | - | 47,XX,+21 | . | . |
| 43 | F | 4.7 | - | 46,XX,del(3)(p25) | . | . |
| 44 | F | 4.7 | - | 46,XX | N | BRAF c.26G>C (het, mat) |
| 45 | M | 5.3 | - | 46,XY | N | N |
| 46 | M | 5.7 | - | 47,XY,+18 | . | . |
| 47 | F | 5.8 | - | 47,XX,+18 | . | . |
| 48 | F | 7.0 | - | 46,XX | N | N |
| 49 | M | 7.7 | - | 46,XY | 15q11.2(22784523_23179948)x1 | N |
| Apparently Isolated iNT | Apparently Isolated Cystic Hygroma | |||
|---|---|---|---|---|
| 2.5–2.9 mm | 3–3.4 mm | ≥3.5 mm | ||
| Karyotype | 11.73% | 4.52% | 34.35% | 48.39% |
| CMA | 1.32% | 1.7% | 4.1% | |
| RASopathy panel | 1.44% | |||
| ES | 2.44% | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastromoro, G.; Guadagnolo, D.; Khaleghi Hashemian, N.; Bernardini, L.; Giancotti, A.; Piacentini, G.; De Luca, A.; Pizzuti, A. A Pain in the Neck: Lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma—Systematic Review of the Literature, Meta-Analysis and Case Series. Diagnostics 2023, 13, 48. https://doi.org/10.3390/diagnostics13010048
Mastromoro G, Guadagnolo D, Khaleghi Hashemian N, Bernardini L, Giancotti A, Piacentini G, De Luca A, Pizzuti A. A Pain in the Neck: Lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma—Systematic Review of the Literature, Meta-Analysis and Case Series. Diagnostics. 2023; 13(1):48. https://doi.org/10.3390/diagnostics13010048
Chicago/Turabian StyleMastromoro, Gioia, Daniele Guadagnolo, Nader Khaleghi Hashemian, Laura Bernardini, Antonella Giancotti, Gerardo Piacentini, Alessandro De Luca, and Antonio Pizzuti. 2023. "A Pain in the Neck: Lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma—Systematic Review of the Literature, Meta-Analysis and Case Series" Diagnostics 13, no. 1: 48. https://doi.org/10.3390/diagnostics13010048
APA StyleMastromoro, G., Guadagnolo, D., Khaleghi Hashemian, N., Bernardini, L., Giancotti, A., Piacentini, G., De Luca, A., & Pizzuti, A. (2023). A Pain in the Neck: Lessons Learnt from Genetic Testing in Fetuses Detected with Nuchal Fluid Collections, Increased Nuchal Translucency versus Cystic Hygroma—Systematic Review of the Literature, Meta-Analysis and Case Series. Diagnostics, 13(1), 48. https://doi.org/10.3390/diagnostics13010048







