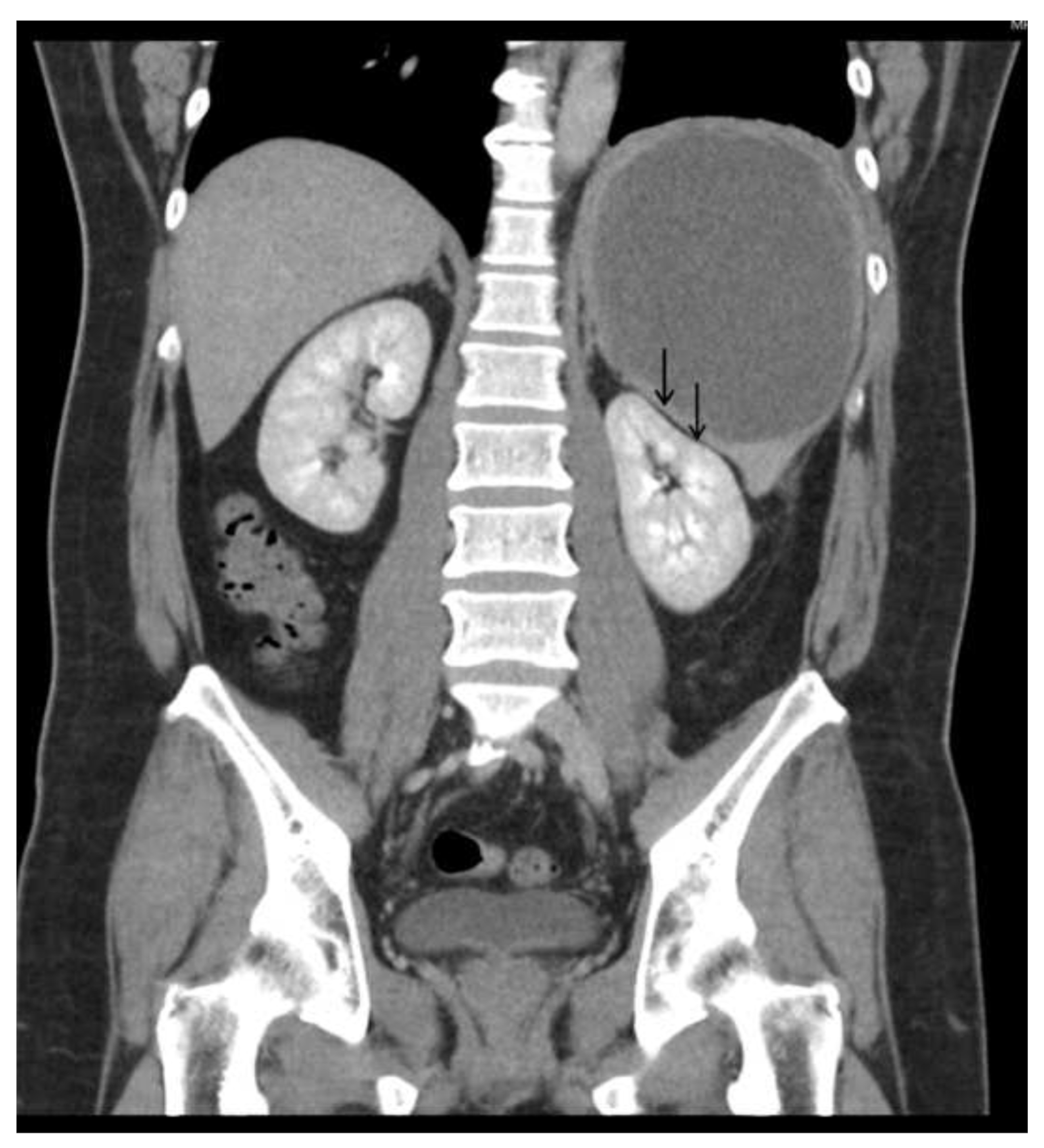
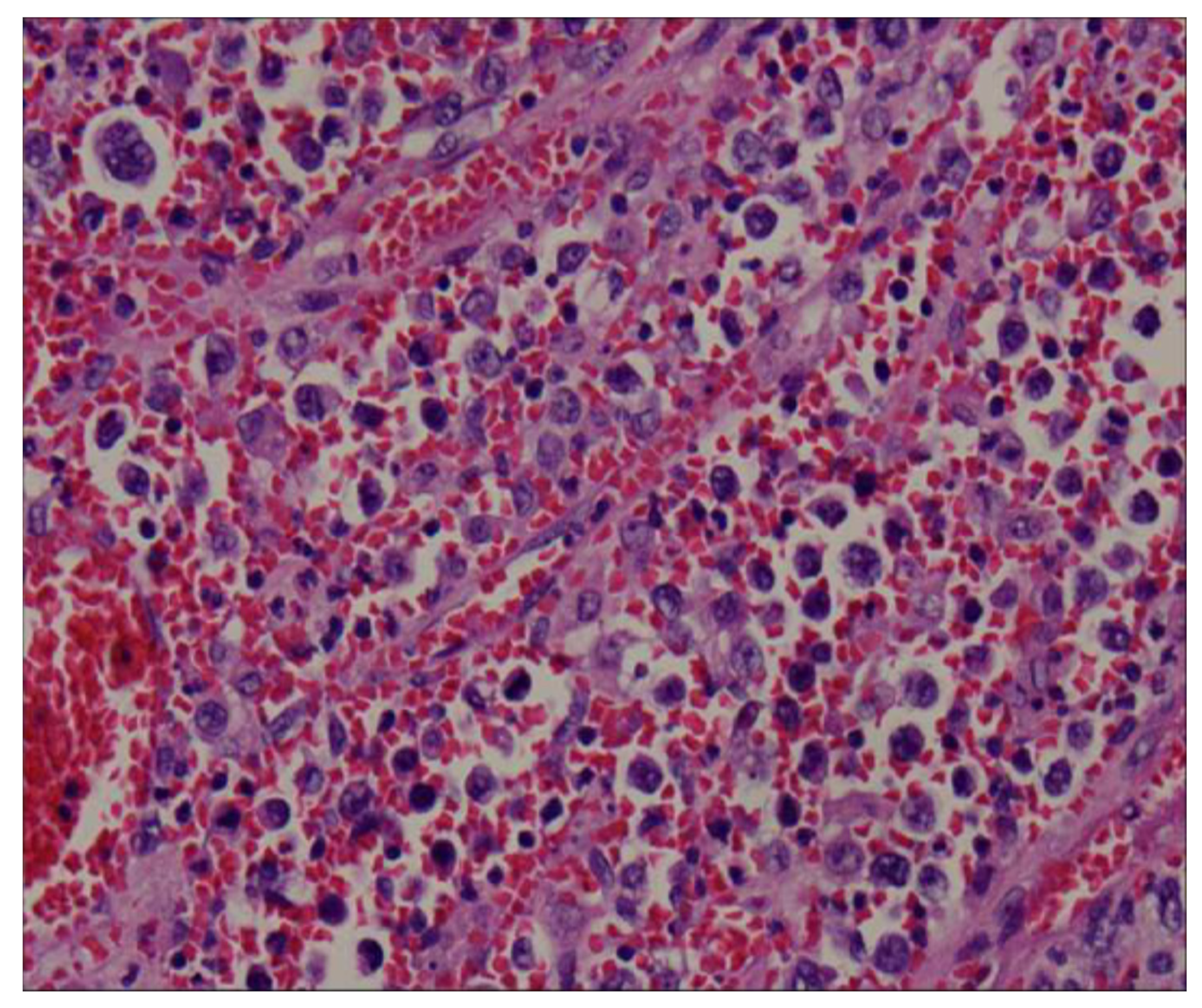
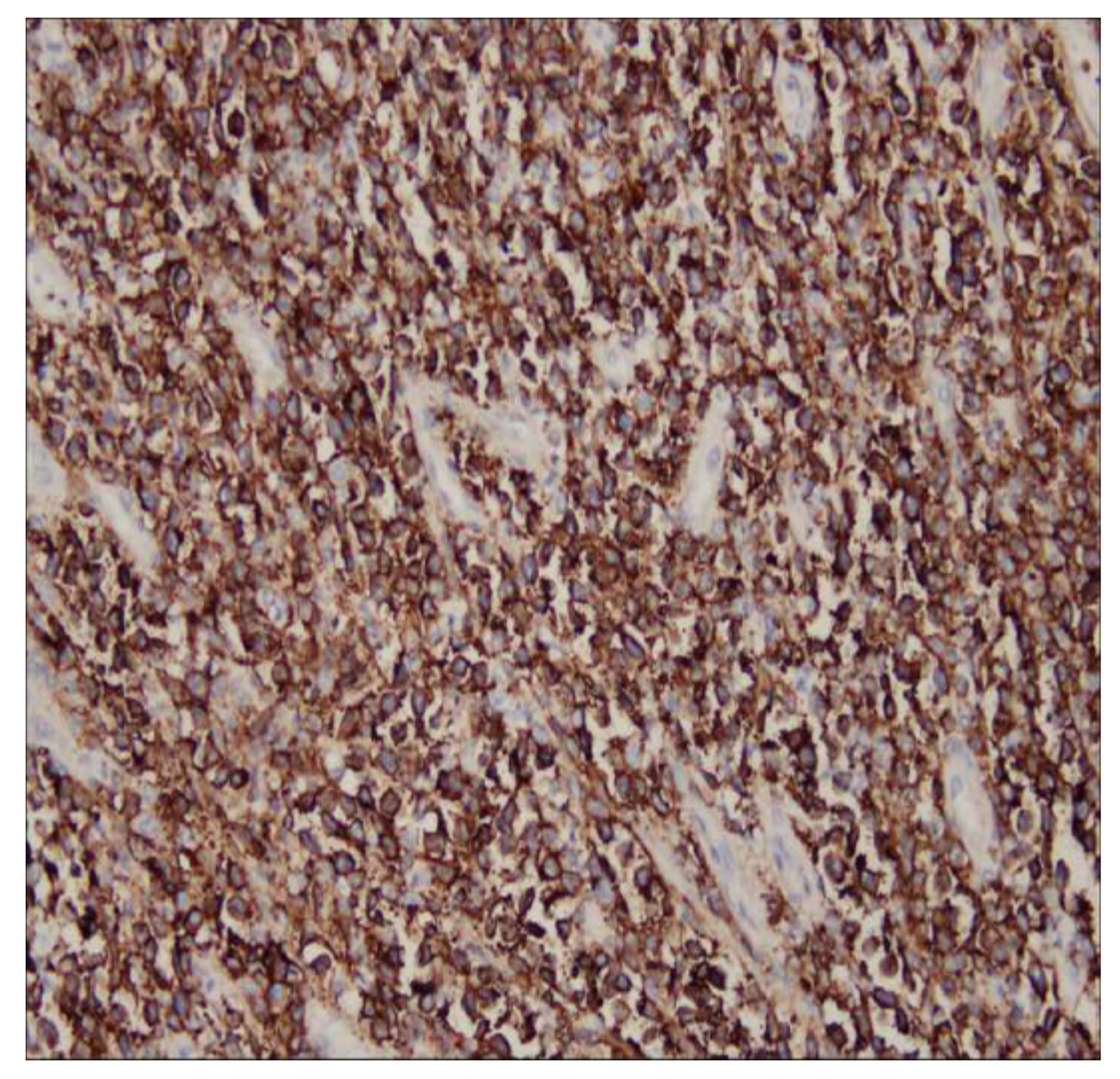
Unusual Rapid Growth of Primary Splenic Diffuse Large B-Cell Lymphoma with Extensive Necrosis
Abstract
:Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das Gupta, T.; Goombes, B.; Brosfeld, R.D. Primary malignant neoplasms of the spleen. Surg. Gynecol. Obstet. 1969, 120, 947–960. [Google Scholar]
- Ahmann, D.L.; Kiely, J.M.; Harrison, E.G.; Payne, W.S. Malignant lymphoma of the spleen. A review of 49 cases in which the diagnosis was made at splenectomy. Cancer 1966, 19, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Ingle, S.B.; Hinge Ingle, C.R. Primary splenic lymphoma: Current diagnostic trends. World J. Clin. Cases 2016, 4, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Kamaya, A.; Weinstein, S.; Desser, T.S. Multiple lesions of the spleen: Differential diagnosis of cystic and solid lesions. Semin. Ultrasound CT MR 2006, 27, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Yocum, B.P.; Hwang, M.; Mesa, H.; Collins, K. Differential Diagnosis of Cystic Lesions of the Spleen: A Review of Clinical, Imaging and Pathological Findings. Int. J. Surg. Pathol. 2022. [CrossRef] [PubMed]
- Taibi, S.; Jabi, R.; Kradi, Y.; Miry, N.; Bouziane, M. Diffuse Large B-Cell Lymphoma Revealed by Splenic Abscess: A Case Report. Cureus 2021, 13, e18771. [Google Scholar] [CrossRef] [PubMed]
- Kehoe, J.; Straus, D.J. Primary lymphoma of the spleen: Clinical features and outcome after splenectomy. Cancer 1988, 62, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Bairey, O.; Shvidel, L.; Perry, C.; Dann, E.J.; Ruchlemer, R.; Tadmor, T.; Goldschmidt, N. Characteristics of primary splenic diffuse large B-cell lymphoma and role of splenectomy in improving survival. Cancer 2015, 121, 2909–2916. [Google Scholar] [CrossRef] [PubMed]
- Isaacson, P.G. Primary splenic lymphoma. Cancer Surv. 1997, 30, 193–212. [Google Scholar] [PubMed]
- Carboni, F.; Covello, R.; Valle, M. Primary Splenic Lymphoma. J. Gastrointest. Surg. 2021, 25, 2423–2425. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.-R.; Wu, H.-K.; Chen, Y.-Y. Unusual Rapid Growth of Primary Splenic Diffuse Large B-Cell Lymphoma with Extensive Necrosis. Diagnostics 2023, 13, 35. https://doi.org/10.3390/diagnostics13010035
Chen Y-R, Wu H-K, Chen Y-Y. Unusual Rapid Growth of Primary Splenic Diffuse Large B-Cell Lymphoma with Extensive Necrosis. Diagnostics. 2023; 13(1):35. https://doi.org/10.3390/diagnostics13010035
Chicago/Turabian StyleChen, Yue-Ren, Hwa-Koon Wu, and Yang-Yuan Chen. 2023. "Unusual Rapid Growth of Primary Splenic Diffuse Large B-Cell Lymphoma with Extensive Necrosis" Diagnostics 13, no. 1: 35. https://doi.org/10.3390/diagnostics13010035
APA StyleChen, Y.-R., Wu, H.-K., & Chen, Y.-Y. (2023). Unusual Rapid Growth of Primary Splenic Diffuse Large B-Cell Lymphoma with Extensive Necrosis. Diagnostics, 13(1), 35. https://doi.org/10.3390/diagnostics13010035





