Performance Evaluation of STANDARD Q COVID/FLU Ag Combo for Detection of SARS-CoV-2 and Influenza A/B
Abstract
1. Introduction
2. Materials and Methods
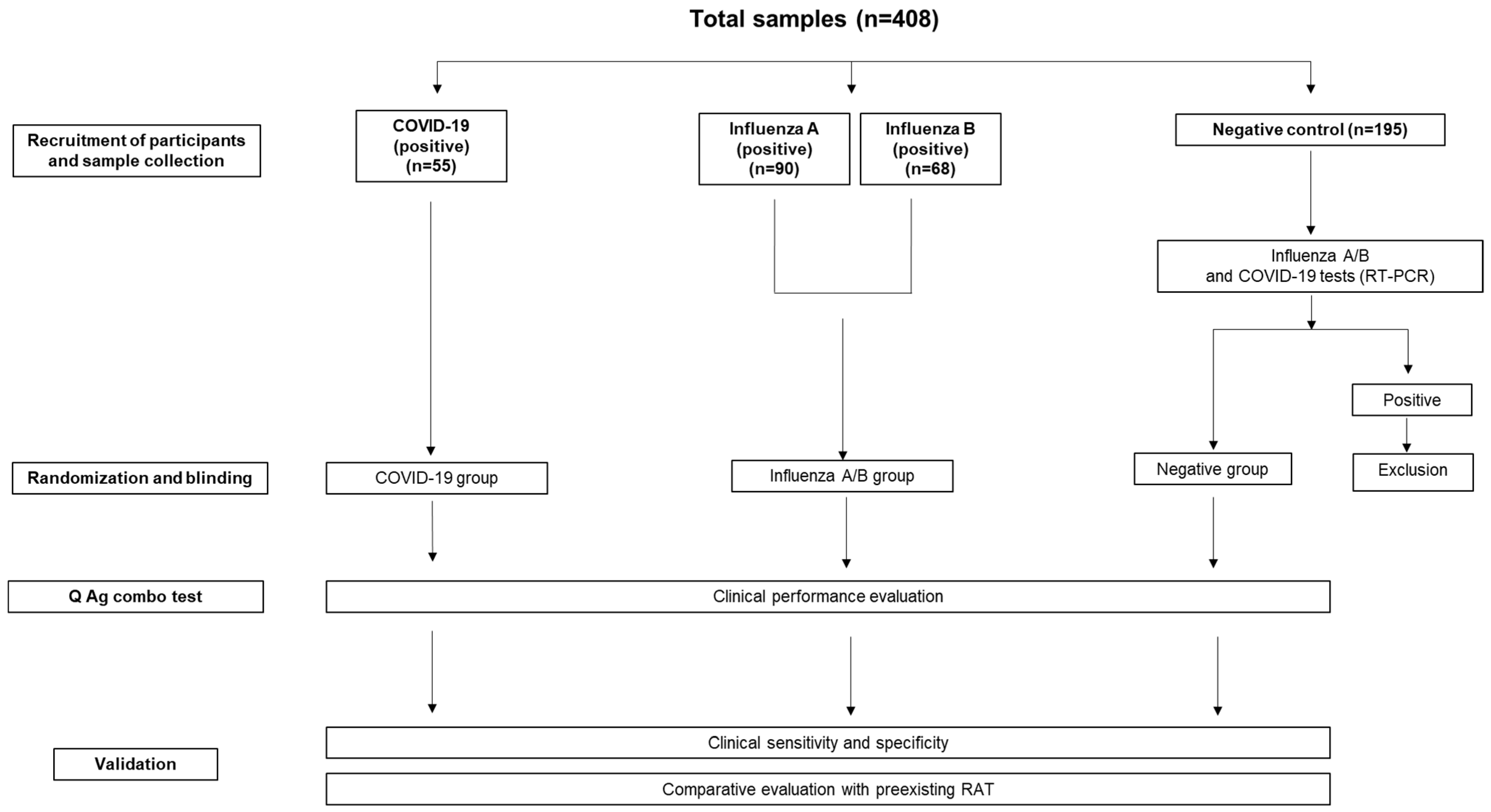
2.1. Subjects
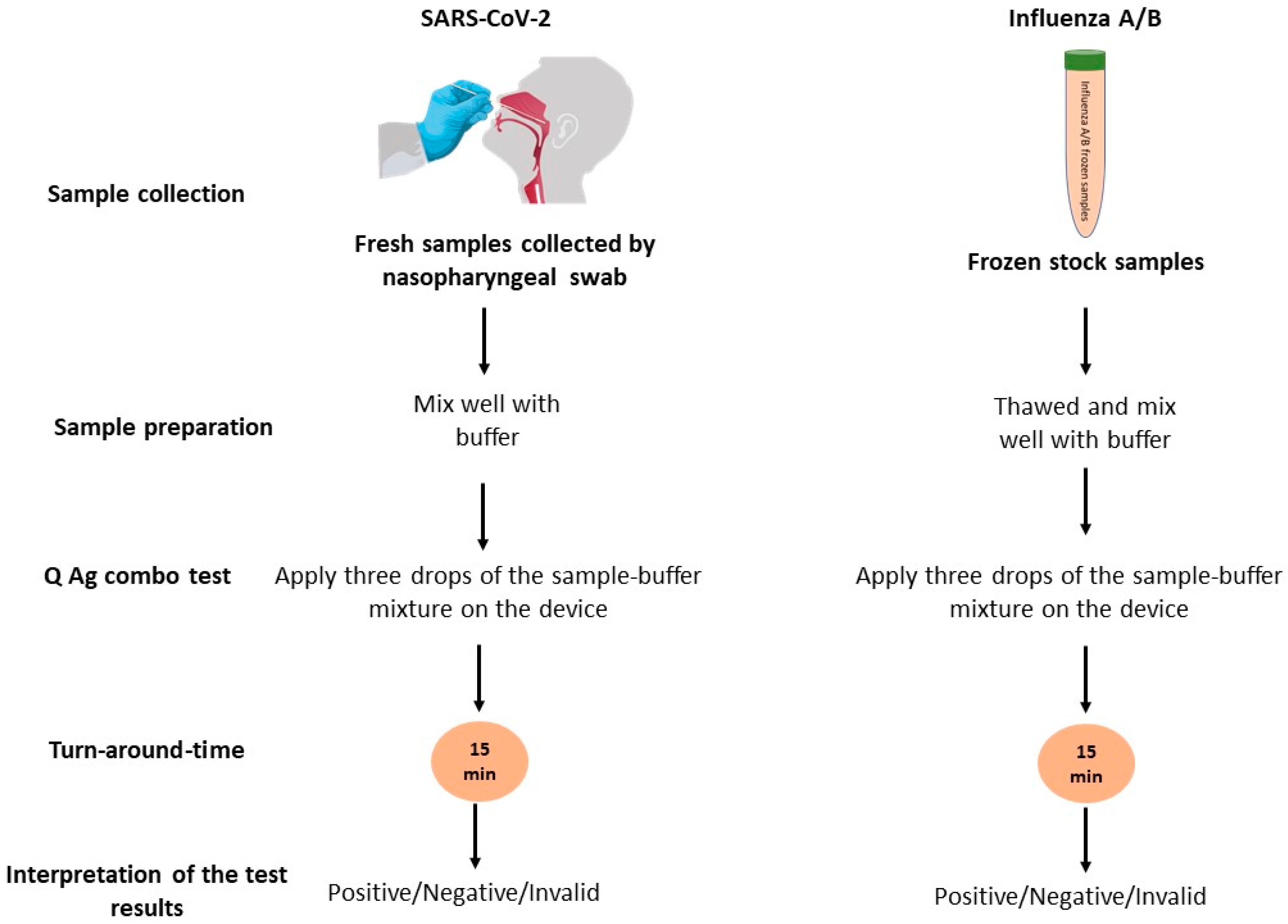
2.2. Sample Collection
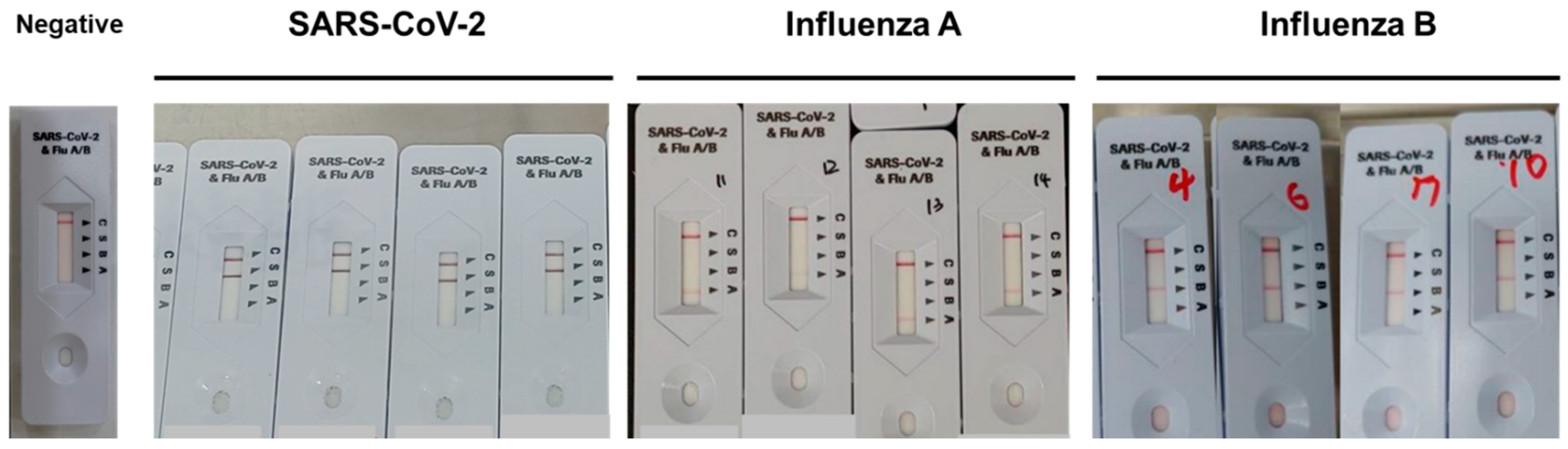
2.3. The Rapid Combo Antigen Test (RCAT)
2.4. The Rapid Antigen Test (RAT)
2.5. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
2.6. Statistical Analysis
3. Results
3.1. Agreement and Diagnostic Performance of STANDARD Q COVID/FLU Ag Combo Compared with RT-PCR for the Detection of SARS-CoV-2
3.2. Agreement and Diagnostic Performance of the STANDARD Q COVID/FLU Ag Combo Compared to the RT-PCR for the Detection of Influenza A
3.3. Agreement and Diagnostic Performance of the STANDARD Q COVID/FLU Ag Combo Compared to RT-PCR for the Detection of Influenza B
3.4. Diagnostic Performance of the STANDARD Q COVID/FLU Ag Combo for the Detection of SARS-CoV-2 by Restricting Positive Cases According to the DSO and Ct Values
3.5. Agreement of the STANDARD Q COVID/FLU Ag Combo with the Pre-Existing Rapid Antigen Tests
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neopane, P.; Nypaver, J.; Shrestha, R.; Beqaj, S. Performance Evaluation of TaqMan SARS-CoV-2, Flu A/B, RSV RT-PCR Multiplex Assay for the Detection of Respiratory Viruses. Infect. Drug Resist. 2022, 15, 5411–5423. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.D. Respiratory Tract Infections: A Clinical Approach. In Molecular Medical Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; pp. 1499–1506. [Google Scholar]
- Tregoning, J.S.; Schwarze, J.r. Respiratory viral infections in infants: Causes, clinical symptoms, virology, and immunology. Clin. Microbiol. Rev. 2010, 23, 74–98. [Google Scholar] [CrossRef] [PubMed]
- Pinky, L.; Dobrovolny, H.M. Coinfections of the respiratory tract: Viral competition for resources. PLoS ONE 2016, 11, e0155589. [Google Scholar] [CrossRef] [PubMed]
- WHO. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/europe/emergencies/situations/covid-19 (accessed on 3 November 2022).
- Jae-hee, L. Cumulative COVID-19 Cases in S. Korea Surpass 20M. Available online: https://english.hani.co.kr/arti/english_edition/e_national/1053399.html (accessed on 3 November 2022).
- Hutchinson, E.C. Influenza virus. Trends Microbiol. 2018, 26, 809–810. [Google Scholar] [CrossRef] [PubMed]
- Center for Disease Control and Prevention. Key Facts about Influenza (Flu). Available online: https://www.cdc.gov/flu/about/keyfacts.htm (accessed on 4 November 2022).
- International SOS. Seasonal Flu Overview. Available online: https://pandemic.internationalsos.com/overview/seasonal-flu (accessed on 4 November 2022).
- Johnson, T.J.; Nishida, R.T.; Sonpar, A.P.; Lin, Y.-C.J.; Watson, K.A.; Smith, S.W.; Conly, J.M.; Evans, D.H.; Olfert, J.S. Viral load of SARS-CoV-2 in droplets and bioaerosols directly captured during breathing, speaking and coughing. Sci. Rep. 2022, 12, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tellier, R. Aerosol transmission of influenza A virus: A review of new studies. J. R. Soc. Interface 2009, 6, S783–S790. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19): Similarities and Differences between COVID-19 and Influenza. Available online: https://www.who.int/news-room/questions-and-answers/item/coronavirus-disease-covid-19-similarities-and-differences-with-influenza (accessed on 5 November 2022).
- Lee, S.; Widyasari, K.; Yang, H.-R.; Jang, J.; Kang, T.; Kim, S. Evaluation of the Diagnostic Accuracy of Nasal Cavity and Nasopharyngeal Swab Specimens for SARS-CoV-2 Detection via Rapid Antigen Test According to Specimen Collection Timing and Viral Load. Diagnostics 2022, 12, 710. [Google Scholar] [CrossRef]
- Shin, H.; Lee, S.; Widyasari, K.; Yi, J.; Bae, E.; Kim, S. Performance evaluation of STANDARD Q COVID-19 Ag home test for the diagnosis of COVID-19 during early symptom onset. J. Clin. Lab. Anal. 2022, 36, e24410. [Google Scholar] [CrossRef]
- Trabattoni, E.; Le, V.; Pilmis, B.; de Ponfilly, G.P.; Caisso, C.; Couzigou, C.; Vidal, B.; Mizrahi, A.; Ganansia, O.; Le Monnier, A. Implementation of Alere i Influenza A & B point of care test for the diagnosis of influenza in an ED. Am. J. Emerg. Med. 2018, 36, 916–921. [Google Scholar]
- SD Biosensor. STANDARD Q COVID/Flu Ag Combo. Available online: https://www.sdbiosensor.com/product/product_view?product_no=235 (accessed on 3 November 2022).
- McHugh, M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Akashi, Y.; Kiyasu, Y.; Terada, N.; Kurihara, Y.; Kato, D.; Miyazawa, T.; Muramatsu, S.; Shinohara, Y.; Ueda, A. A prospective evaluation of the diagnostic performance of a combo rapid antigen test QuickNavi-Flu+ COVID19 Ag. J. Infect. Chemother. 2022, 28, 840–843. [Google Scholar] [CrossRef] [PubMed]
- Nodell, B. NEJM Paper is Compendium on Rapid Tests for COVID-19. Available online: https://newsroom.uw.edu/news/nejm-paper-compendium-rapid-tests-covid-19 (accessed on 4 November 2022).
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Iglὁi, Z.; Velzing, J.; Van Beek, J.; Van de Vijver, D.; Aron, G.; Ensing, R.; Benschop, K.; Han, W.; Boelsums, T.; Koopmans, M. Clinical evaluation of Roche SD Biosensor rapid antigen test for SARS-CoV-2 in municipal health service testing site, the Netherlands. Emerg. Infect. Dis. 2021, 27, 1323. [Google Scholar] [CrossRef]
- Bustin, S.A.; Mueller, R. Real-time reverse transcription PCR (qRT-PCR) and its potential use in clinical diagnosis. Clin. Sci. 2005, 109, 365–379. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, L.-M.; Wan, L.; Xiang, T.-X.; Le, A.; Liu, J.-M.; Peiris, M.; Poon, L.L.; Zhang, W. Viral dynamics in mild and severe cases of COVID-19. Lancet Infect. Dis. 2020, 20, 656–657. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.N.; Manissero, D.; Steele, V.R.; Pareja, J. A systematic review of the clinical utility of cycle threshold values in the context of COVID-19. Infect. Dis. Ther. 2020, 9, 573–586. [Google Scholar] [CrossRef]
- Aykac, K.; Cura Yayla, B.C.; Ozsurekci, Y.; Evren, K.; Oygar, P.D.; Gurlevik, S.L.; Coskun, T.; Tasci, O.; Demirel Kaya, F.; Fidanci, I. The association of viral load and disease severity in children with COVID-19. J. Med. Virol. 2021, 93, 3077–3083. [Google Scholar] [CrossRef]
- Lanser, L.; Bellmann-Weiler, R.; Öttl, K.-W.; Huber, L.; Griesmacher, A.; Theurl, I.; Weiss, G. Evaluating the clinical utility and sensitivity of SARS-CoV-2 antigen testing in relation to RT-PCR Ct values. Infection 2021, 49, 555–557. [Google Scholar] [CrossRef]
- Laferl, H.; Kelani, H.; Seitz, T.; Holzer, B.; Zimpernik, I.; Steinrigl, A.; Schmoll, F.; Wenisch, C.; Allerberger, F. An approach to lifting self-isolation for health care workers with prolonged shedding of SARS-CoV-2 RNA. Infection 2021, 49, 95–101. [Google Scholar] [CrossRef]
- Abbot. Panbio COVID-19 Ag Rapid Test Device (NASAL) [Package Insert]; Abbot Rapid Diagnostics Jena GmbH: Jena, Germany, 2021. [Google Scholar]
- Abbot. Bioline Product Catalog. Available online: https://content.veeabb.com/1d09429b-8373-419f-8f1a-d28f9586863a/87c102d8-0a11-45f2-925f-c3ffae2d2345/87c102d8-0a11-45f2-925f-c3ffae2d2345_source__v.pdf (accessed on 19 December 2022).
- Cubas-Atienzar, A.I.; Kontogianni, K.; Edwards, T.; Wooding, D.; Buist, K.; Thompson, C.R.; Williams, C.T.; Patterson, E.I.; Hughes, G.L.; Baldwin, L. Limit of detection in different matrices of 19 commercially available rapid antigen tests for the detection of SARS-CoV-2. Sci. Rep. 2021, 11, 1–8. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, H.Y.; Lee, M.; Ahn, J.G.; Baek, J.Y.; Kim, M.Y.; Huh, K.; Jung, J.; Kang, J.-M. Respiratory syncytial virus outbreak without influenza in the second year of the coronavirus disease 2019 pandemic: A national sentinel surveillance in Korea, 2021–2022 season. J. Korean Med. Sci. 2022, 37, e258. [Google Scholar] [CrossRef] [PubMed]
| SARS-CoV-2 | PCR Results | Total | Cohen’s Kappa | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| STANDARD Q COVID/Flu Ag Combo | Positive | 51 | 1 | 52 | 0.940 |
| Negative | 4 | 194 | 198 | ||
| Total | 55 | 195 | 250 | ||
| Influenza A | PCR Results | Total | Cohen’s Kappa | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| STANDARD Q COVID/Flu Ag Combo | Positive | 83 | 0 | 83 | 0.941 |
| Negative | 7 | 195 | 202 | ||
| Total | 90 | 195 | 285 | ||
| Influenza B | PCR Results | Total | Cohen’s Kappa | ||
|---|---|---|---|---|---|
| Positive | Negative | ||||
| STANDARD Q COVID/Flu Ag Combo | Positive | 62 | 1 | 63 | 0.928 |
| Negative | 6 | 194 | 200 | ||
| Total | 68 | 195 | 263 | ||
| Positive | Negative | Sensitivity, % | |
|---|---|---|---|
| DSO | |||
| 0–3 days | 22 | 0 | 100% (95% CI, 84.56–100%) |
| 4–6 days | 22 | 0 | 100% (95% CI, 84.56–100%) |
| >7 days | 7 | 4 | 63.64% (95% CI, 30.79–89.07%) |
| Ct Value of RdRp | |||
| ≤20 | 27 | 2 | 93.10% (95% CI, 77.23–99.15%) |
| 20 < Ct ≤ 30 | 16 | 2 | 88.89% (95% CI, 65.29–98.62%) |
| 30 < Ct < 40 | 8 | 0 | 100% (95% CI, 63.06–100%) |
| SARS-CoV-2 | Influenza A | Influenza B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PANBIO COVID-19 | Total | Cohen’s Kappa Value | SD Bioline Influenza | Total | Cohen’s Kappa Value | SD Bioline Influenza | Total | Cohen’s Kappa Value | |||||
| Positive | Negative | Positive | Negative | Positive | Negative | ||||||||
| STANDARD Q COVID/FLU Combo Test | Positive | 46 | 6 | 52 | 0.924 | 83 | 0 | 83 | 0.983 | 14 | 48 | 62 | 0.299 |
| Negative | 0 | 198 | 198 | 2 | 200 | 202 | 1 | 200 | 201 | ||||
| Total | 46 | 204 | 250 | 85 | 200 | 285 | 15 | 248 | 263 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Widyasari, K.; Kim, S.; Kim, S.; Lim, C.S. Performance Evaluation of STANDARD Q COVID/FLU Ag Combo for Detection of SARS-CoV-2 and Influenza A/B. Diagnostics 2023, 13, 32. https://doi.org/10.3390/diagnostics13010032
Widyasari K, Kim S, Kim S, Lim CS. Performance Evaluation of STANDARD Q COVID/FLU Ag Combo for Detection of SARS-CoV-2 and Influenza A/B. Diagnostics. 2023; 13(1):32. https://doi.org/10.3390/diagnostics13010032
Chicago/Turabian StyleWidyasari, Kristin, Soomin Kim, Sunjoo Kim, and Chae Seung Lim. 2023. "Performance Evaluation of STANDARD Q COVID/FLU Ag Combo for Detection of SARS-CoV-2 and Influenza A/B" Diagnostics 13, no. 1: 32. https://doi.org/10.3390/diagnostics13010032
APA StyleWidyasari, K., Kim, S., Kim, S., & Lim, C. S. (2023). Performance Evaluation of STANDARD Q COVID/FLU Ag Combo for Detection of SARS-CoV-2 and Influenza A/B. Diagnostics, 13(1), 32. https://doi.org/10.3390/diagnostics13010032







