Abstract
Although known since the first half of the twentieth century, the evolution of spectroscopic techniques has undergone a strong acceleration after the 2000s, driven by the successful development of new computer technologies suitable for analyzing the large amount of data obtained. Today’s applications are no longer limited to analytical chemistry, but are becoming useful instruments in the medical field. Their versatility, rapidity, the volume of information obtained, especially when applied to biological fluids that are easy to collect, such as urine, could provide a novel diagnostic tool with great potential in the early detection of different diseases. This review aims to summarize the existing literature regarding spectroscopy analyses of urine samples, providing insight into potential future applications.
1. Introduction
The achievement of an early diagnosis is essential for success in the treatment of any disease, including in the oncological field. As a matter of fact, in the absence of clinically detectable signs and symptoms or efficient screening programs for several diseases, new parameters need to be evaluated. New ideas and techniques are evolving to fill this gap, which would otherwise apparently be impassable with traditional methods. Liquid biopsy is one of the latest and most rapidly emerging diagnostic platforms in medicine. The term was introduced by Pantel and Alix-Panabières to identify circulating tumor cells (CTCs) in patients’ blood. This approach was firstly pursued as an early detection method, and its use was extended to the assessment of benign or malignant disease in blood, as well as in other body fluids such as urine, saliva, cerebrospinal fluid (CSF), or pleural effusion [1,2]. Among these, urine analysis has gained interest in recent years as it is easy to collect, non-invasive, and familiar to the patient. Since metabolites can be found in urine, there is the possibility of gaining indirect information about the metabolism of several organs as well as any inflammatory or neoplastic processes [3,4].
In the last two decades, there has been scientific interest in vibrational spectroscopy (VS), an analytical method potentially applicable to urine. While spectroscopy investigates the interaction between electromagnetic radiation (light) and matter, VS identifies the molecular structure of the analyzed sample through its vibrational characteristics interacting with a beam source. The revealed spectrum represents a faithful representation of the unique molecular characteristics investigated [5]. This rapid, non-destructive, minimally invasive, and relatively inexpensive technique has shown to be promising in detecting biomarkers in different biofluids such as urine [6]. Hence, with the emerging progress in technology and machine learning, the analysis and classification of multiple parameters derived from spectroscopic analysis of urine have been facilitated, and its potential exponentially elevated. This paper aims to present the state of the art regarding the spectroscopic analysis of urine samples and to provide insight into its potential medical applications, summarizing what has been achieved so far and looking to the future.
Basics of Vibrational Spectroscopy
Vibrational spectroscopy (VS) identifies the molecular structure and composition of a sample by studying its vibrational characteristics. When a sample is exposed to a beam source, its molecular bindings’ vibrational status can give absorption (studied by infrared spectroscopy, IR) or scattering (studied by Raman spectroscopy, RS) processes.
If a molecule is exposed to an infrared beam that matches in frequency with the natural vibration of the molecule, absorption occurs. In IR spectrometry, to describe it in an extremely simplified way, infrared light passes through a sample and the intensity of the transmitted electromagnetic radiation is measured at each frequency by a detector, resulting in a characteristic spectrum.
RS, on the other hand, is based on the fact that when monochromatic light (light having same frequency, i.e., laser) hits a molecule, most of it is transmitted without change (elastic, Rayleigh scattering), so reaches the detector unaltered. In a small percentage, it is scattered with a different frequency, higher or lower (inelastic, strokes and anti-strokes), and so with different wavelengths. The difference between the energy of the incident photon, from the source, and the energy of the stroke or anti-stroke photon will correspond to the vibratory motion of the molecular bond, and so to its structure.
Due to specific physical principles, transitions which have large Raman intensities often have weak IR intensities and vice versa. This contrasting feature allows vibrational motions that might not be active in IR to be analysed using RS.
The development of these techniques has followed the development of laser systems and methods that allow amplification and better spectra capture (surface-enhanced Raman, resonance Raman, tip-enhanced Raman, polarized Raman, ATR-FTIR, Near IR, etc.), as well as the use of computers and statistical computational systems (Fourier transformation), which help obtain spectra quickly and make them easily interpretable [5,7,8].
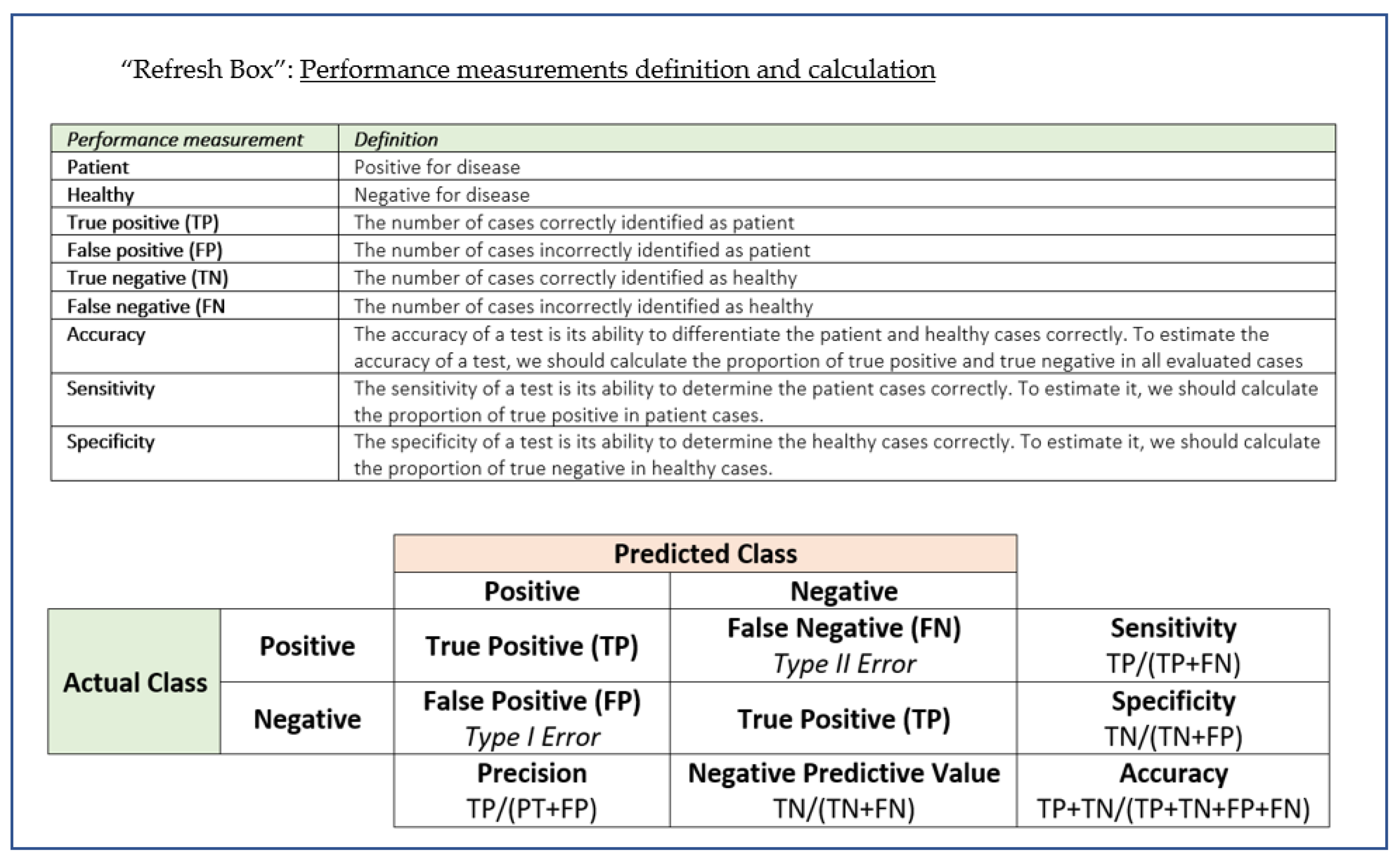
To make it easier to understand the terminology used in evaluating the performance of an instrument/diagnostic technique, we have briefly summarised some key concepts and definitions in a handy “Refresh box”.
2. Search Strategy
We performed literature research in Google Scholar, ResearchGate, and PubMed papers from January 1947 to December 2020 using keyword algorithms to identify manuscripts related to spectroscopy in urine samples; we also reviewed literature citations, following PRISMA guidelines. The search string in PubMed was (title/abstract): Spectroscopy AND/OR Vibrational Spectroscopy AND/OR Infrared Spectroscopy AND/OR Raman Spectroscopy AND/OR Fourier Transform Infrared Spectroscopy AND/OR FTIR AND/OR ATR-FTIR AND/OR SERS AND Urine AND/OR Biofluids.
The inclusion criteria covered any article that explicitly described the spectroscopic methods applied to urine, whether human, animal, or artificial. Exclusion criteria included case reports, letters to editors, publications in non-peer-reviewed journals such as congress papers, and studies with abstract only, or those where the language used was not English. We manually screened each record, removing those ineligible by the previously defined criteria. As reported in Tables S1 and S2 (Supplementary Materials), 169 original articles have been identified, 17 of which were reviews, 61 reporting the use of spectroscopy and urine analysis to identify diseases or clinical conditions in vivo, and 13 applied in the human oncological field. We could also divide them into those using artificial urine (22), and of human (116) or animal origin (17). In total, 91 papers described the use of a spectroscopic method related to its sensitivity and specificity in correctly identifying the most varied substances in urine previously prepared, not exclusively in vivo.
3. Analysis of the Literature
3.1. From the First Attempts to Current Performance
The first described attempt, in 1947, shows how it was already possible to identify, in the considerable time of ca. 10 min, quantities of androsterone, a product of hormonal metabolism, in concentrations of 0.5%, using a primordial IR spectrograph [9]. With the evolution of the technique and available tools, timing has been reduced and detection limits increased; in 2013, using FTIR (Fourier-transform infrared spectroscopy) it was possible to identify concentrations of Ibuprofen up to 0.77 μgml−1 in a few seconds [10] and in 2018, Turzhitsky et al. used SERS (surface-enhanced Raman spectroscopy) to reach a limit of detection for Morphin in water of 5 pg mL−1 (100 ng mL−1 in urine) in less than 2 min, extraction procedure included [11].
3.2. Forensic Medicine Applications
As urine is one of the ways to eliminate exogenous substances, both legal and not, associated with the increasingly limited detection equipment, sports medicine initially took advantage of spectroscopy more than other areas of medicine. This is how the use, mainly with the SERS technique, has been applied for the identification of drugs such as opioids (e.g., morphine, tramadol) [12,13,14], amphetamine [15], methamphetamine, methylenedioxy-methamphetamine (MDMA) [16,17,18,19,20,21,22], cocaine [23], cannabinoids [24,25,26], ketamine [27], antipsychotics such as clozapine [28], benzodiazepines [29,30], and legal but potential doping substances such as erythropoietin [31] phenylethanolamine A [32], or ephedrine [33].
These studies showed that VS had similar, if not superior, capabilities to standard methods in identifying toxic and/or prohibited substances in urine. As a result, the medico-legal field turned its attention to it.
A good example of this interest and of the transition from the laboratory to clinical practice in forensic medicine is the use of the ATR–FTIR (attenuated total reflectance—Fourier-transform infrared) spectrograph by Takamura et al. in 2019, to identify the sex of donors from small portions of urine. Dried urine traces of 101 donors, 61 males and 40 females, were analyzed. The spectrum indicated slight differences between the two sexes in the spectroscopical regions of 1710−1690 cm−1, 1395−1380 cm−1, and 1070−1050 cm−1. A brief clarification is, at this point, appropriate: red radiation is that part of the magnetic spectrum that has wavelengths between those of visible radiation, 13,000 cm−1, and those of microwaves, >1 cm−1. The spectrum obtained from VS is represented as a diagram where in abscissa are the wavelengths, and in ordinate is the intensity of absorption reported in percentage. By visual comparison of the given spectra in this study, these differences were not higher than the standard deviations, so it was infeasible to detect the donors’ sex. Subsequently, a multivariate statistical model was developed using mathematical methods as partial least-squares discriminant analysis (PSD) associated with a genetic algorithm. As a result, the improved discrimination performance enabled the finding of previously undetectable differences, so it could be possible to reach a specificity, sensitivity, and total discrimination accuracy for donor-wise discrimination of 0.99 ± 0.01, 0.92 ± 0.02, and 0.97 ± 0.01, respectively [34].
Recognizing and differentiating biofluids in forensics is increasingly crucial and decisive in the development of an investigation. As reported by W. R. Premasir, SERS spectra of 24 h dried samples of human blood, vaginal fluid, semen, saliva, and urine could provide good quality and reproducible characteristic SERS signatures leading to rapid identification of body fluids (Figure 1) [35].
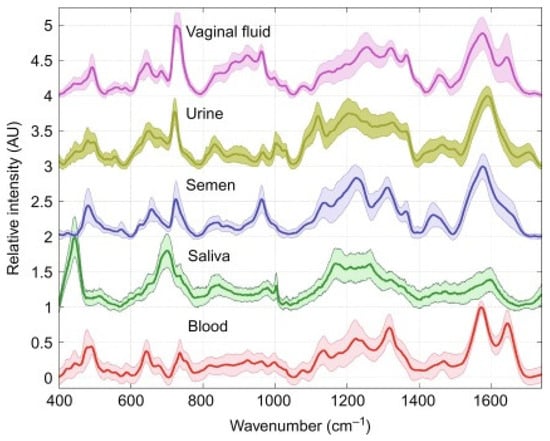
Figure 1.
SERS spectra of dried human body fluids. Shaded regions correspond to standard deviation of 60 spectra (two donors of each body fluid type). Picture reproduced with permission from “Frontiers and Advances in Molecular Spectroscopy”, Jaan Laane, Elsevier, 2018 [36].
Identifying urine from other biofluids as vaginal fluid or sperm was also the question in the research of Gregório et al. featuring FTIR (Fourier-transform infrared spectroscopy) [36]. The authors showed how the IR region from 1480 to 1800 cm−1 was particularly suitable for detecting remains of bodily fluids on the layers of the three different pads tested. Although urine was easily identified because of urea’s molecular vibrations, discrimination between semen and vaginal fluid was limited because of having both similar bands due to proteins within the studied range.
3.3. Non-Oncological Applications
The ability to obtain valid results from both a qualitative and quantitative point of view has also pushed the potential of these techniques in the identification of substances that are omnipresent in urine and play a role in the evaluation of the physiological functioning of the renal system, sometimes demonstrating greater accuracy and precision over standard methods. This is the case of urea [37], creatinine [38,39,40], glucose [41,42], ketone [43], and salts such as sulphate, phosphate [44], and oxalate [45,46]. Also present in urine are amino acids such as tyrosine [47], detected by Yedongo Yo et al. in 2018, or others such as phenylalanine, which, in the case of diseases, can occur in altered concentrations. Phenylketonuria is an illness that, if not detected early, can lead to severe consequences for newborns. An alternative, quick, and effective method to measure it has been proposed by Murugesan et al.: mixing urine samples with a polyvinylpyrrolidone (PVP)-stabilized silver colloidal (AgC) solution. Once evaporated on a solid substrate with a rough surface, this technique could improve SERS sensitivity, yielding an estimated recovery higher than 97%, especially if ZnO powder, able to remove uric acid, the main interfering factor in the phenylalanine spectroscopic band, was added to the mixture [48].
Because of the rapidity in precisely identifying almost all dissolved compounds, VS may also play a role in toxicological investigation, for example, in the detection of acephate [49], a strong pesticide, or for the monitoring of therapy with antibiotics such as sulfamethoxazole [50], cefalosporine (cefazolin, cefoperazone, cefotaxime, ceftriaxone and cefuroxime) [51,52], moxifloxacin [53], painkillers such as paracetamol [54], ibuprofen [10], and antihypertensive drugs such as propranolol [55]. Identifying exogenous substances connected to particular lifestyles can help monitor patients’ compliance. For example, spectrometric identification of biomarkers, such as thiocyanate [56], or nicotine metabolites, such as cotinine and trans-3′hydroxycotinine (3HC) [57], showed the ability to identify smoking patients from non-smokers. Additionally, in these cases, the procedures (SERS) involved the addition of media enhancing the spectroscopic signal: in the first case, a recyclable substrate based on gold–silver nanoparticles and silica; in the second case, a gold colloidal solution.
In parallel to the potential spectral identification of individual new substances in the urine, spectra analysis has developed, mainly thanks to the evolution of mathematical and computational methods applied to capture differences, such as to allow the division into categories and then obtain diagnoses. In case of infectious diseases, the rapidity in the correct identification of the pathogen is directly linked to the correct therapeutic approach and, therefore, to clinical improvement [58]. Although great strides have been made in microbiology and virology, especially concerning the recognition of bacteria and fungi [59,60], the gold standard remains the culture, with consequent longer time for diagnosis and delayed therapy start [61]. Four publications, two of which from in 2013, have shown how, thanks to the use of support vector machines (SVM) and multivariate statistical analysis, pathogens such as Escherichia coli and Enterococcus faecalis can be identified without the use of cultures, with a considerable time advantage, reaching an accuracy of 92% [62,63]. Similar results were reported in a study featuring partial least square-discriminant analysis in the identification of not only E. coli and E. faecalis but also of K. pneumoniaea and S. saprophyticus, with a >95% accuracy and >99% specificity, in less than 1 h, including the filtration and centrifugation procedure [64].
Using FTIR, Steenbeke et al. confirmed the power of spectroscopy in the detection of proteins, lipids, white blood cells (typical of pyuria), and red blood cells (typical of hematuria), as well as in the identification of crystals in urine (Figure 2). Using principal component analysis (PCA) to analyze the obtained spectra, they differentiated between Gram-negative and Gram-positive species, while soft independent modelling of class analogy (SIMCA) revealed promising classification ratios between the different pathogens (Figure 3) [65]. The power of classification of these techniques is of particular interest in cases where distinguishing different patient groups without obvious biomarkers is needed or when invasive methods should be avoided. This is the case of kidney donors, who, in Jingmao’s study in 2017, were correctly classified (accuracy 96.50%) according to the probability of rejection (known by previous biopsy) using only PCA and linear discriminant analysis (LDA) of the spectra obtained from 30 patients’ urine samples [66]. A previous pilot study developed by Somorjaia in 2000 also involved kidney illness and proved, despite a small sample of patients (N = 67), the ability to distinguish between normal renal transplants and rejected allografts, using an optimal region selector method based on the IR spectra of urine [67]. In 2015 the power to find abnormal kidney function related to acute kidney transplant rejection was confirmed by using SERS in 58 patients, suggesting this method could have an earlier and more specific value than the clinically used biomarker, serum creatinine [68]. In 2017, a research group resulting from a collaboration between researchers from Taiwan and the UK identified that spectral features such as 1668 cm−1, 1033 cm−1, and 1545 cm−1 might be useful urinary biomarkers of acute inflammatory renal injury, renal fibrosis, and Glomerulonephritis (GN). The study started with an animal model and then proved its quality in human urine. The intensity of the urinary 1545 cm−1 spectral marker, attributable to the amide II band of the peptide bonds of urinary protein, was consistently elevated in patients with crescentic GN-ANCA-associated vasculitis (N 24) compared to the healthy volunteers (N 11). It was also evident that moderate-to-severe GN patients with a GFR (glomerular filtration rate) of <60 mL/min/1.73 m2, both in active disease or in remission, had a higher 1545 cm−1 band intensity than GN patients with GFR ≥ 60 mL/min/1.73 m2, showing that this spectrometric biomarker may be a good indicator of the severity of renal dysfunction [69].
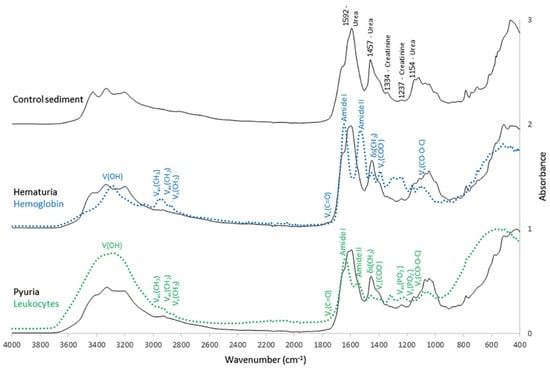
Figure 2.
Mean normalized infrared (IR) spectra of urinary sediment of control subjects, patients with hematuria, and patients with pyuria. Distinctive peaks were present at 1592 cm−1 (urea), 1457 cm−1, (urea), 1334 cm−1 (creatinine), 1237 cm−1 (creatinine), and 1154 cm−1 (urea). The amide I (1655 cm−1 and amide II (1525 cm−1) bands were overlapping in the region from 1510 to 1750 cm−1. A dried suspension of hemoglobin (blue line) and isolated leukocytes (green line) was added to the figure. Typical molecular assignments of a biological IR spectrum were indicated on the IR spectra of hemoglobin and the leukocytes: V = stretching vibrations; δ = bending vibrations; s = symmetrical vibrations and as = asymmetrical vibrations. Picture reproduced with permission from “Exploring the possibilities of infrared spectroscopy for urine sediment examination and detection of pathogenic bacteria in urinary tract infections.” Steenbeke, M. et al., Clinical Chemistry and Laboratory Medicine [65].
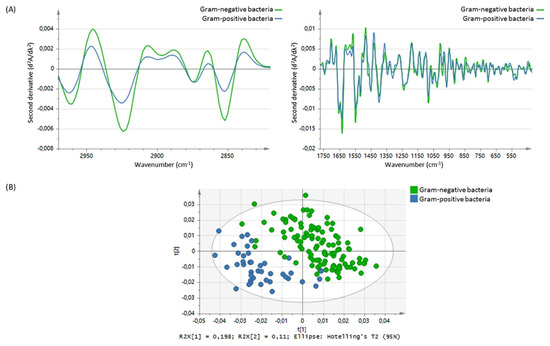
Figure 3.
(A) Mean spectral differences in the second derivative from 2970 to 2820 cm−1 and from 1772 to 420 cm−1 between Gram-negative bacteria (green line) and Gram-positive bacteria (n = 41, blue line). (B) Score plot showing clustering of the Gram-negative bacteria (green dots) and Gram-positive bacteria (blue dots), based on principal component analysis in the 2970–2820 cm−1 and 1772–420 cm−1 spectral range. Picture reproduced with permission from “Exploring the possibilities of infrared spectroscopy for urine sediment examination and detection of pathogenic bacteria in urinary tract infections. Steenbeke, M. et al., Clinical Chemistry and Laboratory Medicine [65].
An ever-present topic around spectroscopy in urine is recognizing and measuring specific physiological components in urine, such as urea, creatinine, and glucose, because variation in their concentrations has significant clinical consequences. Recently, Bispo et al. used a dispersive Raman spectrometer to recognize and measure these substances to correlate them with the risk of kidney failure in diabetic and hypertensive patients. Comparing the differences in the intensities of several peaks, they properly calculated the difference in the concentration of the urine biochemical components in each group. They reported a progressive decrease in creatinine and urea (p < 0.05) and an increase in glucose (p < 0.05) as a biomarker of disease progression. The principal component analysis and the discriminating model build-up showed an overall classification rate of 70% [70].
A good example of the use of spectrometric biomarkers applied to the evaluation of a disease and its evolution is the association made by Yang et al. in 2018 between the wavelength of 1509 cm−1 and the presence of coronary occlusion greater than 70%. Trying to correctly classify cardiovascular patients operated on with percutaneous coronary intervention, unoperated, and healthy through the SERS analysis of urine, this wavelength allowed a sensitivity and specificity of 90% and 78.9%, respectively. A possible explanation, whose reasoning links from the spectrometric results back to the biochemical counterpart, was identified in the platelet-derived growth factor-BB (PDGF-BB), a molecule known to be associated with ischemic heart disease. The subsequent spiked urine experiment of this PDGF-BB showed that this substance was precisely associated with this spectrum variation. Moreover, the measured SERS spectra of all urine samples from 87 patients with coronary heart disease were compared with the clinical data provided by the hospital, and they revealed that the appearance of 1509 cm−1 in SERS spectra was in good agreement with the results of coronary angiography technology where cardiovascular congestion was above 70% (Figure 4). In this subgroup classification, sensitivity and specificity were 87.9% and 87.0%, respectively [71].
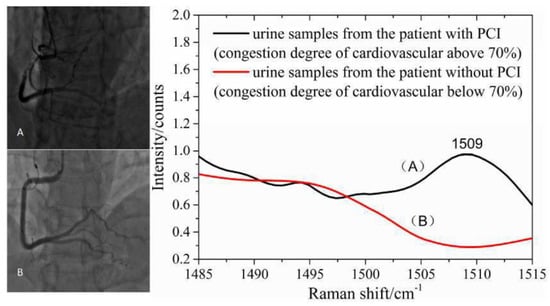
Figure 4.
The SERS spectra of human urine could provide valuable information for noninvasive and prospective diagnosis of suspected cases of coronary heart disease. (A) urine samples from the patient with PCI (congestion degree of cardiovascular above 70%; (B) urine samples from the patient without PCI (congestion degree of cardiovascular below 70%). Picture reproduced with permission from “Noninvasive and prospective diagnosis of coronary heart disease with urine using surface-enhanced Raman spectroscopy”, Yang, H. et al., Analyst, 2018 [71].
3.4. Oncological Applications
Published data on spectral analysis of the urine of cancer patients are sparse. In our research, we found 13 publications wherein spectroscopy was applied to human cancer pathologies. We report below the seven with the most significant clinical relevance. Paraskevaidi et al. used FTIR spectroscopy to analyze urine samples after 6–8 h of fasting from 10 ovarian cancer and 10 endometrial cancer patients and compared the spectra with similarly collected urine samples from control patients undergoing hysterectomy for benign conditions. Using algorithms such as partial least squares discriminant analysis (PLS-DA), principal component analysis with support vector machines (PCA-SVM) (Figure 5), and genetic algorithm with linear discriminant analysis (GA-LDA), the authors reported high levels of accuracy for both endometrial (95% sensitivity, 100% specificity, 95% accuracy) and ovarian cancer (100% sensitivity, 96.3% specificity, 100% accuracy). The responsible discriminating spectral peaks were in the wavelengths of proteins and nucleic acids [72].
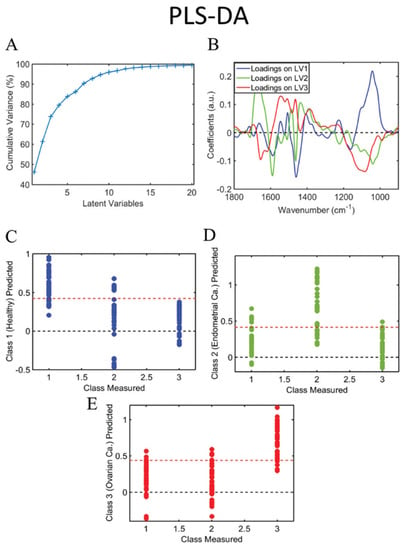
Figure 5.
Cumulative explained variance using PCA (A); PCA loadings on PC1, 2 and 3 (B); predicted probability of healthy class versus endometrial and ovarian cancer (C); predicted probability of endometrial cancer class versus healthy and ovarian cancer (D); predicted probability of ovarian cancer class versus healthy and endometrial cancer (E). Class measured 1 = healthy control; 2 = endometrial cancer; 3 = ovarian cancer. PC: principal component. Picture reproduced with permission from “Potential of mid-infrared spectroscopy as a noninvasive diagnostic test in urine for endometrial or ovarian cancer”, Analyst, Paraskevaidi M. et al. 2018 [72].
Gynecologic cancer was also the target of the analysis of Lin et al. in 2019, who analyzed the spectra of 43 breast cancer patients and compared them with 48 healthy volunteers and 50 gastric cancer patients. Using urine spectra and linear discriminative analysis, they could achieve a diagnostic specificity of 87.5%, 95.8%, and 95.7%, respectively [73]. Del Mistro et al., using Raman spectroscopy, compared urine samples from nine prostate cancer patients with nine healthy controls and also reported differences in the intensity of the spectra. Their algorithms using principal component analysis (PCA) and linear discriminant analysis (LDA) led to a sensitivity of 100%, a specificity of 89 %, and an overall diagnostic accuracy of 95 %. The authors also used urine after fasting, which was frozen within 4–5 h after collection and, in order to remove traces of proteins (e.g., hemoglobin) potentially interfering with SERS analysis, centrifuged at 14,000 g at 4 °C for 15 min before measurement [74]. Two published studies compared oral cancer patients with healthy controls, both using Raman spectroscopy and PCA/LDA algorithms. Elulamai et al. used first-voided morning urine samples, which were stored in a refrigerator at 4 °C for a maximum of 48 h after collection and were thawed to room temperature before measurements, and reported a sensitivity and specificity of 98.6% and 87.1%, respectively, with an overall accuracy of 93.7% in right classification of the presented urine [75]. Jaychandran et al. reported on 50 oral cancer patients, comparing them to 21 healthy controls. They used urine samples collected between 9 and 11 o’clock, which were then stored in an ice box. Measurements were performed using Raman spectroscopy and the PCA/LDA analysis showed an accuracy of 90.5% [6]. In 2016, Feng et al. analyzed Raman-enhanced spectra from 68 nasopharyngeal cancer patients, 55 esophageal cancer patients, and 52 healthy volunteers. They reported a diagnostic sensitivity of 95.5%, 90.9% and 98.1% and specificity 97.2%, 98.2% and 95.7%, respectively. They used a modified method for detecting nucleosides in urine with the help of Raman spectroscopy (RS), while the samples had been treated with affinity chromatography and supplemented with gold as a substrate for the spectroscopy analysis [76]. Similarly, Wang et al. used a modified technique enhancing RNA detection in urine for Raman spectroscopic detection. In a training cohort of 43 prostate cancer patients, they could demonstrate 93% specificity, 95.3% sensitivity, and 94.2% accuracy [77].
4. Discussion
The results of our search revealed a growing interest in the use of the physical properties of urine, as well as the potential substances dissolved in it, to recognize physiological and pathological processes. Compared to other biofluids, urine shows great advantages: its collection is completely non-invasive and low-cost, it does not require special medical or laboratory expertise, it has a quite stable and pure composition since it is derived from the renal filtration process, and it can be used simultaneously for various diagnostic purposes [78]. Conversely, the collection of blood, saliva, tears, and other biofluids, such as amniotic fluid, is more invasive, often requires special transportation and manipulation (e.g., blood clotting), and needs specialized staff and/or expensive instrumentation. This work shows the potential of the application of VS on urine samples for various diagnostic purposes, with the potential of a further improvement in accuracy if associated with the developing computational method. The increasing use of artificial intelligence in daily life (e.g., marketing) is the basis for the subsequent widespread use in clinical routine. Unfortunately, the need to collect millions of data to train algorithms that can provide credible results is currently rarely met due to the scarcity of extensive generalized studies, usually inhomogeneous in methods and techniques. As shown in this paper, published studies present considerable variability regarding every step of the process, from the collection, processing, and storage of the samples (for example, urine after fastening or random samples, freezing, centrifuging, etc.) to the chosen spectroscopy approach (e.g., SERS versus ATR-FTIR), the different machine learning algorithms used, and the statistical processing applied. These factors contribute to a significant inhomogeneity of the existing databases, which limits their interactions. The next step to overcome this limit could be to develop standard data analysis methods at every process step. Only accurate pre-processed data and appropriate use of machine learning and AI methods can lead to accurate analysis and classification of raw data obtained from the spectroscopy and spread the use of this analysis tool. Of note, advances in algorithm and computational power allow for gaining results that were not achievable some years ago. Mostly from 2000 onwards, the use of computationally intensive statistical models and algorithms such as PCA (principal component analysis), PLS (partial least squares), GA (genetic algorithm) or LDA (linear discriminant analysis) led to highly accurate measurements in various clinical studies including oncological diagnoses, treatment, and prognosis. The possibility of using artificial intelligence to classify patients according to their state of health or the state of response to a therapy or a surgical treatment through the analysis of urine characteristics represents an opportunity, although not entirely feasible in the present, too precious to be missed. We are now at the beginning of a new era wherein AI will play a major role in assisting physicians in clinical practice. The first, encouraging results highlighted in this review should suggest redoubling our effort to improve the accuracy of AI applications, especially if coupled with such an inexpensive, non-invasive, easy-to-use analytical technique as vibrational spectroscopy.
5. Conclusions
The repeatability of the results and the ability to obtain them rapidly and with high accuracy with low-cost equipment makes vibrational spectroscopy in urine samples a perfect candidate for promising future research in the medical field. However, the standardization of the method, the initiation of larger translational and clinical studies as well as further exploration of the machine learning methods are needed to achieve the desired high levels of reliability and consistency.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/diagnostics13010027/s1. Tables S1 and S2.
Author Contributions
The authors confirm contribution to the paper as follows: study conception and design: F.V., A.T. and T.K.; literature review and data collection: F.V.; analysis and screening process of the collected papers: F.V., M.D. and A.G. under supervision of T.K.; draft manuscript preparation: F.V. and A.T.; technical: V.K. The authors confirm sole responsibility for the following: study conception and design, data collection, analysis and interpretation of results, and manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partially funded by Krebs Liga Beider Basel (KLbB-5068-02-2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Klaus Pantel, C.A.-P. Circulating tumour cells in cancer patients: Challenges and perspectives. Trends Mol. Med. 2010, 16, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Siravegna, G.; Marsoni, S.; Siena, S.; Bardelli, A. Integrating liquid biopsies into the management of cancer. Nat. Rev. Clin. Oncol. 2017, 14, 531–548. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, C.; Meng, Q.; Hu, Z.; Hu, M.; Zhang, M. MicroRNAs in urine as diagnostic biomarkers for multiple myeloma. Int. J. Lab. Hematol. 2020, 43, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Brouwer-Brolsma, E.M.; Brennan, L.; Drevon, C.A.; van Kranen, H.; Manach, C.; Dragsted, L.O.; Roche, H.M.; Andres-Lacueva, C.; Bakker, S.J.L.; Bouwman, J.; et al. Combining traditional dietary assessment methods with novel metabolomics techniques: Present efforts by the Food Biomarker Alliance. Proc. Nutr. Soc. 2017, 76, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Banwell, C.N. Fundamentals of Molecular Spectroscopy; McGraw-Hill: London, UK, 1983. [Google Scholar]
- Jaychandran, S.M.P.; Bharanidharan, G. Raman Spectroscopic Analysis of Blood, Urine, Saliva and Tissue of Oral Potentially Malignant Disorders and Malignancy—A Diagnostic Study. Int. J. Oral Craniofacial Sci. 2016, 2, 11–14. [Google Scholar]
- Clark, J. Infrared Spectroscopy. R Spectroscopy Background. 2020. LibreTexts Truro School in Cornwall. Available online: https://chem.libretexts.org/@go/page/3732 (accessed on 16 April 2022).
- Yang, L.; Hong, G. Raman Spectroscopy. 2016. LibreTexts. Department of Chemistry, UC Davis. Available online: https://chem.libretexts.org/@go/page/1848 (accessed on 16 April 2022).
- Dobriner, K.; Lieberman, S.; Rhoads, C.P.; Jones, R.N.; Williams, V.Z.; Barnes, R.B. Studies in steroid metabosim III. The Application of infra-red spectrometry to the fractionation of urinary ketosteroidsm. J. Biol. Chem. 1947, 172, 297–311. [Google Scholar] [CrossRef]
- Khaskheli, A.R.; Sirajuddin; Sherazi, S.T.H.; Mahesar, S.A.; Kandhro, A.A.; Kalwar, N.H.; Mallah, M.A. Estimation of ibuprofen in urine and tablet formulations by transmission Fourier Transform Infrared spectroscopy by partial least square. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 102, 403–407. [Google Scholar] [CrossRef]
- Turzhitsky, V.; Zhang, L.; Horowitz, G.L.; Vitkin, E.; Khan, U.; Zakharov, Y.; Qiu, L.; Itzkan, I.; Perelman, L.T. Picoanalysis of Drugs in Biofluids with Quantitative Label-Free Surface-Enhanced Raman Spectroscopy. Small 2018, 14, 1802392. [Google Scholar] [CrossRef]
- Wang, K.; Xu, B.; Wu, J.; Zhu, Y.; Guo, L.; Xie, J. Elucidating fentanyls differentiation from morphines in chemical and biological samples with surface-enhanced Raman spectroscopy. Electrophoresis 2019, 40, 2193–2203. [Google Scholar] [CrossRef]
- Yu, B.; Cao, C.; Li, P.; Mao, M.; Xie, Q.; Yang, L. Sensitive and simple determination of zwitterionic morphine in human urine based on liquid-liquid micro-extraction coupled with surface-enhanced Raman spectroscopy. Talanta 2018, 186, 427–432. [Google Scholar] [CrossRef]
- Alharbi, O.; Xu, Y.; Goodacre, R. Detection and quantification of the opioid tramadol in urine using surface enhanced Raman scattering. Analyst 2015, 140, 5965–5970. [Google Scholar] [CrossRef]
- Kalasinsky, K.S.; Levine, B.; Smith, M.L.; Magluilo, J.; Schaefer, T. Detection of Amphetamine and Methamphetamine in Urine by Gas Chromatography/Fourier Transform Infrared (GC/FTIR)Spectroscopy. J. Anal. Toxicol. 1993, 17, 359–364. [Google Scholar] [CrossRef]
- Hong, Y.; Zhou, X.; Xu, B.; Huang, Y.; He, W.; Wang, S.; Wang, C.; Zhou, G.; Chen, Y.; Gong, T. Optoplasmonic Hybrid Materials for Trace Detection of Methamphetamine in Biological Fluids through SERS. ACS Appl. Mater. Interfaces 2020, 12, 24192–24200. [Google Scholar] [CrossRef]
- Weng, S.; Dong, R.; Zhu, Z.; Zhang, D.; Zhao, J.; Huang, L.; Liang, D. Dynamic surface-enhanced Raman spectroscopy and Chemometric methods for fast detection and intelligent identification of methamphetamine and 3, 4-Methylenedioxy methamphetamine in human urine. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2018, 189, 1–7. [Google Scholar] [CrossRef]
- Mao, K.; Zhou, Z.; Han, S.; Zhou, X.; Hu, J.; Li, X.; Yang, Z. A novel biosensor based on Au@Ag core-shell nanoparticles for sensitive detection of methylamphetamine with surface enhanced Raman scattering. Talanta 2018, 190, 263–268. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, H.; Mao, M.; Meng, J.; Yang, L.; Liu, J. Surface-Enhanced Raman Spectroscopy on Liquid Interfacial Nanoparticle Arrays for Multiplex Detecting Drugs in Urine. Anal. Chem. 2016, 88, 8145–8151. [Google Scholar] [CrossRef]
- Dong, R.; Weng, S.; Yang, L.; Liu, J. Detection and Direct Readout of Drugs in Human Urine Using Dynamic Surface-Enhanced Raman Spectroscopy and Support Vector Machines. Anal. Chem. 2015, 87, 2937–2944. [Google Scholar] [CrossRef]
- Han, Z.; Liu, H.; Meng, J.; Yang, L.; Liu, J.; Liu, J. Portable Kit for Identification and Detection of Drugs in Human Urine Using Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2015, 87, 9500–9506. [Google Scholar] [CrossRef]
- Han, Z.; Liu, H.; Wang, B.; Weng, S.; Yang, L.; Liu, J. Three-Dimensional Surface-Enhanced Raman Scattering Hotspots in Spherical Colloidal Superstructure for Identification and Detection of Drugs in Human Urine. Anal. Chem. 2015, 87, 4821–4828. [Google Scholar] [CrossRef]
- Meng, J.; Tang, X.; Zhou, B.; Xie, Q.; Yang, L. Designing of ordered two-dimensional gold nanoparticles film for cocaine detection in human urine using surface-enhanced Raman spectroscopy. Talanta 2017, 164, 693–699. [Google Scholar] [CrossRef]
- Muhamadali, H.; Watt, A.; Xu, Y.; Chisanga, M.; Subaihi, A.; Jones, C.; Ellis, D.; Sutcliffe, O.B.; Goodacre, R. Rapid Detection and Quantification of Novel Psychoactive Substances (NPS) Using Raman Spectroscopy and Surface-Enhanced Raman Scattering. Front. Chem. 2019, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Sivashanmugan, K.; Zhao, Y.; Wang, A.X. Tetrahydrocannabinol Sensing in Complex Biofluid with Portable Raman Spectrometer Using Diatomaceous SERS Substrates. Biosensors 2019, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Mostowtt, T.; McCord, B. Surface enhanced Raman spectroscopy (SERS) as a method for the toxicological analysis of synthetic cannabinoids. Talanta 2017, 164, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Han, S.-Q.; Chen, X.; Zhang, C.; Zhao, H.; Lin, S.; Zhang, Y.; Hasi, W.-L. Rapid and Sensitive Surface-enhanced Raman Spectroscopy Method for Determination of Ketamine in Urine. Anal. Sci. 2019, 35, 1209–1213. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Yu, X.; Wu, Z.; Lu, F.; Yuan, Y. Antipsychotic drug poisoning monitoring of clozapine in urine by using coffee ring effect based surface-enhanced Raman spectroscopy. Anal. Chim. Acta 2018, 1014, 64–70. [Google Scholar] [CrossRef]
- Doctor, E.L.; McCord, B. The application of supported liquid extraction in the analysis of benzodiazepines using surface enhanced Raman spectroscopy. Talanta 2015, 144, 938–943. [Google Scholar] [CrossRef]
- Doctor, E.L.; McCord, B. Comparison of aggregating agents for the surface-enhanced Raman analysis of benzodiazepines. Anal. 2013, 138, 5926–5932. [Google Scholar] [CrossRef]
- Selbes, Y.S.; Caglayan, M.G.; Eryılmaz, M.; Boyaci, I.H.; Saglam, N.; Basaran, A.A.; Tamer, U. Surface-enhanced Raman probe for rapid nanoextraction and detection of erythropoietin in urine. Anal. Bioanal. Chem. 2016, 408, 8447–8456. [Google Scholar] [CrossRef]
- Li, M.; Yang, H.; Li, S.; Zhao, K.; Li, J.; Jiang, D.; Sun, L.; Deng, A. Ultrasensitive and Quantitative Detection of a New β-Agonist Phenylethanolamine A by a Novel Immunochromatographic Assay Based on Surface-Enhanced Raman Scattering (SERS). J. Agric. Food Chem. 2014, 62, 10896–10902. [Google Scholar] [CrossRef]
- Guimarães, A.E.; Pacheco, M.T.T.; Silveira, L.S., Jr.; Barsottini, D.; Duarte, J.; Villaverde, A.B.; Zângaro, R.A. Near Infrared Raman Spectroscopy (NIRS): A technique for doping control. Spectroscopy 2006, 20, 185–194. [Google Scholar] [CrossRef]
- Takamura, A.; Halamkova, L.; Ozawa, T.; Lednev, I.K. Phenotype Profiling for Forensic Purposes: Determining Donor Sex Based on Fourier Transform Infrared Spectroscopy of Urine Traces. Anal. Chem. 2019, 91, 6288–6295. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Chen, Y.; Fore, J.; Brodeur, A.; Ziegler, L.D. Chapter 10—SERS Biomedical Applications: Diagnostics, Forensics, and Metabolomics; Frontiers and Advances in Molecular Spectroscopy; Laane, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 327–367. ISBN 9780128112205. [Google Scholar]
- Gregório, I.; Zapata, F.; García-Ruiz, C. Analysis of human bodily fluids on superabsorbent pads by ATR-FTIR. Talanta 2017, 162, 634–640. [Google Scholar] [CrossRef]
- Shaw, R.; Kotowich, S.; Mantsch, H.H.; Leroux, M. Quantitation of protein, creatinine, and urea in urine by near-infrared spectroscopy. Clin. Biochem. 1996, 29, 11–19. [Google Scholar] [CrossRef]
- Wang, T.-L.; Chiang, H.K.; Lu, H.-H.; Peng, F.-Y. Semi-quantitative Surface Enhanced Raman Scattering Spectroscopic Creatinine Measurement in Human Urine Samples. Opt. Quantum Electron. 2005, 37, 1415–1422. [Google Scholar] [CrossRef]
- Qi, D.; Berger, B.A. Quantitative concentration measurements of creatininedissolved in water and urine using Ramanspectroscopy and a liquid core optical fiber. J. Biomed. Opt. 2005, 10, 031115. [Google Scholar] [CrossRef]
- McMurdy, J.W.; Berger, A.J. Raman Spectroscopy-Based Creatinine Measurement in Urine Samples from a Multipatient Population. Appl. Spectrosc. 2003, 57, 522–525. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, T.; You, R.; Wu, Y.; Shen, H.; Feng, S.; Su, J. Fabrication and Characterization of a Highly-Sensitive Surface-Enhanced Raman Scattering Nanosensor for Detecting Glucose in Urine. Nanomaterials 2018, 8, 629. [Google Scholar] [CrossRef]
- Chen, Q.; Fu, Y.; Zhang, W.; Ye, S.; Zhang, H.; Xie, F.; Gong, L.; Wei, Z.; Jin, H.; Chen, J. Highly sensitive detection of glucose: A quantitative approach employing nanorods assembled plasmonic substrate. Talanta 2017, 165, 516–521. [Google Scholar] [CrossRef]
- Pezzaniti, J.; Jeng, T.-W.; McDowell, L.; Oosta, G.M. Preliminary investigation of near-infrared spectroscopic measurements of urea, creatinine, glucose, protein, and ketone in urine. Clin. Biochem. 2001, 34, 239–246. [Google Scholar] [CrossRef]
- Heise, H.M.; Voigt, G.; Rudloff, S.; Werner, G. Quantitation of Metabolites and Salts in Urine by Attenuated Total Reflection Infrared Spectroscopy. Fourier Transformed Spectroscopy. In Proceedings of the Twelth International Conference, Tokyo, Japan, 22–27 August 1999. [Google Scholar]
- Kim, Y.E.; Kim, J.W.; Lee, J.Y. Evaluation of Fourier transform near-infrared spectrometer for determination of oxalate in standard urinary solution. Prev. Med. Public Health 2006, 39, 165–170. [Google Scholar]
- Cytron, S.; Kravchick, S.; Sela, B.A.; Shulzinger, E.; Vasserman, I.; Raichlin, Y.; Katzir, A. Fiberoptic infrared spectroscopy: A Novel tool for the analysis of urine and urinary salts in situ in real time. Urology 2003, 61, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Lu, D.; You, R.; Liu, J.; Huang, L.; Su, J.; Feng, S. Diazotization-Coupling Reaction-BasedDetermination of Tyrosine in Urine Using AgNanocubes by Surface-Enhanced Raman Spectroscopy. Nanomaterials 2018, 8, 400. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, B.; Yang, J. Tunable Coffee Ring Formation on Polycarbonate Nanofiber Film for Sensitive SERS Detection of Phenylalanine in Urine. ACS Omega 2019, 4, 14928–14936. [Google Scholar] [CrossRef] [PubMed]
- Clauson, S.L.; Sylvia, J.M.; Arcury, T.A.; Summers, P.; Spencer, K.M. Detection of Pesticides and Metabolites Using Surface-Enhanced Raman Spectroscopy (SERS): Acephate. Appl. Spectrosc. 2015, 69, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Markina, N.E.; Markin, A.V.; Weber, K.; Popp, J.; Cialla-May, D. Liquid-liquid extraction-assisted SERS-based determination of sulfamethoxazole in spiked human urine. Anal. Chim. Acta 2020, 1109, 61–68. [Google Scholar] [CrossRef]
- Markina, N.E.; Markin, A.V. Application of Aluminum Hydroxide for Improvement of Label-Free SERS Detection of Some Cephalosporin Antibiotics in Urine. Biosensors 2019, 9, 91. [Google Scholar] [CrossRef]
- Markina, N.E.; Goryacheva, I.Y.; Markin, A.V. Sample pretreatment and SERS-based detection of ceftriaxone in urine. Anal. Bioanal. Chem. 2018, 410, 2221–2227. [Google Scholar] [CrossRef]
- Mamián-López, M.B.; Poppi, R.J. Quantification of moxifloxacin in urine using surface-enhanced Raman spectroscopy (SERS) and multivariate curve resolution on a nanostructured gold surface. Anal. Bioanal. Chem. 2013, 405, 7671–7677. [Google Scholar] [CrossRef]
- Mukanova, Z.; Gudun, K.; Elemessova, Z.; Khamkhash, L.; Ralchenko, E.; Bukasov, R. Detection of Paracetamol in Water and Urea in Artificial Urine with Gold Nanoparticle@Al Foil Cost-efficient SERS Substrate. Anal. Sci. 2018, 34, 183–187. [Google Scholar] [CrossRef]
- Subaihi, A.; Muhamadali, H.; AlMasoud, N.; Ellis, D.I.; Trivedi, D.K.; Hollywood, K.A.; Xu, Y.; Goodacre, R. Rapid, Accurate, and Quantitative Detection of Propranolol inMultiple Human Biofluids via Surface-Enhanced Raman Scattering. Anal. Chem. 2016, 88, 10884–10892. [Google Scholar] [CrossRef]
- Ankudze, B.; Philip, A.; Pakkanen, T.T. Ultrasensitive and recyclable superstructure of Au SiO2@Ag wire for surfaceenhanced Raman scattering detection of thiocyanate in urine and human serum. Anal. Chim. Acta 2018, 1049, 179–187. [Google Scholar] [CrossRef]
- Huang, R.; Han, S.; Li, X. Detection of tobacco-related biomarkers in urine samples by surface-enhanced Raman spectroscopy coupled with thin-layer chromatography. Anal. Bioanal. Chem. 2013, 405, 6815–6822. [Google Scholar] [CrossRef]
- Committee on Diagnostic Error in Health Care; Board on Health Care Services; Institute of Medicine; The National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care; Balogh, E.P., Miller, B.T., Ball, J.R., Eds.; National Academies Press (US): Washington, DC, USA, 2015. [Google Scholar]
- Jabri, H.; Lakhdar, N.; El Khattabi, W.; Afif, H. Diagnostic means for tuberculosis. Rev. Pneumol. Clin. 2016, 72, 320–325. [Google Scholar] [CrossRef]
- Anjos, L.M.M.; Marcondes, M.B.; Lima, M.F.; Mondelli, A.L.; Okoshi, M.P. Streptococcal acute pharyngitis. Rev. Soc. Bras. Med.Trop. 2014, 47, 409–413. [Google Scholar] [CrossRef]
- Schmiemann, G.; Kniehl, E.; Gebhardt, K.; Matejczyk, M.M.; Hummers-Pradier, E. The Diagnosis of Urinary Tract Infection. Dtsch. Ärzteblatt Int. 2010, 107, 361. [Google Scholar] [CrossRef]
- Kloß, S.; Kampe, B.; Sachse, S.; Rösch, P.; Straube, E.; Pfister, W.; Kiehntopf, M.; Popp, J. Culture Independent Raman Spectroscopic Identification of Urinary Tract Infection Pathogens: A Proof of Principle Study. Anal. Chem. 2013, 85, 9610–9616. [Google Scholar] [CrossRef]
- Schröder, U.-C.; Ramoji, A.; Glaser, U.; Sachse, S.; Leiterer, C.; Csaki, A.; Hübner, U.; Fritzsche, W.; Pfister, W.; Bauer, M.; et al. Combined Dielectrophoresis–Raman Setup for the Classification of Pathogens Recovered from the Urinary Tract. Anal. Chem. 2013, 85, 10717–10724. [Google Scholar] [CrossRef]
- Premasiri, W.R.; Chen, Y.; Williamson, P.M.; Bandarage, D.C.; Pyles, C.; Ziegler, L.D. Rapid urinary tract infection diagnostics by surface-enhanced Raman spectroscopy (SERS): Identification and antibiotic susceptibilities. Anal. Bioanal. Chem. 2017, 409, 3043–3054. [Google Scholar] [CrossRef]
- Steenbeke, M.; De Bruyne, S.; Boelens, J.; Oyaert, M.; Glorieux, G.; Van Biesen, W.; Linjala, J.; Delanghe, J.R.; Speeckaert, M.M. Exploring the possibilities of infrared spectroscopy for urine sediment examination and detection of pathogenic bacteria in urinary tract infections. Clin. Chem. Lab. Med. 2020, 58, 1759–1767. [Google Scholar] [CrossRef]
- Chi, J.; Ma, Y.; Weng, F.L.; Thiessen-Philbrook, H.; Parikh, C.R.; Du, H. Surface-enhanced Raman scattering analysis of urine from deceased donors as a prognostic tool for kidney transplant outcome. J. Biophotonics 2017, 10, 1743–1755. [Google Scholar] [CrossRef]
- Somorjai, R.; Dolenko, B.; Nikulin, A.; Nickerson, P.; Rush, D.; Shaw, A.; Glogowski, M.; Rendell, J.; Deslauriers, R. Distinguishing normal from rejecting renal allografts: Application of a three—Stage classification strategy to MR and IR spectra of urine. Vib. Spectrosc. 2002, 28, 97–102. [Google Scholar] [CrossRef]
- Chi, J.; Zaw, T.; Cardona, I.; Hosnain, M.; Garg, N.; Lefkowitz, H.R.; Tolias, P.; Du, H. Use of surface-enhanced Raman scattering as a prognostic indicator of acute kidney transplant rejection. Biomed. Opt. Express 2015, 6, 761–769. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yu, M.C.; Rich, P.; Foreman, L.; Smith, J.; Yu, M.S.; Tanna, A.; Dibbur, V.; Unwin, R.; Tam, F.W. Label Free Detection of Sensitive Mid-Infrared Biomarkers of Glomerulonephritis in Urine Using Fourier Transform Infrared Spectroscopy. Sci. Rep. 2017, 7, 4601. [Google Scholar] [CrossRef] [PubMed]
- Bispo, J.A.M.; Vieira, E.E.D.S.; Silveira, J.L.; Fernandes, A.B. Correlating the amount of urea, creatinine, and glucose in urine from patients with diabetes mellitus and hypertension with the risk of developing renal lesions by means of Raman spectroscopy and principal component analysis. J. Biomed. Opt. 2013, 18, 087004. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhao, C.; Li, R.; Shen, C.; Cai, X.; Sun, L.; Luo, C.; Yin, Y. Noninvasive and prospective diagnosis of coronary heart disease with urine using surface-enhanced Raman spectroscopy. Analyst 2018, 143, 2235–2242. [Google Scholar] [CrossRef]
- Paraskevaidi, M.; Morais, C.L.; Lima, K.M.; Ashton, K.M.; Stringfellow, H.F.; Martin-Hirsch, P.L.; Martin, F.L. Potential of mid-infrared spectroscopy as a noninvasive diagnostic test in urine for endometrial or ovarian cancer. Analyst 2018, 143, 3156. [Google Scholar] [CrossRef]
- Lin, X.; Wang, L.; Lin, H.; Lin, D.; Lin, J.; Liu, X.; Qiu, S.; Xu, Y.; Chen, G.; Feng, S. A novel urine analysis technique combining affinitychromatography with Au nanoparticle based surface enhancedRaman spectroscopy for potential applications in non-invasivecancer screening. J. Biophotonics 2019, 12, e201800327. [Google Scholar] [CrossRef]
- Del Mistro, G.; Cervo, S.; Mansutti, E.; Spizzo, R.; Colombatti, A.; Belmonte, P.; Zucconelli, R.; Steffan, A.; Sergo, V.; Bonifacio, A. Surface-enhanced Raman spectroscopy of urine for prostate cancer detection: A preliminary study. Anal. Bioanal. Chem. 2015, 407, 3271–3275. [Google Scholar] [CrossRef]
- Elumalai, B.; Prakasarao, A.; Ganesan, B.; Dornadula, K.; Ganesan, S. Raman spectroscopic characterization of urine of normal and oral cancer subjects. J. Raman Spectrosc. 2014, 46, 84–93. [Google Scholar] [CrossRef]
- Feng, S.; Zheng, Z.; Xu, Y.; Lin, J.; Chen, G.; Weng, C.; Lin, D.; Qiu, S.; Cheng, M.; Huang, Z.; et al. A noninvasive cancer detection strategy based on gold nanoparticle surface-enhanced raman spectroscopy of urinary modified nucleosides isolated by affinity chromatography. Biosens. Bioelectron. 2017, 91, 616–622. [Google Scholar] [CrossRef]
- Wang, J.; Koo, K.M.; Wee, E.J.H.; Wang, Y.; Trau, M. A nanoplasmonic label-free surface-enhanced Raman scattering strategy for non-invasive cancer genetic subtyping in patient samples. Nanoscale 2017, 9, 3496–3503. [Google Scholar] [CrossRef]
- Remer, T.; Montenegro-Bethancourt, G.; Shi, L. Long-term urine biobanking: Storage stability of clinical chemical parameters under moderate freezing conditions without use of preservatives. Clin. Biochem. 2014, 47, 307–311. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).