Abstract
Since December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has spread rapidly throughout the world causing health, social and economic instability. The severity and prognosis of patients with SARS-CoV-2 infection are associated with the presence of comorbidities such as cardiovascular disease, hypertension, chronic lung disease, cerebrovascular disease, diabetes, chronic kidney disease, and malignancy. Thrombosis is one of the most serious complications that can occur in patients with COVID-19. Homocysteine is a non-proteinogenic α-amino acid considered a potential marker of thrombotic diseases. Our review aims to provide an updated analysis of the data on the involvement of homocysteine in COVID-19 to highlight the correlation of this amino acid with disease severity and the possible mechanisms by which it intervenes.
1. Introduction
The World Health Organization (WHO) declared in early 2020 that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection was a public health emergency of international concern. To date, considerable efforts have been made to both prevent and diagnose and treat SARS-CoV-2 infection. SARS-CoV-2 enters host cells via the angiotensin-converting enzyme 2 (ACE2) receptor and can infect the heart, vascular tissues, and circulating cells [1,2].
Pneumonia, multiorgan failure, or even death was highlighted in patients with SARS-CoV-2 infection. In order to manage and treat patients with Coronavirus disease 2019 (COVID-19), it is really important to know the risk factors (comorbidities) and specific biomarkers. Hypertension, cardiovascular diseases, diabetes, kidney diseases, lung diseases, and cancer were the comorbidities associated with the severity of COVID-19 [3,4,5,6]. Laboratory biomarkers of organ damage have an important role in the diagnosis and prognosis of patients infected with SARS-CoV-2 because the virus has been identified in endothelial, liver, kidney, lung and neuronal cells [7,8,9,10]. Proinflammatory cytokines, neuron-specific enolase, lactate dehydrogenase, aspartate transaminase, neutrophil count, neutrophil-lymphocyte ratio, troponins, creatine kinase, myoglobin, D-dimer, brain natriuretic peptide and its N-terminal prohormone are the most widely used biomarkers to predict disease severity [11,12,13].
Thrombosis is one of the most serious complications that can occur in patients infected with SARS-CoV-2 [14,15,16,17]. Helms et al. [18] evaluated the thrombotic risk in a number of 150 patients with COVID-19 and reported a percentage of 42% with thrombotic complications, mainly pulmonary embolisms. It is clear that patients with SARS-CoV-2 may have coagulation abnormalities leading to a hypercoagulability state.
Homocysteine is a non-proteogenic α-amino acid that is formed in the metabolism of methionine. The increase in serum homocysteine values can be caused either by the excessive intake of methionine, or most frequently, by the blocking of one of the metabolic pathways through dietary deficiency of folic acid, vitamin B12 and vitamin B6. Elevated serum homocysteine concentration is thought to be involved in many diseases, including neurological diseases [19,20,21], cardiovascular diseases [22,23,24], osteoporosis [25,26], and diabetes [27] (Figure 1).
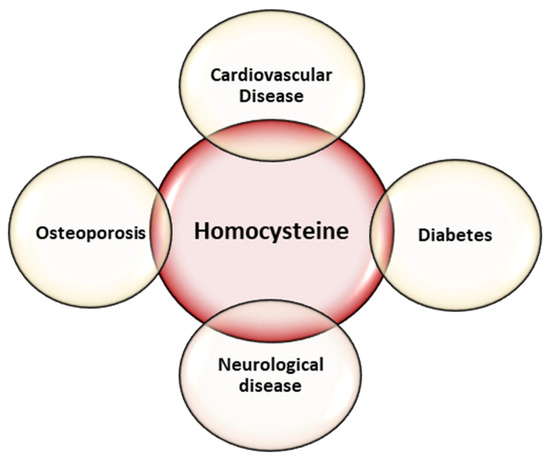
Figure 1.
Diseases associated with homocysteine.
Numerous studies indicate that elevated serum homocysteine levels have toxic effects on the vascular endothelium, causing cellular dysfunction [27,28,29,30,31,32,33]. Damage to the vascular endothelium leads to thrombus formation, generating a hypercoagulability state.
Considering the previously mentioned data on the state of hypercoagulability in patients with SARS-CoV-2 infection and that homocysteine is a marker of thrombotic diseases, we aimed to review the available data on the involvement of hyperhomocysteinemia (HHCY) in the prognosis of patients infected with this virus.
2. Homocysteine Metabolism
Homocysteine is a sulfated amino acid, formed during the metabolism of methionine, an essential amino acid present in proteins of animal origin [34]. Homocysteine is metabolized in two ways, namely: remethylation (through which methionine is regenerated) and transsulfuration (through which it degrades to cysteine) (Figure 2). The serum level varies according to the two metabolic pathways.
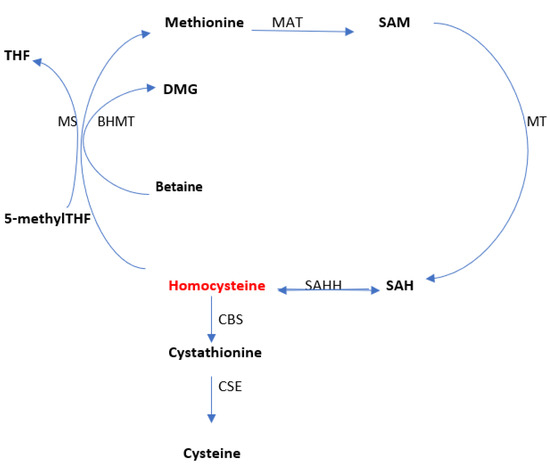
Figure 2.
The overview of homocysteine metabolism. Methionine adenosyltransferase (MAT); methyltransferase (MT); S-adenosylhomocysteine hydrolase (SAHH); betaine-homocysteine S-methyl transferase (BHMT); Cystathionine β-synthase (CBS); Cystathionine γ-lyase (CSE); methionine synthase (MS). Adapted from Moretti et al. [34].
Methionine is the body’s main donor of methyl radicals in the form of S-adenosylmethionine (SAM) which is then converted into S-adenosylhomocysteine (SAH). Homocysteine is generated by the cleavage of SAH, the reaction catalyzed by S-adenosylhomocysteine hydrolase (SAHH; EC 3.3.1.1). Once synthesized, homocysteine quickly undergoes remethylation to methionine in a reaction catalyzed by methionine synthase (MS; EC 2.1.1.13) (which uses N5-methyltetrahydrofolate as a methyl donor and cobalamin as the cofactor). N5-methyltetrahydrofolate is formed by the reduction of N5,10-methylenetetrahydrofolate in a reaction catalyzed by N5,10-methylenetetrahydrofolate reductase (MTHFR; EC 1.5.1.20) [35,36,37].
Remethylation of homocysteine is also achieved via betaine (derived from choline) under the action of betaine-homocysteine S-methyl transferase (BHMT; EC 2.1.1.5), betaine being then converted to dimethylglycine. This last reaction occurs only in the liver and kidneys, while remethylation via methionine synthase is distributed in all tissues [38,39].
Methylcobalamin receives the methyl group from S-adenosylmethionine (SAM) or 5-methyltetrahydrofolate (5-methylTHF), the active form of folic acid. After remethylation, methionine can be reused to produce SAM, the body’s “universal methyl group donor,” which actively participates in numerous metabolic pathways that include myelin methylation, the synthesis of carnitine, coenzyme Q10, creatine, epinephrine, melatonin, methylcobalamin, and phosphatidylcholine [40,41,42].
Research has shown that the accumulation of high amounts of homocysteine and adenosine at the cellular level causes all methylation reactions to be completely inhibited [43,44,45]. Homocysteine is converted to cysteine via cystathionine. Cystathionine β-synthase (CBS; EC 4.2.1.22) catalyzes the first step of transsulfuration and allows the condensation of homocysteine and serine to generate cystathionine. It uses vitamin B6 in its active form (pyridoxal 5-phosphate) as a co-factor. Cystathionine γ-lyase (CSE; EC 4.4.1.1) catalyzes the hydrolysis of cystathionine to form α-ketobutyrate and cysteine, and is also dependent on pyridoxal-5-phosphate [46,47].
The amino acids cysteine and taurine are important compounds at the cardiac level, and for liver detoxification, cholesterol excretion, bile salt formation and glutathione production [48,49]. Recent studies mention that 5-methylTHF, methylcobalamin, betaine, pyridoxal 5-phosphate, and N-acetylcysteine significantly lower elevated homocysteine levels [50,51,52].
3. Hyperhomocysteinemia and Vascular Damage
3.1. Overview of Hyperhomocysteinemia
In plasma, homocysteine can be found bound to proteins, but also in free, oxidized, or disulfide forms [53]. Plasma homocysteine concentrations of less than 15 μM are considered normal [54,55]. The increase in plasma homocysteine concentration above the normal values is defined as hyperhomocysteinemia. Depending on the concentration of homocysteine, it can be of several types, namely: moderate hyperhomocysteinemia (15–30 μM), intermediate hyperhomocysteinemia (30–100 μM) and severe hyperhomocysteinemia (>100 μM) [56].
The increase in the plasma level of homocysteine can be due to genetic, nutritional, or pathological factors (Table 1).

Table 1.
Causes of elevated homocysteine.
Changes in homocysteine plasma levels are directly related to the genetic background.
The activity of enzymes involved in homocysteine metabolism is modulated by variants of the genes that encode these enzymes. A C677T point mutation in the gene encoding MTHFR is the most common genetic cause of HHCY. Homozygous carriers may have a moderate increase in homocysteine levels and may experience varying degrees of symptoms caused by venous or arterial thrombosis [79,80].
Another mutation in the MTHFR gene, polymorphism 1298A > C (p. E429A) is associated with decreased MTHFR activity that is more pronounced in the homozygous compared with the heterozygous state, leading to HHCY [81]. Another enzyme involved in the homocysteine remethylation pathway is methionine synthase (MS). The MTR 2756A > G (p. D919G) and MTRR 66A > G polymorphisms can affect the levels of homocysteine [82]. In addition to genetic factors, HHCY can be attributed to a deficiency of vitamins B6, B12 or folate, which plays a crucial role in the homocysteine remethylation pathway, without which, homocysteine would fail [83]. The prevalence of HHCY can vary significantly between populations and, in addition to the previously discussed factors, also depends on age, sex, alcoholism, smoking and medication [28,46].
Since the 1950s, it has been highlighted through numerous studies that megaloblastic anemias caused by folate or vitamin B12 deficiencies can be associated with thromboembolic accidents [84]. It has been shown that there is a directly proportional relationship between folic acid deficiency and elevated homocysteine levels in the risk of coronary heart disease. Low plasma concentration of folic acid is associated with an increased risk of fatal coronary heart disease and an increase in plasma homocysteine [85]. Epidemiological data have shown that homocysteine can be considered a coronary risk factor because homocysteine plasma concentrations are constantly increased in patients with cardiovascular diseases compared to normal subjects.
The coenzymes pyridoxal phosphate, methyl-tetrahydrofolate and cobalamin have an essential role for the enzymes involved in the metabolism of homocysteine and are dependent on the consumption of vitamin B6, vitamin B9 and vitamin B12, respectively. There is a non-linear and inversely proportional association between homocysteine plasma concentration and folate level as well as vitamin intake. Likewise, weaker inversely proportional associations were observed between the concentration of vitamin B12 and plasma pyridoxal phosphate, with the intake of B6, but not with the intake of vitamin B12. It has been shown that a diet rich in cereals and folic acid derivatives contributes to an increase in folate plasma levels and implicitly a decrease in homocysteine concentration [86]. In patients infected with Helicobacter pylori, a deficient absorption of vitamin B6, B12 and acid-causing HHCY have been noted. According to Selhub [87], the prevalence of HHCY would reach an average of 30% among subjects aged between 65 and 80 years and 40–60% in patients over 80 years of age. This increased frequency of HHCY among the geriatric population was explained by a deficiency in B vitamins, estimated at 29% in the elderly and 55% in the very old [88]. It should be noted that in elderly people, folic acid supplementation must be systematically associated with vitamin B12, because it is shown that strong doses of folic acid can lead to a deterioration of cognitive functions, masking the possible deficiency in vitamin B12 [89]. Men have a higher level of homocysteine than women, due to the muscle mass that is better developed and the effects of sex hormones, a fact that was confirmed by a study conducted on a group of people of both sexes, male and female. [90]. Part of the relationship between women’s age and HHCY could be explained by the onset of menopause. In females, an increase in homocysteine is observed after menopause [91].
3.2. Hyperhomocysteinemia and Endothelial Dysfunction
Alterations in the balance between vasodilators and vasoconstrictors produced by the endothelium are defined as endothelial dysfunction, associated with atherosclerosis and cardiovascular diseases [92]. High homocysteine levels have toxic effects on the vascular endothelium, damaging it and causing cellular dysfunction, followed by platelet activation, and thrombus formation, thus creating a hypercoagulable state [93]. Regarding venous thromboembolic disease, an increase in the risk of venous thrombosis of 60% (retrospective studies) and 27% (prospective studies) was found for an increase in plasma homocysteine level of 5 mmol/L. The same study showed a 20% increased risk of venous thrombosis in subjects carrying the MTHFR677TT genotype compared to patients with the MTHFR677CC genotype. Therefore, this study incriminates homocysteine as a risk factor for venous thromboembolic disease [94]. Several mechanisms have been proposed through which homocysteine affects the function of the vascular endothelium, among which we mention: increasing oxidative stress, limiting the bioavailability of nitric oxide, stimulating smooth cell proliferation and changing the properties of the elastic wall [95]. Oxidative inactivation of nitric oxide, an important vasodilator, can be a mechanism for endothelial dysfunction in HHCY [96]. The loss of endothelium-mediated vasodilator ability leads to the tilting of the vascular balance towards an abnormally constrictive inflammatory prothrombotic state and is considered to be one of the most rapid manifestations of cardiovascular damage and precedes the formation of atherosclerotic plaques [92]. More research is needed to better understand the exact mechanism by which homocysteine affects endothelial function.
3.3. Hyperhomocysteinemia and Cardiovascular Disease
Cardiovascular disease (CVD) is the leading global cause of mortality, with thrombotic complications playing a major role [97]. One of the most incriminated mechanisms of CVD is the atherosclerotic process, in which lipids accumulate at the subendothelial level, resulting in a low-grade inflammatory lesion and transforming the endothelium into a phenotype prone to inflammation and thrombosis. Two hypotheses have been formulated to explain the atherogenicity of the increase in the plasma level of homocysteine: the lipid hypothesis, according to which the alteration of lipoprotein metabolism secondarily induces a touch of the vascular wall, and the inflammatory hypothesis dominated by the direct aggression of cells and vascular tissue.
Lipid peroxidation of low-density lipoproteins (LDL) induces fragmentation of apolipoproteins (apo) B100 initially for their capture by macrophage scavenger receptors. This phenomenon would be responsible to a certain extent for atherogenicity. Sulfated derivatives possessing a free thiol group are likely to cause the oxidation of LDL by endothelial cells and macrophages in vitro [98]. McCully [99] developed the hypothesis that homocysteine would be the main sulfate compound favoring the oxidation of LDL in vivo, a property of its vascular pathogenicity. The ability of homocysteine to modify the physicochemical and biological properties of LDL in vitro has been confirmed. Incubation of LDL in the presence of homocysteine induces a decrease in their content in polyunsaturated fatty acids, the formation of terminal substances of lipid peroxidation (substances that react to thiobarbituric acid) and the fragmentation of apolipoproteins B100 [100]. On the other hand, the thiolation of LDL by homocysteine confers certain pro-atherogenic properties similar to oxidized LDL [101]. Additionally, clinical trial results support a relationship between HHCY and lipid peroxidation in humans [102]. Therefore, the existence of a positive correlation between homocysteinemia and the plasma concentration of a new marker of lipid peroxidation, F2-isoprostane, was reported [103].
In contrast, other studies have provided hypotheses going against the lipid hypothesis. Indeed, the oxidation susceptibility of LDL from patients homozygous for CBS deficiency is similar to that of LDL from control subjects [104]. In vitro, homocysteine concentrations between 25 and 500 nmol/L would protect LDL against oxidation, arguing in favor of a paradoxical antioxidant effect of homocysteine [105].
In conclusion, most of the in vitro studies prove that homocysteine induces structural changes at the LDL level, but most of the in vivo results do not confirm the hypothesis that the atherogenicity of HHCY could be related to its ability to oxidize lipoproteins. These studies do not even allow to disprove the lipid hypothesis since first of all the existence of circulating oxidized LDL is controversial, the degree of oxidation of LDL is evaluated by determining their content in thiobarbituric acid which does not allow to highlight the low variations due to its vast variability. Another reason is that the LDL of homozygous CBS-deficient patients is not more susceptible to oxidation in vitro than control subjects [106].
The atherogenicity of HHCY could also result from a primitive activity of vascular endothelial cells leading to their functional dysregulation, followed by platelet and leukocyte activation and proliferation of smooth muscle cells simultaneously with the modification of the sub-endothelial matrix [44,45].
The deleterious action of homocysteine on endothelial cell function has been demonstrated both in vitro and in vivo. In vitro, non-toxic concentrations (less than 1mmol/l) of homocysteine alter the production or activation by endothelial cells of mediators involved in platelet aggregation processes, coagulation and fibrinolysis [107]. They alter the production and bioavailability of endothelial vasodilators and vasoconstrictor mediators.
These experimental results were confirmed by clinical studies indicating that HHCY is accompanied by variations in the plasma concentration and activity of certain endothelium-derived mediators: increased concentrations of von Willebrand factor, endothelin-1 and adhesion molecules such as ICAM-1 (intercellular adhesion molecule-1) and VCAM-1 (vascular cell adhesion molecule-1), decreasing the activity of antithrombin III [108]. At the same time, HHCY is accompanied by alteration of endothelium-dependent vasodilatation, without alteration of endothelium-independent vasodilatation. The pro-atherogenic action of HHCY could come from altering the functionality of the vascular endothelium, causing the induction of a procoagulant and proinflammatory state as well as the deregulation of vascular tone.
The mitogenic action of homocysteine on smooth muscle cells and its toxicity on vascular connective tissue was revealed by histological analysis of the arteries of homocystinuric patients, then confirmed experimentally in animals and in vitro [109]. The mitogenic action would be directly related to the induction of the expression of the regulatory cyclins D1 and A as well as the activation of the transcription factor NF-κB in smooth muscle cells [110]. This action would be indirectly the result of the previously evoked endothelial lesions, as well as the associated thrombotic processes, causing the intraparietal release of platelet factors such as PDGF (platelet-derived growth factor). The aggression of the vascular connective tissue by homocysteine could achieve multiple mechanisms. These include stimulation of collagen synthesis by smooth muscle cells, inhibition of intrachain bond formation between primitive collagen and elastin molecules, induction of collagenases, acceleration of elastin fiber degradation, and induction of cellular secretion of insoluble proteoglycans due to their exclusively sulfated character.
The lipid and inflammatory hypotheses come together to explain the deleterious effects of HHCY. In fact, modified (by homocysteine) low-density lipoproteins (LDL) exhibit biological properties that lead to a dysregulation of the functional state of endothelial cells, comparable to that described for the direct effect of homocysteine on the vascular endothelium. Free radicals originating from the autoxidation of homocysteine or by deregulation of the thiol-redox status, interact with LDL, oxidizing them, which potentiates the harmful effects on the arterial wall. The pathophysiology of the homocysteine-vascular wall interaction is complex and multifactorial.
Since the identification of the main “traditional” risk factors (diabetes mellitus, hypertension, dyslipidemia) for CVD, more than 100 “novel” risk factors have been proposed and studied as potential causative agents, triggers or therapeutic targets [111]. Homocysteine represents one of these “novel” markers that have generated significant interest. Severe HHCY has been observed in individuals with homozygous MTHFR mutations and has been associated with premature cardiovascular disease [112,113,114]. The link between homocysteine and cardiovascular disease can be explained by several different mechanisms, such as endothelial dysfunction, oxidative damage, increased collagen synthesis and increased vascular smooth muscle cell proliferation [112,115,116,117]. Recently, cross-sectional studies have looked for an association between the increase in plasma homocysteine level and the presence of structural and functional alterations of the arterial wall in asymptomatic subjects with cardiovascular pathologies [118]. From a functional point of view, elevated plasma homocysteine has been associated with impaired endothelium-dependent vasodilatation. From a structural point of view, it was highlighted that the increase in homocysteinemia would be associated with the restoration of the carotid artery independently of the known determining factors, of arterial diameter and thickness, and suggests that HHCY could equally constitute a marker of atherosclerosis. It has been shown that folate supplementation would allow control of moderate HHCY.
A direct causal relationship between HHCY induction and accelerated atherosclerosis has been reported in apolipoprotein E (apoE)-deficient mice [119]. It has been reported that the myocardium is particularly vulnerable to damage by HHCY, which is associated with the production of reactive oxygen species and causes the progression of cardiovascular disease [120]. The correlation between HHCY and atherosclerosis comes from the comparative analysis of the clinical and biological profiles described for the three enzyme deficiencies of genetic origin, associated with severe HHCY in humans. The pro-atherogenic role of HHCY has been confirmed by clinical and epidemiological studies. Most studies have reported a strong association between HHCY and total occlusive arterial disease, ischemic heart disease, and peripheral arterial disease. In parallel, the total of prospective studies performed in patients affected by declared cardiovascular diseases demonstrated that homocysteinemia is correlated with the occurrence of cardiovascular accidents. However, prospective studies have provided conflicting results, some of them considering HHCY to be causal in the clinical manifestations of atherosclerosis, and others noting the absence of a causal relationship.
If these different opinions do not allow us to state that HHCY is an independent cardiovascular risk factor, they prove that HHCY actively contributes to the development of cardiovascular diseases in high-risk patients.
Ponti et al. [121] reported that they started a prospective study of 500 patients to evaluate the predictive value of homocysteine as a specific marker for cardiovascular risk in patients with COVID-19, grouped into “usual,” “sub intensive,” and “intensive” [121].
4. Homocysteine: A Closer Look at the Correlation with SARS-CoV-2 Infection
To date, there are few studies on the involvement of homocysteine in the COVID-19 disease. Table 2 schematically presents the data published in the literature regarding plasma homocysteine levels and SARS-CoV-2 infection.

Table 2.
Studies indicating a correlation between HCY levels and COVID-19 patients.
The first prospective single-center cohort study on this subject is presented by Yang et al. [122]. The authors investigated different parameters such as homocysteine and predictors of imaging progression on lung computed tomography scans in the case of patients infected with SARS-CoV-2. Their results indicate that homocysteine levels were correlated with the severity of lung lesions (assessed by chest CT scans) [122]. Until now, few studies have been presented in the literature regarding the correlation of homocysteine with pulmonary imaging progression in patients with SARS-CoV-2 infection. Ponti et al., [123] in a study conducted on Italian-nationality patients hospitalized with COVID-19 investigated the role of homocysteine in this disease. In agreement with the obtained results, they propose plasma HCY levels and MTHFR gene sequencing as markers for the clinical management of SARS-CoV-2 infection [123]. Although several studies report homocysteine as a strong predictive marker for the severity of SARS-CoV-2 infection, the results of the study by Khalid et al. [128] reported that it is a moderate predictive marker for the disease.
The renin-angiotensin-aldosterone system is central to circulatory blood volume and peripheral vascular resistance regulation; its main components are ACE, angiotensin II/angiotensin II receptors, types AT1 and AT2. ACE’s main role is converting angiotensin I to angiotensin II which binds to type I Ang II receptors thus triggering an intracellular pathway leading to a plethora of potentially detrimental vascular effects (sodium and water retention, vasoconstriction, inflammation, oxidative stress, apoptosis) [131,132]. Conversely, the ACE2 enzyme is involved in angiotensin II proteolysis; the resulting Ang (1–7) peptide coupled to Mas receptors has opposite biological effects to angiotensin II. SARS-CoV and SARS-CoV-2 spike proteins have an affinity for ACE2 which acts as a receptor and mediates membrane fusion and cell entry [133,134]. Cell entry, aside from viral ACE2 binding, alters the balance between angiotensin II and Ang (1–7) effects leading to pro-inflammatory events and triggering cytokine cascade activation [135,136]. A possible correlation between HHCY and SARS-CoV-2 infection may be explained by the direct involvement of homocysteine angiotensin II type I receptor activation, contributing to the severity of vascular/endothelial lesions [137]. There are multiple mechanisms accounting for AT1 activation—homocysteine-triggered upregulation of AT1 transcription has been described but also direct interaction between homocysteine and the AT1 receptor with intracellular conformational changes and subsequent activation [137]. There are three biological active forms of homocysteine (free, oxidized and reduced) [138,139,140]. There are no clear data on the differences between them in terms of intensity of interaction with AT1 and cardiovascular effects. Considering these interactions, it would be useful to find out if patients with comorbidities associated with high plasma homocysteine levels (such as megaloblastic anemia) have worse SARS-CoV-2 infection outcomes. Should this be the case therapeutic approaches may be developed although reducing homocysteine plasma levels by vitamin B supplementation seemed not to alleviate cardiovascular risk in renal transplant patients [141].
Another possible correlation between HHCY and the severity of SARS-CoV-2 infection may be related to the presence of the C677T point mutation in the gene encoding MTHFR [142]. Herrera et al. [143] performed a retrospective study on 334 patients having had coronary, pulmonary, or other location arterial thrombosis to investigate the relationship between the C677T polymorphism, homocysteine concentration and prothrombotic biomarkers. Their results indicate the correlation of HHCY in the presence of the T allele in the C677T gene with pulmonary embolism and acute myocardial ischemia [143]. The study by Ponti et al. [144] showed, in the Latino population, a strong correlation between the C677 T variant and death due to SARS-CoV-2 infection.
5. Conclusions
Although it is known that SARS-CoV-2 infection causes severe coagulation disorders and that these play a crucial role in the evolution of patients, currently many aspects of the underlying pathophysiology remain unclear. Homocysteine has been proposed as a marker of cardiovascular risk and complications in hospitalized patients with SARS-CoV-2 infection. Its inclusion in laboratory markers may be of particular importance to predict disease severity.
Author Contributions
Conceptualization, N.F. and I.L.S.; methodology, E.C.; investigation, O.V.B. and G.B.; resources, A.C. and C.E.I.; writing—original draft preparation, A.E.J.; writing—review and editing, A.C.P., I.A.S. and M.A.M.; visualization, C.F.; supervision, E.C., A.C.P. and M.A.M.; project administration, N.F. and I.L.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef] [PubMed]
- Gadanec, L.K.; McSweeney, K.R.; Qaradakhi, T.; Ali, B.; Zulli, A.; Apostolopoulos, V. Can SARS-CoV-2 Virus Use Multiple Receptors to Enter Host Cells? Int. J. Mol. Sci. 2021, 22, 992. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Zanon, M.; Montanaro, M.; Radaelli, D.; Sessa, F.; Di Mizio, G.; Montana, A.; Corrao, S.; Salerno, M.; Pomara, C. More than Pneumonia: Distinctive Features of SARS-CoV-2 Infection. From Autopsy Findings to Clinical Implications: A Systematic Review. Microorganisms 2020, 8, 1642. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, M.; Mei, F.; Skrami, E.; Latini, L.L.; Amico, D.; Balestro, E.; Bini, F.; Bonifazi, F.; Caminati, A.; Candoli, P.; et al. Predictors of Worse Prognosis in Young and Middle-Aged Adults Hospitalized with COVID-19 Pneumonia: A Multi-Center Italian Study (COVID-UNDER50). J. Clin. Med. 2021, 10, 1218. [Google Scholar] [CrossRef]
- Laurenzi, A.; Caretto, A.; Molinari, C.; Bazzigaluppi, E.; Brigatti, C.; Marzinotto, I.; Mercalli, A.; Melzi, R.; Nano, R.; Tresoldi, C.; et al. Pre-Existing Diabetes and COVID-Associated Hyperglycaemia in Patients with COVID-19 Pneumonia. Biology 2021, 10, 754. [Google Scholar] [CrossRef]
- Armaly, Z.; Kinaneh, S.; Skorecki, K. Renal Manifestations of COVID-19: Physiology and Pathophysiology. J. Clin. Med. 2021, 10, 1216. [Google Scholar] [CrossRef]
- Mokhtari, T.; Hassani, F.; Ghaffari, N.; Ebrahimi, B.; Yarahmadi, A.; Hassanzadeh, G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. J. Mol. Histol. 2020, 51, 613–628. [Google Scholar] [CrossRef]
- Xu, L.; Liu, J.; Lu, M.; Yang, D.; Zheng, X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020, 40, 998–1004. [Google Scholar] [CrossRef]
- Barbosa, L.C.; Goncalves, T.L.; de Araujo, L.P.; de Oliveira Rosario, L.V.; Ferrer, V.P. Endothelial cells and SARS-CoV-2: An intimate relationship. Vasc. Pharmacol. 2021, 137, 106829. [Google Scholar] [CrossRef]
- Polidoro, R.B.; Hagan, R.S.; de Santis Santiago, R.; Schmidt, N.W. Overview: Systemic inflammatory response derived from lung injury caused by SARS-CoV-2 infection explains severe outcomes in COVID-19. Front. Immunol. 2020, 11, 1626. [Google Scholar] [CrossRef]
- Battaglini, D.; Lopes-Pacheco, M.; Castro-Faria-Neto, H.C.; Pelosi, P.; Rocco, P.R. Laboratory Biomarkers for Diagnosis and Prognosis in COVID-19. Front. Immunol. 2022, 13, 857573. [Google Scholar] [CrossRef] [PubMed]
- Kermali, M.; Khalsa, R.K.; Pillai, K.; Ismail, Z.; Harky, A. The role of biomarkers in diagnosis of COVID-19–A systematic review. Life Sci. 2020, 254, 117788. [Google Scholar] [CrossRef] [PubMed]
- Tjendra, Y.; Al Mana, A.F.; Espejo, A.P.; Akgun, Y.; Millan, N.C.; Gomez-Fernandez, C.; Cray, C. Predicting disease severity and outcome in COVID-19 patients: A review of multiple biomarkers. Arch. Pathol. Lab. Med. 2020, 144, 1465–1474. [Google Scholar] [CrossRef] [PubMed]
- Moschonas, I.C.; Tselepis, A.D. SARS-CoV-2 infection and thrombotic complications: A narrative review. J. Thromb. Thrombolysis 2021, 52, 111–123. [Google Scholar] [CrossRef]
- Badulescu, O.V.; Sirbu, P.D.; Filip, N.; Bordeianu, G.; Cojocaru, E.; Budacu, C.C.; Badescu, M.C.; Bararu-Bojan, I.; Veliceasa, B.; Ciocoiu, M. Hereditary Thrombophilia in the Era of COVID-19. Healthcare 2022, 10, 993. [Google Scholar] [CrossRef]
- Kander, T. Coagulation disorder in COVID-19. Lancet Haematol. 2020, 7, e630–e632. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Helms, J.; Tacquard, C.; Severac, F.; Leonard-Lorant, I.; Ohana, M.; Delabranche, X.; Merdji, H.; Clere-Jehl, R.; Schenk, M.; Fagot Gandet, F.; et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: A multicenter prospective cohort study. Intensive Care Med. 2020, 46, 1089–1098. [Google Scholar] [CrossRef]
- Towfighi, A.; Markovic, D.; Ovbiagele, B. Pronounced association of elevated serum homocysteine with stroke in subgroups of individuals: A nationwide study. J. Neurol. Sci. 2010, 298, 153–157. [Google Scholar] [CrossRef]
- Seshadri, S. Elevated plasma homocysteine levels: Risk factor or risk marker for the development of dementia and Alzheimer’s disease? J. Alzheimer’s Dis. 2006, 9, 393–398. [Google Scholar] [CrossRef]
- Azzini, E.; Ruggeri, S.; Polito, A. Homocysteine: Its Possible Emerging Role in At-Risk Population Groups. Int. J. Mol. Sci. 2020, 21, 1421. [Google Scholar] [CrossRef]
- Brattström, L.; Wilcken, D.E. Homocysteine and cardiovascular disease: Cause or effect? Am. J. Clin. Nutr. 2020, 72, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Baggott, J.E.; Tamura, T. Homocysteine, Iron and Cardiovascular Disease: A Hypothesis. Nutrients 2015, 7, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Filip, C.; Albu, E.; Lupascu, D.; Filip, N. The influence of a new rutin derivative in an experimental model of induced hyperhomocysteinemia in rats. Farmacia 2017, 65, 596–599. [Google Scholar]
- Fratoni, V.; Brandi, M.L. B Vitamins, Homocysteine and Bone Health. Nutrients 2015, 7, 2176–2192. [Google Scholar] [CrossRef] [PubMed]
- Filip, A.; Badulescu, O.V.; Sirbu, P.D.; Cojocaru, E.; Filip, N.; Puha, G.; Trandafir, L.; Iancu, C.; Trandafirescu, M.F.; Alexa, O. Serum homocysteine and reactive species levels in fragility fractures of the pelvis. Rev. De Chim. 2019, 70, 3216–3219. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Y.; Wang, G. Homocysteine levels are associated with endothelial function in newly diagnosed type 2 diabetes mellitus patients. Metab. Syndr. Relat. Disord. 2019, 17, 323–327. [Google Scholar] [CrossRef]
- Tawfik, A.; Samra, Y.A.; Elsherbiny, N.M.; Al-Shabrawey, M. Implication of Hyperhomocysteinemia in Blood Retinal Barrier (BRB) Dysfunction. Biomolecules 2020, 10, 1119. [Google Scholar] [CrossRef]
- Splaver, A.; Lamas, G.A.; Hennekens, C.H. Homocysteine and cardiovascular disease: Biological mechanisms, observational epidemiology, and the need for randomized trials. Am. Heart J. 2004, 148, 34–40. [Google Scholar] [CrossRef]
- Balint, B.; Jepchumba, V.K.; Guéant, J.L.; Guéant-Rodriguez, R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef]
- Albu, E.; Filip, C.; Zamosteanu, N.; Jaba, I.M.; Linic, I.S.; Sosa, I. Hyperhomocysteinemia is an indicator of oxidant stress. Med. Hypotheses 2012, 78, 554–555. [Google Scholar] [CrossRef] [PubMed]
- Albu, E.; Lupascu, D.; Filip, C.; Jaba, I.M.; Zamosteanu, N. The influence of a new rutin derivative on homocysteine, cholesterol and total antioxidative status in experimental diabetes in rat. Farmacia 2013, 61, 1167–1177. [Google Scholar]
- Moretti, R.; Caruso, P. The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. Int. J. Mol. Sci. 2019, 20, 231. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, J.D. Metabolic regulatory properties of S-adenosylmethionine and S-adenosylhomocysteine. Clin. Chem. Lab. Med. 2007, 45, 1694–1699. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Simile, M.M.; Calvisi, D.F.; Feo, C.F.; Feo, F. S-Adenosylmethionine: From the Discovery of Its Inhibition of Tumorigenesis to Its Use as a Therapeutic Agent. Cells 2022, 11, 409. [Google Scholar] [CrossRef]
- Mato, J.M.; Martínez-Chantar, M.L.; Lu, S.C. S-adenosylmethionine metabolism and liver disease. Ann. Hepatol. 2015, 12, 183–189. [Google Scholar] [CrossRef]
- Imbard, A.; Benoist, J.F.; Esse, R.; Gupta, S.; Lebon, S.; de Vriese, A.S.; de Baulny, H.O.; Kruger, W.; Schiff, M.; Blom, H.J. High homocysteine induces betaine depletion. Biosci. Rep. 2015, 35, e00222. [Google Scholar] [CrossRef]
- Eussen, S.J.; Ueland, P.M.; Clarke, R.; Blom, H.J.; Hoefnagels, W.H.; Van Staveren, W.A.; De Groot, L.C. The association of betaine, homocysteine and related metabolites with cognitive function in Dutch elderly people. Br. J. Nutr. 2007, 98, 960–968. [Google Scholar] [CrossRef]
- Li, H.; Lu, H.; Tang, W.; Zuo, J. Targeting methionine cycle as a potential therapeutic strategy for immune disorders. Expert Opin. Ther. Targets 2017, 21, 861–877. [Google Scholar] [CrossRef]
- Berardis, D.D.; Orsolini, L.; Iasevoli, F.; Tomasetti, C.; Mazza, M.; Valchera, A.; Fornaro, M.; Perna, G.; Piersanti, M.; Nicola, M.D.; et al. S-Adenosyl-L-Methionine for Major Depressive Disorder. In Melatonin, Neuroprotective Agents and Antidepressant, Therapy; Springer: New Delhi, India, 2016; pp. 847–854. [Google Scholar]
- Román, G.C.; Mancera-Páez, O.; Bernal, C. Epigenetic Factors in Late-Onset Alzheimer’s Disease: MTHFR and CTH Gene Polymorphisms, Metabolic Transsulfuration and Methylation Pathways, and B Vitamins. Int. J. Mol. Sci. 2019, 20, 319. [Google Scholar] [CrossRef]
- Tehlivets, O.; Malanovic, N.; Visram, M.; Pavkov-Keller, T.; Keller, W. S-adenosyl-L-homocysteine hydrolase and methylation disorders: Yeast as a model system. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.E.; Wang, H. Homocysteine and hypomethylation: A novel link to vascular disease. Trends Cardiovasc. Med. 1999, 9, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Paganelli, F.; Mottola, G.; Fromonot, J.; Marlinge, M.; Deharo, P.; Guieu, R.; Ruf, J. Hyperhomocysteinemia and Cardiovascular Disease: Is the Adenosinergic System the Missing Link? Int. J. Mol. Sci. 2021, 22, 1690. [Google Scholar] [CrossRef] [PubMed]
- Durand, P.; Prost, M.; Loreau, N.; Lussier-Cacan, S.; Blache, D. Impaired homocysteine metabolism and atherothrombotic disease. Lab. Investig. 2001, 81, 645–672. [Google Scholar] [CrossRef]
- Škovierová, H.; Vidomanová, E.; Mahmood, S.; Sopková, J.; Drgová, A.; Červeňová, T.; Halašová, E.; Lehotský, J. The Molecular and Cellular Effect of Homocysteine Metabolism Imbalance on Human Health. Int. J. Mol. Sci. 2016, 17, 1733. [Google Scholar] [CrossRef]
- Jong, C.J.; Sandal, P.; Schaffer, S.W. The Role of Taurine in Mitochondria Health: More than Just an Antioxidant. Molecules 2021, 26, 4913. [Google Scholar] [CrossRef]
- Clemente Plaza, N.; Reig García-Galbis, M.; Martínez-Espinosa, R.M. Effects of the Usage of l-Cysteine (l-Cys) on Human Health. Molecules 2018, 23, 575. [Google Scholar] [CrossRef]
- Maclean, K.N.; Jiang, H.; Phinney, W.N.; Mclagan, B.M.; Roede, J.R.; Stabler, S.P. Derangement of hepatic polyamine, folate, and methionine cycle metabolism in cystathionine beta-synthase-deficient homocystinuria in the presence and absence of treatment: Possible implications for pathogenesis. Mol. Genet. Metab. 2021, 132, 128–138. [Google Scholar] [CrossRef]
- McCaddon, A.; Regland, B. COVID-19: A methyl-group assault? Med. Hypotheses 2021, 149, 110543. [Google Scholar] [CrossRef]
- Dattilo, M.; Fontanarosa, C.; Spinelli, M.; Bini, V.; Amoresano, A. Modulation of human hydrogen sulfide Metabolism by Micronutrients, preliminary Data. Nutr. Metab. Insights 2022, 15, 11786388211065372. [Google Scholar] [CrossRef]
- Rehman, T.; Shabbir, M.A.; Inam-Ur-Raheem, M.; Manzoor, M.F.; Ahmad, N.; Liu, Z.W.; Ahmad, M.H.; Siddeeg, A.; Abid, M.; Aadil, R.M. Cysteine and homocysteine as biomarker of various diseases. Food Sci. Nutr. 2020, 8, 4696–4707. [Google Scholar] [CrossRef]
- Wang, N.; Chen, M.; Gao, J.; Ji, X.; He, J.; Zhang, J.; Zhao, W. A series of BODIPY-based probes for the detection of cysteine and homocysteine in living cells. Talanta 2019, 195, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Price, B.R.; Wilcock, D.M.; Weekman, E.M. Hyeprhomocysteinemia as a risk factor for vascular contributions to cognitive impairment and dementia. Front. Aging Neurosci. 2018, 10, 305. [Google Scholar] [CrossRef] [PubMed]
- Kostic, S.; Micovic, Z.; Andrejevic, L.; Cvetkovic, S.; Stamenkovic, A.; Stankovic, S.; Obrenovic, R.; Labudovic-Borovic, M.; Hrncic, D.; Jakovljevic, V.; et al. The effects of L-cysteine and N-acetyl-L-cysteine on homocysteine metabolism and haemostatic markers, and on cardiac and aortic histology in subchronically methionine-treated Wistar male rats. Mol. Cell. Biochem. 2019, 451, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Dean, L. Methylenetetrahydrofolate Reductase Deficiency. In Medical Genetics Summaries; National Center for Biotechnology Information (US): Bethesda, MD, USA, 2012. [Google Scholar]
- Rainero, I.; Vacca, A.; Roveta, F.; Govone, F.; Gai, A.; Rubino, E. Targeting MTHFR for the treatment of migraines. Expert. Opin. Ther. Targets 2019, 23, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Sosic, G.; Sretenovic, S.; Radivojevic, D.; Jovic, N.; Varjacic, M. The impact of the gene variants FV Leiden, FII G20210A, MTHFR C677T and PAI-1 4G/5G on pregnancy loss in women from Central Serbia. Serb. J. Exp. Clin. Res. 2020, 21, 19–25. [Google Scholar] [CrossRef]
- Dwivedi, M.K.; Tripathi, A.K.; Shukla, S.; Khan, S.; Chauhan, U.K. Homocysteine and cardiovascular disease. Biotechnol. Mol. Biol. Rev. 2011, 6, 101–107. [Google Scholar]
- Sibani, S.; Leclerc, D.; Weisberg, I.S.; O’Ferrall, E.; Watkins, D.; Artigas, C.; Rosenblatt, D.S.; Rozen, R. Characterization of mutations in severe methylenetetrahydrofolate reductase deficiency reveals an FAD-responsive mutation. Hum. Mutat. 2003, 21, 509–520. [Google Scholar] [CrossRef]
- Arruda, V.R.; von Zuben, P.M.; Chiaparini, L.C.; Annichino-Bizzacchi, J.M.; Costa, F.F. The mutation Ala677 → Val in the methylene tetrahydrofolate reductase gene: A risk factor for arterial disease and venous thrombosis. Thromb. Haemost. 1997, 77, 818–821. [Google Scholar] [CrossRef]
- Doshi, S.N.; Goodfellow, J.; Lewis, M.J.; McDowell, I.F. Homocysteine and endothelial function. Cardiovasc. Res. 1999, 42, 578–582. [Google Scholar] [CrossRef]
- Israelsson, B.; Brattström, L.E.; Hultberg, B.L. Homocysteine and myocardial infarction. Atherosclerosis 1988, 71, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Fowler, B. Disorders of homocysteine metabolism. J. Inherit. Metab. Dis. 1997, 20, 270–285. [Google Scholar] [CrossRef]
- Kripps, K.A.; Sremba, L.; Larson, A.A.; Van Hove, J.L.; Nguyen, H.; Wright, E.L.; Mirsky, D.M.; Watkins, D.; Rosenblatt, D.S.; Ketteridge, D.; et al. Methionine synthase deficiency: Variable clinical presentation and benefit of early diagnosis and treatment. J. Inherit. Metab. Dis. 2022, 45, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Harmon, D.L.; Shields, D.C.; Woodside, J.V.; McMaster, D.; Yarnell, J.W.; Young, I.S.; Peng, K.; Shane, B.; Evans, A.E.; Whitehead, A.S. Methionine synthase D919G polymorphism is a significant but modest determinant of circulating homocysteine concentrations. Genet. Epidemiol. 1999, 17, 298–309. [Google Scholar] [CrossRef]
- Kožich, V.; Stabler, S. Lessons learned from inherited metabolic disorders of sulfur-containing amino acids metabolism. J. Nutr. 2020, 150 (Suppl. S1), 2506S–2517S. [Google Scholar] [CrossRef] [PubMed]
- Ghose, M.; Das, M.; Das, R.; Barua, A.R.; Deka, P.; Barman, A.; Lahan, V.; Choudhury, D.J.; Sharma, J.P.; Mathur, M.; et al. Homocysteine, Vitamins B6, B12, and Folate and the Risk of Ischemic and Hemorrhagic Stroke: A Case-control Study from Northeast India. Ann. Neurosci. 2022. [Google Scholar] [CrossRef]
- Ubbink, J.B.; van der Merwe, A.; Delport, R.; Allen, R.H.; Stabler, S.P.; Riezler, R.; Vermaak, W.J. The effect of a subnormal vitamin B-6 status on homocysteine metabolism. J. Clin. Investig. 1996, 98, 177–184. [Google Scholar] [CrossRef]
- Allen, R.H.; Stabler, S.P.; Savage, D.G.; Lindenbaum, J. Diagnosis of cobalamin deficiency I: Usefulness of serum methylmalonic acid and total homocysteine concentrations. Am. J. Hematol. 1990, 34, 90–98. [Google Scholar] [CrossRef]
- Monsen, A.L.B.; Refsum, H.; Markestad, T.; Ueland, P.M. Cobalamin status and its biochemical markers methylmalonic acid and homocysteine in different age groups from 4 days to 19 years. Clin. Chem. 2003, 49, 2067–2075. [Google Scholar] [CrossRef]
- Jang, S.; Han, J.W.; Shin, J.; Kim, T.H.; Kwak, K.P.; Kim, K.; Kim, B.J.; Kim, S.G.; Kim, J.L.; Kim, T.H.; et al. Normal-but-low serum folate levels and the risks for cognitive impairment. Psychiatry Investig. 2019, 16, 532. [Google Scholar] [CrossRef]
- Muyitdinovna, X.S. The role of hyperhomocysteinemia in the development of cognitive impairment in chronic cerebral ischemia. Web Sci. Int. Sci. Res. J. 2022, 3, 421–428. [Google Scholar]
- Abdelgawad, F.E.; Bakri, G.M.M. Serum Vitamin B12 and Homocysteine Levels in Type 2 Diabetic Patients with and without Metformin Therapy. J. Biomed. Sci. Eng. 2019, 12, 557. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Roh, H.; Kwon, Y. Causes of hyperhomocysteinemia and its pathological significance. Arch. Pharmacal Res. 2018, 41, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.W.; Park, S.Y.; Kim, H. Drug-Induced Vitamin Deficiency. Ann. Clin. Nutr. Metab. 2022, 14, 20–31. [Google Scholar] [CrossRef]
- Infante, M.; Leoni, M.; Caprio, M.; Fabbri, A. Long-term metformin therapy and vitamin B12 deficiency: An association to bear in mind. World J. Diabetes 2021, 12, 916. [Google Scholar] [CrossRef]
- Laraqui, A.; Allami, A.; Carrié, A.; Raisonnier, A.; Coiffard, A.S.; Benkouka, F.; Bendriss, A.; Benjouad, A.; Bennouar, N.; El Kadiri, N.; et al. Relation between plasma homocysteine, gene polymorphisms of homocysteine metabolism-related enzymes, and angiographically proven coronary artery disease. Eur. J. Intern. Med. 2007, 18, 474–483. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H.; Wang, X.; Wei, B.; Wu, Z.; Chen, S.; Wang, B.; Huang, H.; Jin, L. Association of homocysteine with IVF/ICSI outcomes stratified by MTHFR C677T polymorphisms: A prospective cohort study. Reprod. BioMedicine Online 2021, 43, 52–61. [Google Scholar] [CrossRef]
- Nefic, H.; Mackic-Djurovic, M.; Eminovic, I. The Frequency of the 677C>T and 1298A>C Polymorphisms in the Methylenetetrahydrofolate Reductase (MTHFR) Gene in the Population. Med. Arch. 2018, 72, 164–169. [Google Scholar] [CrossRef]
- Caldeira-Araújo, H.; Ramos, R.; Florindo, C.; Rivera, I.; Castro, R.; Tavares de Almeida, I. Homocysteine Metabolism in Children and Adolescents: Influence of Age on Plasma Biomarkers and Correspondent Genotype Interactions. Nutrients 2019, 11, 646. [Google Scholar] [CrossRef]
- Cordaro, M.; Siracusa, R.; Fusco, R.; Cuzzocrea, S.; Di Paola, R.; Impellizzeri, D. Involvements of Hyperhomocysteinemia in Neurological Disorders. Metabolites 2021, 11, 37. [Google Scholar] [CrossRef]
- Lai, W.K.; Kan, M.Y. Homocysteine-Induced Endothelial Dysfunction. Ann. Nutr. Metab. 2015, 67, 2335. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Miller, J.W. Folate deficiency beyond megaloblastic anemia: Hyperhomocysteinemia and other manifestations of dysfunctional folate status. Semin. Hematology 1999, 36, 47–64. [Google Scholar]
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Zappulo, F.; Tondolo, F.; Cappuccilli, M.; La Manna, G. Folic Acid and Vitamin B12 Administration in CKD, Why Not? Nutrients 2019, 11, 383. [Google Scholar] [CrossRef]
- Rondanelli, M.; Faliva, M.A.; Barrile, G.C.; Cavioni, A.; Mansueto, F.; Mazzola, G.; Oberto, L.; Patelli, Z.; Pirola, M.; Tartara, A.; et al. Nutrition, Physical Activity, and Dietary Supplementation to Prevent Bone Mineral Density Loss: A Food Pyramid. Nutrients 2022, 14, 74. [Google Scholar] [CrossRef]
- Selhub, J.; Jacques, P.F.; Wilson, P.W.; Rush, D.; Rosenberg, I.H. Vitamin status and intake as primary determinants of homocysteinemia in an elderly population. Jama 1993, 270, 2693–2698. [Google Scholar] [CrossRef]
- Herrmann, W.; Quast, S.; Ullrich, M.; Schultze, H.; Bodis, M.; Geisel, J. Hyperhomocysteinemia in high-aged subjects: Relation of B-vitamins, folic acid, renal function and the methylenetetrahydrofolate reductase mutation. Atherosclerosis 1999, 144, 91–101. [Google Scholar] [CrossRef]
- De Koning, E.J.; Van der Zwaluw, N.L.; Van Wijngaarden, J.P.; Sohl, E.; Brouwer-Brolsma, E.M.; Van Marwijk, H.W.J.; Enneman, A.W.; Swart, K.M.A.; Van Dijk, S.C.; Ham, A.C.; et al. Effects of Two-Year Vitamin B12 and Folic Acid Supplementation on Depressive Symptoms and Quality of Life in Older Adults with Elevated Homocysteine Concentrations: Additional Results from the B-PROOF Study, an RCT. Nutrients 2016, 8, 748. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Pang, H.; Guo, H.; Zhang, M.; He, J.; Yan, Y.; Niu, Q.; Muratbek; Rui, D.; Li, S.; et al. Ethnic Differences in the Prevalence of High Homocysteine Levels Among Low-Income Rural Kazakh and Uyghur Adults in Far Western China and Its Implications for Preventive Public Health. Int. J. Environ. Res. Public Health 2015, 12, 5373–5385. [Google Scholar] [CrossRef]
- Bourgonje, A.R.; Abdulle, A.E.; Al-Rawas, A.M.; Al-Maqbali, M.; Al-Saleh, M.; Enriquez, M.B.; Al-Siyabi, S.; Al-Hashmi, K.; Al-Lawati, I.; Bulthuis, M.L.C.; et al. Systemic Oxidative Stress Is Increased in Postmenopausal Women and Independently Associates with Homocysteine Levels. Int. J. Mol. Sci. 2020, 21, 314. [Google Scholar] [CrossRef]
- Pushpakumar, S.; Kundu, S.; Sen, U. Endothelial dysfunction: The link between homocysteine and hydrogen sulfide. Curr. Med. Chem. 2014, 21, 3662–3672. [Google Scholar] [CrossRef]
- Van Guldener, C.O.E.N.; Stehouwer, C. Hyperhomocysteinemia, vascular pathology, and endothelial dysfunction. In Seminars in Thrombosis and Hemostasis; Thieme Medical Publishers, Inc.: New York, NY, USA, 2000; pp. 281–290. [Google Scholar]
- Den Heijer, M.; Lewington, S.; Clarke, R. Homocysteine, MTHFR and risk of venous thrombosis: A meta-analysis of published epidemiological studies. J. Thromb. Haemost. 2005, 3, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Dayal, S.; Lentz, S.R. ADMA and hyperhomocysteinemia. Vasc. Med. 2005, 10 (Suppl. S2), S27–S33. [Google Scholar] [CrossRef] [PubMed]
- Jamison, D.T.; Breman, J.G.; Measham, A.R.; Alleyne, G.; Claeson, M.; Evans, D.B.; Jha, P.; Mills, A.; Musgrove, P. Disease Control Priorities in Developing Countries; Oxford University Press: New York, NY, USA, 2006. [Google Scholar]
- De Goma, E.M.; Knowles, J.W.; Angeli, F.; Budoff, M.J.; Rader, D.J. The evolution and refinement of traditional risk factors for cardiovascular disease. Cardiol. Rev. 2012, 20, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Robenek, H.; Severs, N.J. Endocytosis of lipoproteins and cholesterol homeostasis. In Cell Interaction in Atherosclerosisosis; CRC Press: Boca Raton, FL, USA, 1992; pp. 288–292. [Google Scholar]
- McCully, K.S. Homocysteine and the pathogenesis of atherosclerosis. Expert Rev. Clin. Pharmacol. 2015, 8, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Alul, R.H.; Wood, M.; Longo, J.; Marcotte, A.L.; Campione, A.L.; Moore, M.K.; Lynch, S.M. Vitamin C protects low-density lipoprotein from homocysteine-mediated oxidation. Free. Radic. Biol. Med. 2003, 34, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Nardulli, M.; Durlach, V.; Pepe, G.; Anglés-Cano, E. Mechanism for the ho-mocysteine-enhanced antifibrinolytic potential of lipoprotein (a) in human plasma. Thromb. Haemost. 2005, 94, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, S.; Morrow, J.D.; Roberts, L.J.; Alfthan, G.; Alho, H.; Nyyssönen, K.; Salonen, J.T. Enhanced in vivo lipid peroxidation at elevated plasma total homocysteine levels. Arterioscler. Thromb. Vasc. Biol. 1999, 19, 1263–1266. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fava, F.; Ulaszewska, M.M.; Scholz, M.; Stanstrup, J.; Nissen, L.; Mattivi, F.; Tuohy, K.M. Impact of wheat aleurone on biomarkers of cardiovascular disease, gut microbiota and metabolites in adults with high body mass index: A double-blind, placebo-controlled, randomized clinical trial. Eur. J. Nutr. 2022, 61, 3. [Google Scholar] [CrossRef]
- Fonseca, V.; Guba, S.C.; Fink, L.M. Hyperhomocysteinemia and the endocrine system: Implications for atherosclerosis and thrombosis. Endocr. Rev. 1999, 20, 738–759. [Google Scholar] [CrossRef]
- Nicoll, R.; Howard, J.M.; Henein, M.Y. A review of the effect of diet on cardiovascular calcification. Int. J. Mol. Sci. 2015, 16, 8861–8883. [Google Scholar] [CrossRef]
- Meleady, R.A.; Graham, I.M. Homocysteine as a risk factor for coronary artery disease. Eur. J. Cardiovasc. Prev. Rehabil. 1995, 2, 216–221. [Google Scholar] [CrossRef]
- Pate, M.; Damarla, V.; Chi, D.S.; Negi, S.; Krishnaswamy, G. Endothelial cell biology: Role in the inflammatory response. Adv. Clin. Chem. 2010, 52, 109–130. [Google Scholar] [PubMed]
- Singh, R.B.; Mengi, S.A.; Xu, Y.J.; Arneja, A.S.; Dhalla, N.S. Pathogenesis of atherosclerosis: A multifactorial process. Exp. Clin. Cardiol. 2002, 7, 40. [Google Scholar] [PubMed]
- Zou, C.G.; Banerjee, R. Homocysteine and redox signaling. Antioxid. Redox Signal. 2005, 7, 547–559. [Google Scholar] [CrossRef]
- Thambyrajah, J.; Townend, J.N. Homocysteine and atherothrombosis—Mechanisms for injury. Eur. Heart J. 2000, 21, 967–974. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef]
- Austin, R.; Lentz, S.; Werstuck, G. Role of hyperhomocysteinemia in endothelial dysfunction and atherothrombotic disease. Cell Death Differ. 2004, 11 (Suppl. S1), S56–S64. [Google Scholar] [CrossRef]
- Zaghloul, A.; Iorgoveanu, C.; Desai, A.; Balakumaran, K.; Chen, K. Methylenetetrahydrofolate reductase polymorphism and premature coronary artery disease. Cureus 2019, 11, e5014. [Google Scholar] [CrossRef]
- Raghubeer, S.; Matsha, T.E. Methylenetetrahydrofolate (MTHFR), the One-Carbon Cycle, and Cardiovascular Risks. Nutrients 2021, 13, 4562. [Google Scholar] [CrossRef]
- Shih, C.C.; Shih, Y.L.; Chen, J.Y. The association between homocysteine levels and cardiovascular disease risk among middle-aged and elderly adults in Taiwan. BMC Cardiovasc. Disord. 2021, 21, 191. [Google Scholar] [CrossRef]
- Miao, L.; Deng, G.X.; Yin, R.X.; Nie, R.J.; Yang, S.; Wang, Y.; Li, H. No causal effects of plasma homocysteine levels on the risk of coronary heart disease or acute myocardial infarction: A Mendelian randomization study. Eur. J. Prev. Cardiol. 2021, 28, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Chen, S.; Yang, F.; Wang, Y.; Zhang, K.; Fu, G.; Zhang, W. The impact of homocysteine on the risk of coronary artery diseases in individuals with diabetes: A Mendelian randomization study. Acta Diabetol. 2021, 58, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Liu, Y.; Wang, H. Clinical Evaluation Tool for Vascular Health–Endothelial Function and Cardiovascular Disease Management. Cells 2022, 11, 3363. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Liu, S.; Cheng, L.; Huang, T.; Guo, H.; Wang, D.; Xia, M.; Ling, W.; Xiao, Y. Betaine Supplementation Attenuates S-Adenosylhomocysteine Hydrolase-Deficiency-Accelerated Atherosclerosis in Apolipoprotein E-Deficient Mice. Nutrients 2022, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical Insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520962230. [Google Scholar] [CrossRef]
- Ponti, G.; Ruini, C.; Tomasi, A. Homocysteine as a potential predictor of cardiovascular risk in patients with COVID-19. Med. Hypotheses 2020, 143, 109859. [Google Scholar] [CrossRef]
- Yang, Z.; Shi, J.; He, Z.; Lü, Y.; Xu, Q.; Ye, C.; Chen, S.; Tang, B.; Yin, K.; Lu, Y.; et al. Predictors for imaging progression on chest CT from coronavirus disease 2019 (COVID-19) patients. Aging (Albany NY) 2020, 12, 6037–6048. [Google Scholar] [CrossRef]
- Ponti, G.; Roli, L.; Oliva, G.; Manfredini, M.; Trenti, T.; Kaleci, S.; Ianella, R.; Balzano, B.; Coppola, A.; Fiorentino, G.; et al. Homocysteine (Hcy) assessment to predict outcomes of hospitalized COVID-19 patients: A multicenter study on 313 COVID-19 patients. Clin. Chem. Lab. Med. (CCLM) 2021, 59, e354–e357. [Google Scholar] [CrossRef]
- Smirnova, O.; Matvienko, O.; Korsakova, N.; Lerner, A.; Shvedova, T.; Golovina, O.; Papayan, L. Correlation of coagulation parameters with prognosis of COVID-19. Res. Pract. Thromb. Haemost. 2021, 5, 618929. [Google Scholar] [CrossRef]
- Ali, H.Y.; Al-bayati, M.A.; Alqaraghuli, H.A. Estimation of Serum Amyloid A, CRP, Homocysteine, and Neutrophils to Lymphocyte Ratio as an Inflammatory biomarker and their correlation with Cardiovascular risk in a sample of Iraqi COVID-19 Patients. J. Univ. Shanghai Sci. Technol. 2021, 23, 1475. [Google Scholar] [CrossRef]
- Petelina, T.; Musikhina, N.; Avdeeva, K.; Leonovich, S.; Gapon, L.; Gorbatenko, E.; Sharoyan, Y.; Zueva, E.; Yaroslavskaya, E.; Gultyaeva, E.; et al. Prospective analysis of inflammatory response markers, endothelial dysfunction and hemostasis parameters in COVID-19 associated pneumonia patients with and without type 2 diabetes mellitus. Eur. Heart J. 2021, 42, ehab724–3394. [Google Scholar] [CrossRef]
- Fouda, E.M.; Wahba, N.S.; Elsharawy, A.I.; Ishak, S.R. Serum homocysteine level in pediatric patients with COVID-19 and its correlation with the disease severity. Pediatr. Pulmonol. 2022, 57, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Sabah Khalid, S.; Mohamed Ali, Z.; Faris Raheem, M. Serum Levels of Homocysteine, Troponin-I, and High Sensitive C-Reactive Protein in Iraqi COVID-19 patients. J. Contemp. Med. Sci. 2022, 8. [Google Scholar] [CrossRef]
- Khidoyatovna, I.F.; Chutbaevna, K.Z.; Agzamovna, B.S. Relationship between mthfr gene rs1801133 and rs1801131 polymorphisms with disease severity of covid-19 and homocystein levels in uzbek patients. J. Pharm. Negat. Results 2022, 13, 1879–1888. [Google Scholar]
- Keskin, A.; U Ustun, G.; Aci, R.; Duran, U. Homocysteine as a marker for predicting disease severity in patients with COVID-19. Biomark. Med. 2022, 16, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Povlsen, A.L.; Grimm, D.; Wehland, M.; Infanger, M.; Krüger, M. The Vasoactive Mas Receptor in Essential Hypertension. J. Clin. Med. 2020, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Griendling, K.K. Angiotensin II cell signaling: Physiological and pathological effects in the cardiovascular system. Am. J. Physiol. Cell Physiol. 2007, 292, C82–C97. [Google Scholar] [CrossRef]
- Yang, J.; Petitjean, S.J.L.; Koehler, M.; Zhang, Q.; Dumitru, A.C.; Chen, W.; Derclaye, S.; Vincent, S.P.; Soumillion, P.; Alsteens, D. Molecular interaction and inhibition of SARS-CoV-2 binding to the ACE2 receptor. Nat. Commun. 2020, 11, 4541. [Google Scholar] [CrossRef]
- Oz, M.; Lorke, D.E. Multifunctional angiotensin converting enzyme 2, the SARS-CoV-2 entry receptor, and critical appraisal of its role in acute lung injury. Biomed. Pharmacother. 2021, 136, 111193. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Thepmankorn, P.; Bach, J.; Lasfar, A.; Zhao, X.; Souayah, S.; Chong, Z.Z.; Souayah, N. Cytokine storm induced by SARS-CoV-2 infection: The spectrum of its neurological manifestations. Cytokine 2021, 138, 155404. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yu, B.; Liu, Z.; Li, J.; Ma, M.; Wang, Y.; Zhu, M.; Yin, H.; Wang, X.; Fu, Y.; et al. Homocysteine directly interacts and activates the angiotensin II type I receptor to aggravate vascular injury. Nat. Commun. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Wang, L.; Si, X.; Tian, J.L.; Zhang, Y.; Gui, H.L.; Li, B.; Tan, D.H. Current progress on the mechanisms of hyperhomocysteinemia-induced vascular injury and use of natural polyphenol compounds. Eur. J. Pharmacol. 2021, 905, 174168. [Google Scholar] [CrossRef] [PubMed]
- Chubarov, A.S. Homocysteine Thiolactone: Biology and Chemistry. Encyclopedia 2021, 1, 445–459. [Google Scholar] [CrossRef]
- Nyui, M.; Shoji, Y.; Ueno, M.; Nakanishi, I.; Matsumoto, K.I. Reduction of molecular oxygen by redox active thiols: Comparison of glutathione, N-acetylcysteine, cysteine, and homocysteine. J. Clin. Biochem. Nutr. 2019, 65, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.G.; Carpenter, M.A.; Kusek, J.W.; Levey, A.S.; Hunsicker, L.; Pfeffer, M.A.; Selhub, J.; Jacques, P.F.; Cole, E.; Gravens-Mueller, L.; et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: Primary results from the Folic Acid for Vascular Outcome Reduction in Transplantation trial. Circulation 2011, 123, 1763–1770. [Google Scholar] [CrossRef]
- Karst, M.; Hollenhorst, J.; Achenbach, J. Life-threatening course in coronavirus disease 2019 (COVID-19): Is there a link to methylenetetrahydrofolic acid reductase (MTHFR) polymorphism and hyperhomocysteinemia? Med. Hypotheses 2020, 144, 110234. [Google Scholar] [CrossRef]
- Lupi-Herrera, E.; Soto-López, M.E.; Lugo-Dimas, A.J.; Núñez-Martínez, M.E.; Gamboa, R.; Huesca-Gómez, C.; Sierra-Galán, L.M.; Guarner-Lans, V. Polymorphisms C677T and A1298C of MTHFR Gene: Homocysteine Levels and Prothrombotic Biomarkers in Coronary and Pulmonary Thromboembolic Disease. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029618780344. [Google Scholar] [CrossRef]
- Ponti, G.; Pastorino, L.; Manfredini, M.; Ozben, T.; Oliva, G.; Kaleci, S.; Iannella, R.; Tomasi, A. COVID-19 spreading across world correlates with C677T allele of the methylenetetrahydrofolate reductase (MTHFR) gene prevalence. J. Clin. Lab. Anal. 2021, 35, e23798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).