Triglyceride and Glucose Index Predicts Acute Coronary Syndrome in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Included
2.2. Variables at AAV Diagnosis
2.3. Variables during Follow-Up
2.4. Definition of ACS
2.5. Equation of the TyG Index
2.6. Classification as MetS
2.7. Optimal Cut-Off of the TyG Index
2.8. Statistical Analyses
3. Results
3.1. Characteristics
3.2. Correlation of the TyG Index with Continuous Variables
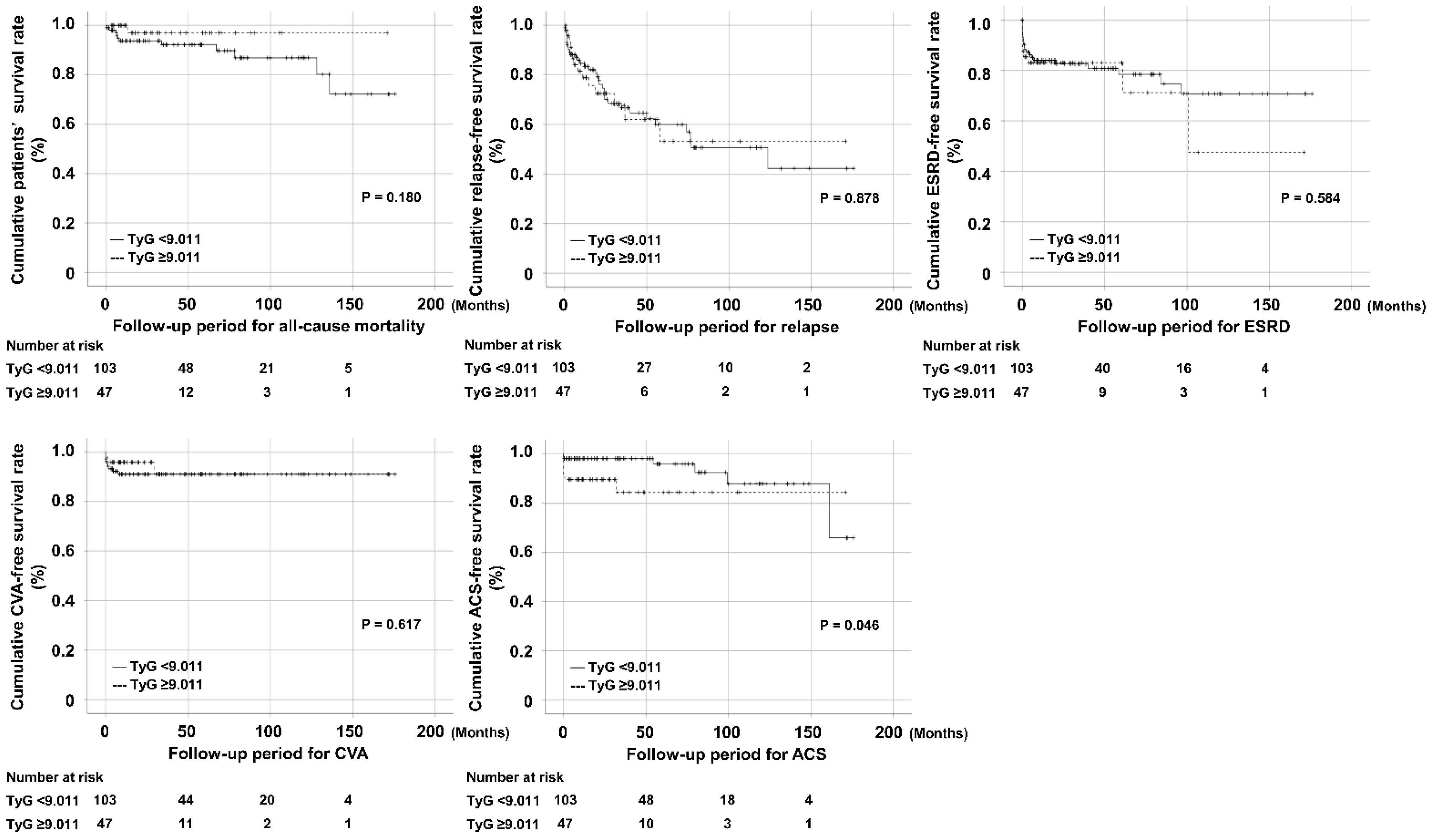
3.3. Comparison of Cumulative Survival Rates According to the TyG Index
3.4. Cox Hazards Model Analyses for ACS Occurrence
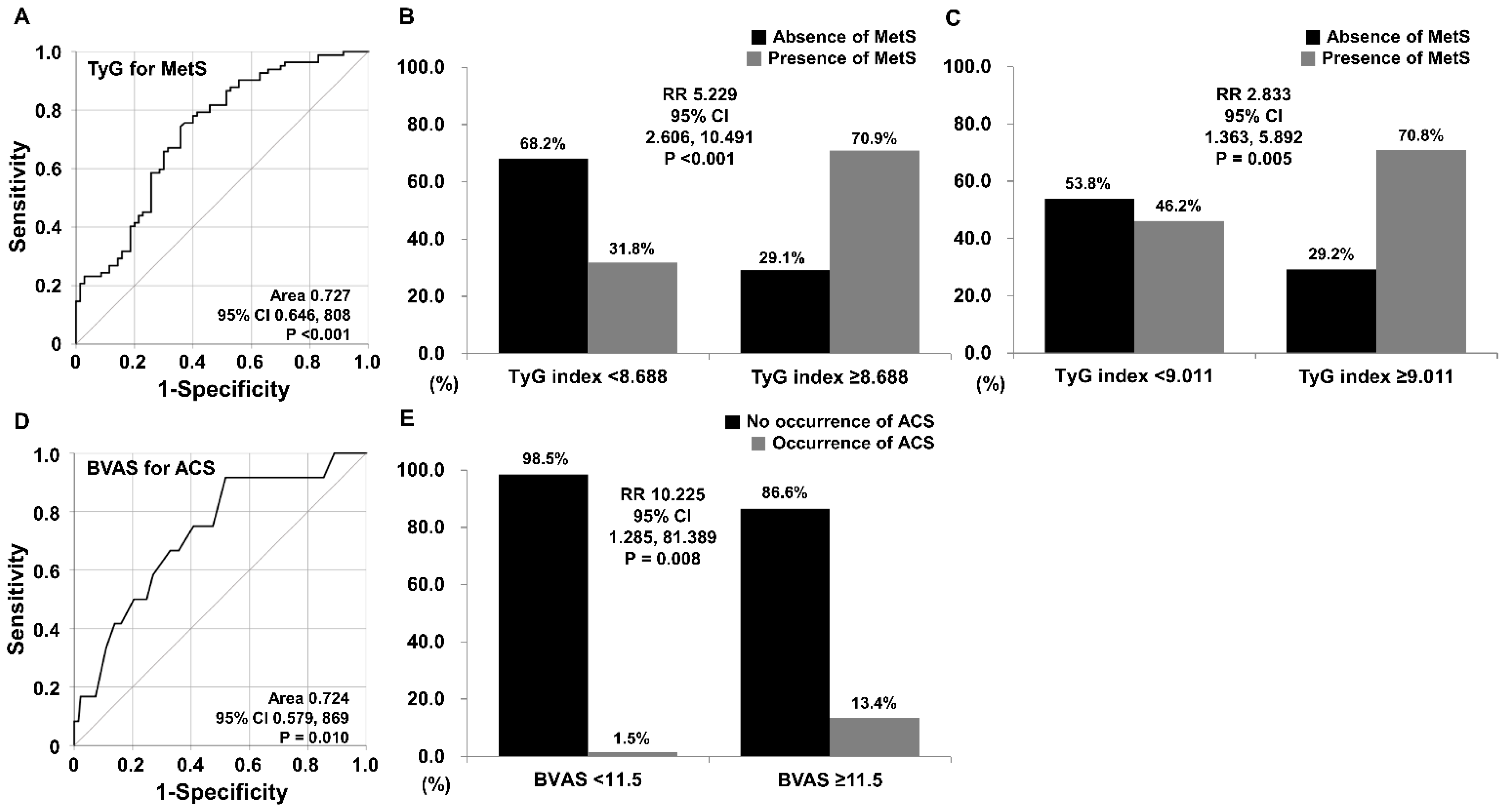
3.5. TyG Index Estimating MetS
3.6. Logistic Regression Analyses for MetS
3.7. BVAS Anticipating ACS Occurrence
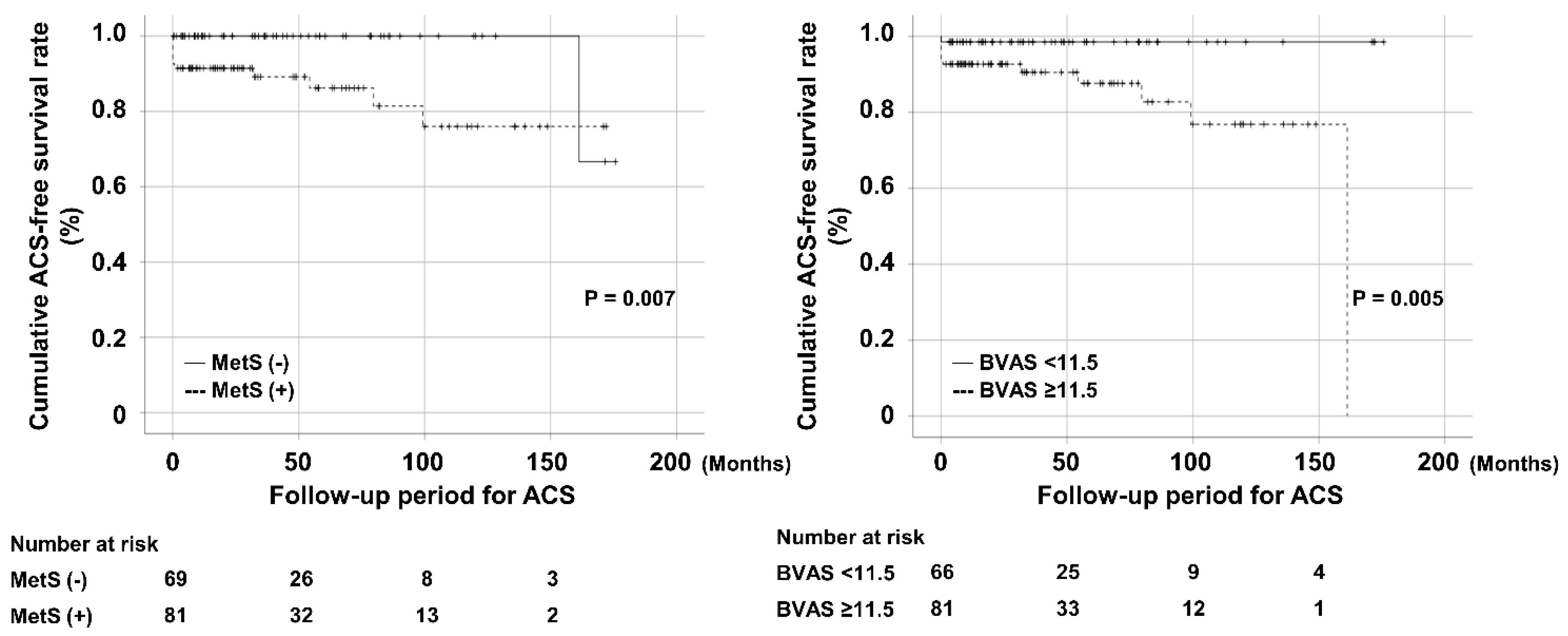
3.8. Comparison of Cumulative Survival Rates According to MetS and BVAS
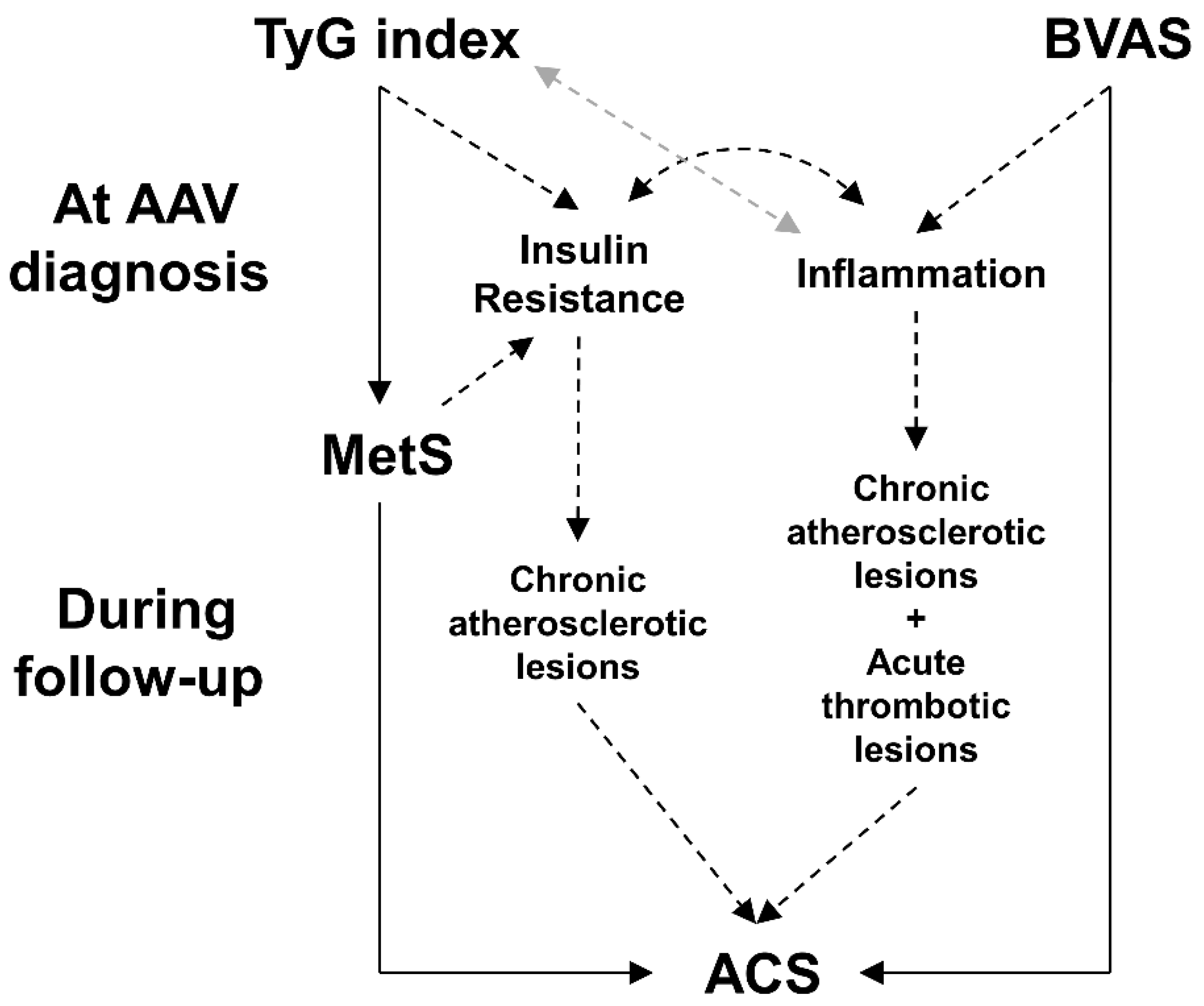
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kitching, A.R.; Anders, H.-J.; Basu, N.; Brouwer, E.; Gordon, J.; Jayne, D.R.; Kullman, J.; Lyons, P.A.; Merkel, P.A.; Savage, C.O.S.; et al. ANCA-associated vasculitis. Nat. Rev. Dis. Prim. 2020, 6, 71. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J. Pathogenesis of antineutrophil cytoplasmic autoantibody-mediated disease. Nat. Rev. Rheumatol. 2014, 10, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Watts, R.; Lane, S.; Hanslik, T.; Hauser, T.; Hellmich, B.; Koldingsnes, W.; Mahr, A.; Segelmark, M.; Cohen-Tervaert, J.W.; Scott, D. Development and validation of a consensus methodology for the classification of the ANCA-associated vasculitides and polyarteritis nodosa for epidemiological studies. Ann. Rheum. Dis. 2007, 66, 222–227. [Google Scholar] [CrossRef]
- Mukhtyar, C.; Lee, R.; Brown, D.; Carruthers, D.; Dasgupta, B.; Dubey, S.; Flossmann, O.; Hall, C.; Hollywood, J.; Jayne, D.; et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann. Rheum. Dis. 2009, 68, 1827–1832. [Google Scholar] [CrossRef] [Green Version]
- Millet, A.; Pederzoli-Ribeil, M.; Guillevin, L.; Witko-Sarsat, V.; Mouthon, L. Antineutrophil cytoplasmic antibody-associated vasculitides: Is it time to split up the group? Ann. Rheum. Dis. 2013, 72, 1273–1279. [Google Scholar] [CrossRef] [Green Version]
- Lee, L.E.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.; Lee, S. Clinical significance of large unstained cell count in estimating the current activity of antineutrophil cytoplasmic antibody-associated vasculitis. Int. J. Clin. Pract. 2021, 75, e14512. [Google Scholar] [CrossRef]
- Singh, J.A. Infection versus cardiovascular disease as leading causes of hospitalisations and associated mortality in vasculitis in the U.S.: A national study. Clin. Exp. Rheumatol. 2021, 39 (Suppl. 129), 56–61. [Google Scholar] [CrossRef]
- Sánchez-García, A.; Rodríguez-Gutiérrez, R.; Mancillas-Adame, L.; Gonzalez-Nava, V.; González-Colmenero, A.D.; Solis, R.C.; Álvarez-Villalobos, N.A.; González-González, J.G. Diagnostic Accuracy of the Triglyceride and Glucose Index for Insulin Resistance: A Systematic Review. Int. J. Endocrinol. 2020, 2020, 4678526. [Google Scholar] [CrossRef]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Khan, S.H.; Sobia, F.; Niazi, N.K.; Manzoor, S.M.; Fazal, N.; Ahmad, F. Metabolic clustering of risk factors: Evaluation of Triglyceride-glucose index (TyG index) for evaluation of insulin resistance. Diabetol. Metab. Syndr. 2018, 10, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Q.; Zhou, D.; Li, Y.; Wang, Y.; Xu, S.-C.; Zhao, X.-H. The Triglyceride-Glucose Index Predicts Coronary Artery Disease Severity and Cardiovascular Outcomes in Patients with Non-ST-Segment Elevation Acute Coronary Syndrome. Dis. Markers 2019, 2019, 6891537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Dong, L.; Shao, Q.; Cheng, Y.; Lv, S.; Sun, Y.; Shen, H.; Wang, Z.; Zhou, Y.; Liu, X. Triglyceride glucose index for predicting cardiovascular outcomes after percutaneous coronary intervention in patients with type 2 diabetes mellitus and acute coronary syndrome. Cardiovasc. Diabetol. 2020, 19, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.H.; Hoffman, G.S.; Merkel, P.A.; Min, Y.I.; Uhlfelder, M.L.; Hellmann, D.B.; Specks, U.; Allen, N.B.; Davis, J.C.; Spiera, R.F.; et al. A disease- specific activity index for Wegener’s granulomatosis: Modification of the Birmingham Vasculitis Activity Score. International Network for the Study of the Systemic Vasculitides (INSSYS). Arthritis Rheum. 2001, 44, 912–920. [Google Scholar] [CrossRef]
- Guillevin, L.; Pagnoux, C.; Seror, R.; Mahr, A.; Mouthon, L.; Le Toumelin, P.; French Vasculitis Study Group (FVSG). The Five-Factor Score Revisited: Assessment of prognoses of systemic necrotizing vasculitides based on the French Vasculitis Study Group (FVSG) cohort. Medicine 2011, 90, 19–27. [Google Scholar] [CrossRef]
- Levey, A.S.; de Jong, P.E.; Coresh, J.; El Nahas, M.; Astor, B.C.; Matsushita, K.; Gansevoort, R.T.; Kasiske, B.L.; Eckardt, K.-U. The definition, classification, and prognosis of chronic kidney disease: A KDIGO Controversies Conference report. Kidney Int. 2011, 80, 17–28. [Google Scholar] [CrossRef] [Green Version]
- Pagidipati, N.J.; Peterson, E.D. Acute coronary syndromes in women and men. Nat. Rev. Cardiol. 2016, 13, 471–480. [Google Scholar] [CrossRef]
- Guerrero-Romero, F.; Simental-Mendía, L.E.; González-Ortiz, M.; Martínez-Abundis, E.; Ramos-Zavala, M.G.; Hernández-González, S.O.; Jacques-Camarena, O.; Rodríguez-Morán, M. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison with the Euglycemic-Hyperinsulinemic Clamp. J. Clin. Endocrinol. Metab. 2010, 95, 3347–3351. [Google Scholar] [CrossRef] [Green Version]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Huh, J.H.; Kang, D.R.; Jang, J.-Y.; Shin, J.-H.; Kim, J.Y.; Choi, S.; Cho, E.J.; Park, J.-S.; Sohn, I.S.; Jo, S.-H.; et al. Metabolic syndrome epidemic among Korean adults: Korean survey of Cardiometabolic Syndrome (2018). Atherosclerosis 2018, 277, 47–52. [Google Scholar] [CrossRef]
- Lee, S.-H.; Yang, H.K.; Ha, H.-S.; Lee, J.-H.; Kwon, H.-S.; Park, Y.-M.; Yim, H.-W.; Kang, M.-I.; Lee, W.-C.; Son, H.-Y.; et al. Changes in Metabolic Health Status Over Time and Risk of Developing Type 2 Diabetes: A Prospective Cohort Study. Medicine 2015, 94, e1705. [Google Scholar] [CrossRef] [PubMed]
- Mottillo, S.; Filion, K.B.; Genest, J.; Joseph, L.; Pilote, L.; Poirier, P.; Rinfret, S.; Schiffrin, E.L.; Eisenberg, M.J. The Metabolic Syndrome and Cardiovascular Risk: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henning, R.J. Type-2 diabetes mellitus and cardiovascular disease. Futur. Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Katakami, N. Mechanism of Development of Atherosclerosis and Cardiovascular Disease in Diabetes Mellitus. J. Atheroscler. Thromb. 2018, 25, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Nakase, M.; Yahagi, K.; Oshima, A.; Kikushima, H.; Ninomiya, K.; Tanaka, T.; Horiuchi, Y.; Asami, M.; Yuzawa, H.; Komiyama, K.; et al. Acute Coronary Syndrome Due to Myeloperoxidase-Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. JACC Cardiovasc. Interv. 2020, 13, e215–e216. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kwon, H.C.; Kang, M.I.; Park, Y.-B.; Park, J.Y.; Lee, S.-W. Increased prevalence rate of metabolic syndrome is an independent predictor of cardiovascular disease in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Rheumatol. Int. 2022, 42, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Hanley, A.J.G.; Williams, K.; Stern, M.P.; Haffner, S.M. Homeostasis Model Assessment of Insulin Resistance in Relation to the Incidence of Cardiovascular Disease: The San Antonio Heart Study. Diabetes Care 2002, 25, 1177–1184. [Google Scholar] [CrossRef] [Green Version]
- Vasques, A.C.J.; Novaes, F.S.; de Oliveira, M.D.S.; Souza, J.R.M.; Yamanaka, A.; Pareja, J.C.; Tambascia, M.A.; Saad, M.J.A.; Geloneze, B. TyG index performs better than HOMA in a Brazilian population: A hyperglycemic clamp validated study. Diabetes Res. Clin. Pract. 2011, 93, e98–e100. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The Diabetes Mellitus–Atherosclerosis Connection: The Role of Lipid and Glucose Metabolism and Chronic Inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [Green Version]
| Variables | Values |
|---|---|
| At diagnosis | |
| Basic clinical data | |
| Age (years) | 57.2 (20.9) |
| Male sex (N, (%)) | 50 (32.9) |
| BMI (kg/m2) | 22.1 (4.6) |
| AAV subtypes (N, (%)) | |
| MPA | 84 (55.3) |
| GPA | 35 (23.0) |
| EGPA | 33 (21.7) |
| ANCA positivity (N, (%)) | |
| MPO-ANCA (or P-ANCA) positive | 102 (67.1) |
| PR3-ANCA (or C-ANCA) positive | 22 (14.5) |
| Both ANCA positive | 5 (3.3) |
| ANCA positive | 119 (78.3) |
| AAV-specific indices | |
| BVAS | 12.0 (12.0) |
| FFS | 1.0 (2.0) |
| Comorbidities (N, (%)) | |
| T2DM | 44 (28.9) |
| Hypertension | 46 (30.3) |
| Laboratory results | |
| White blood cell count (/mm3) | 8640.0 (6835.0) |
| Haemoglobin (g/dL) | 11.4 (4.0) |
| Platelet count (×1000/mm3) | 286.0 (158.0) |
| Blood urea nitrogen (mg/dL) | 18.9 (23.0) |
| Serum creatinine (mg/dL) | 1.0 (1.4) |
| Serum albumin (g/dL) | 3.6 (0.9) |
| Total cholesterol (mg/dL) | 171.5 (65.0) |
| HDL-cholesterol (mg/dL) | 48.0 (25.0) |
| LDL-cholesterol (mg/dL) | 91.4 (47.0) |
| Acute phase reactants | |
| ESR (mm/h) | 56.0 (66.0) |
| CRP (mg/L) | 7.0 (66.7) |
| TyG index-related variables | |
| Fasting glucose (mg/dL) | 103.0 (36.0) |
| Triglyceride (mg/dL) | 114.5 (74.0) |
| TyG index | 8.8 (0.7) |
| During follow-up | |
| Poor prognoses (N, (%)) | |
| All-cause mortality | 12 (7.9) |
| Relapse | 48 (31.6) |
| ESKD | 31 (20.4) |
| CVA | 12 (7.9) |
| ACS | 12 (7.9) |
| Follow-up period for each poor prognosis (months) | |
| All-cause mortality | 33.4 (66.0) |
| Relapse | 20.1 (42.8) |
| ESKD | 20.8 (62.1) |
| CVA | 30.0 (60.8) |
| ACS | 32.6 (63.4) |
| Medications (N, (%)) | |
| Glucocorticoids | 144 (94.7) |
| Cyclophosphamide | 78 (51.3) |
| Rituximab | 24 (15.8) |
| Mycophenolate mofetil | 23 (15.1) |
| Azathioprine | 76 (50.0) |
| Tacrolimus | 11 (7.2) |
| Methotrexate | 15 (9.9) |
| Plasma exchange | 10 (6.6) |
| Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Age | 1.024 | 0.978, 1.071 | 0.309 | |||
| Male sex | 4.933 | 1.403, 17.345 | 0.013 | 5.548 | 1.254, 24.541 | 0.024 |
| BMI | 1.131 | 0.940, 1.359 | 0.192 | |||
| MPA | 1.595 | 0.475, 5.354 | 0.450 | |||
| GPA | 0.730 | 0.159, 3.340 | 0.685 | |||
| EGPA | 0.674 | 0.144, 3.151 | 0.616 | |||
| MPO-ANCA (or P-ANCA) positive | 2.930 | 0.622, 13.804 | 0.174 | |||
| PR3-ANCA (or C-ANCA) positive | 1.954 | 0.249, 15.314 | 0.524 | |||
| BVAS | 1.120 | 1.033, 1.215 | 0.006 | 1.099 | 0.972, 1.242 | 0.131 |
| FFS | 1.936 | 1.147, 3.265 | 0.013 | 1.357 | 0.722, 2.550 | 0.343 |
| T2DM | 4.255 | 1.245, 14.537 | 0.021 | 1.583 | 0.411, 6.093 | 0.504 |
| Hypertension | 3.086 | 0.971, 9.805 | 0.056 | 2.748 | 0.796, 9.485 | 0.110 |
| White blood cell count | 1.000 | 1.000, 1.000 | 0.320 | |||
| Haemoglobin | 0.807 | 0.615, 1.059 | 0.123 | |||
| Platelet count | 1.000 | 0.997, 1.004 | 0.842 | |||
| Blood urea nitrogen | 1.013 | 1.000, 1.027 | 0.054 | 0.997 | 0.978, 1.017 | 0.800 |
| Serum creatinine | 1.142 | 0.911, 1.432 | 0.249 | |||
| Serum albumin | 0.600 | 0.273, 1.320 | 0.204 | |||
| Total cholesterol | 1.005 | 0.992, 1.017 | 0.456 | |||
| HDL-cholesterol | 0.986 | 0.954, 1.019 | 0.401 | |||
| LDL-cholesterol | 1.007 | 0.993, 1.020 | 0.318 | |||
| ESR | 1.007 | 0.992, 1.022 | 0.350 | |||
| CRP | 1.005 | 0.997, 1.013 | 0.249 | |||
| TyG ≥ 9.011 | 3.054 | 0.959, 9.726 | 0.059 | 2.312 | 0.576, 9.281 | 0.237 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, P.G.; Pyo, J.Y.; Ahn, S.S.; Song, J.J.; Park, Y.-B.; Huh, J.H.; Lee, S.-W. Triglyceride and Glucose Index Predicts Acute Coronary Syndrome in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics 2022, 12, 1486. https://doi.org/10.3390/diagnostics12061486
Park PG, Pyo JY, Ahn SS, Song JJ, Park Y-B, Huh JH, Lee S-W. Triglyceride and Glucose Index Predicts Acute Coronary Syndrome in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics. 2022; 12(6):1486. https://doi.org/10.3390/diagnostics12061486
Chicago/Turabian StylePark, Pil Gyu, Jung Yoon Pyo, Sung Soo Ahn, Jason Jungsik Song, Yong-Beom Park, Ji Hye Huh, and Sang-Won Lee. 2022. "Triglyceride and Glucose Index Predicts Acute Coronary Syndrome in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis" Diagnostics 12, no. 6: 1486. https://doi.org/10.3390/diagnostics12061486
APA StylePark, P. G., Pyo, J. Y., Ahn, S. S., Song, J. J., Park, Y.-B., Huh, J. H., & Lee, S.-W. (2022). Triglyceride and Glucose Index Predicts Acute Coronary Syndrome in Patients with Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Diagnostics, 12(6), 1486. https://doi.org/10.3390/diagnostics12061486






