Diagnosis of Human Leptospirosis: Comparison of Microscopic Agglutination Test with Recombinant LigA/B Antigen-Based In-House IgM Dot ELISA Dipstick Test and Latex Agglutination Test Using Bayesian Latent Class Model and MAT as Gold Standard
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Approval
2.2. Serum Sample Collection and Processing
2.3. Leptospiral Serovars and Strains Used in Microscopic Agglutination Test (MAT)
2.4. Microscopic Agglutination Test (MAT)
2.5. PCR Amplification and Cloning
2.6. Induction of Expression and Purification of Recombinant LigA/BCon1-5 Antigen
2.7. Recombinant LigA/BCon1-5-Based IgM Dot ELISA Dipstick Test
2.8. Recombinant LigA/BCon1-5 Based Latex Agglutination Test
2.9. Statistical Analysis
3. Results
3.1. Microscopic Agglutination Test
3.2. Recombinant LigA/BCon1-5 Antigen Expression
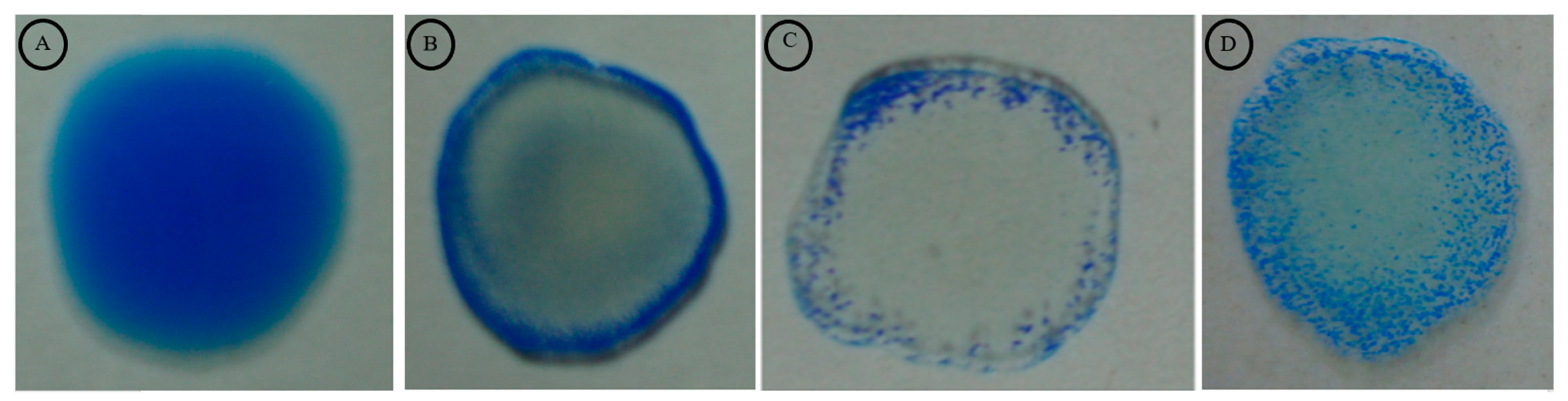
3.3. Recombinant LigA/BCon1-5-Based IgM Dot ELISA Dipstick Test
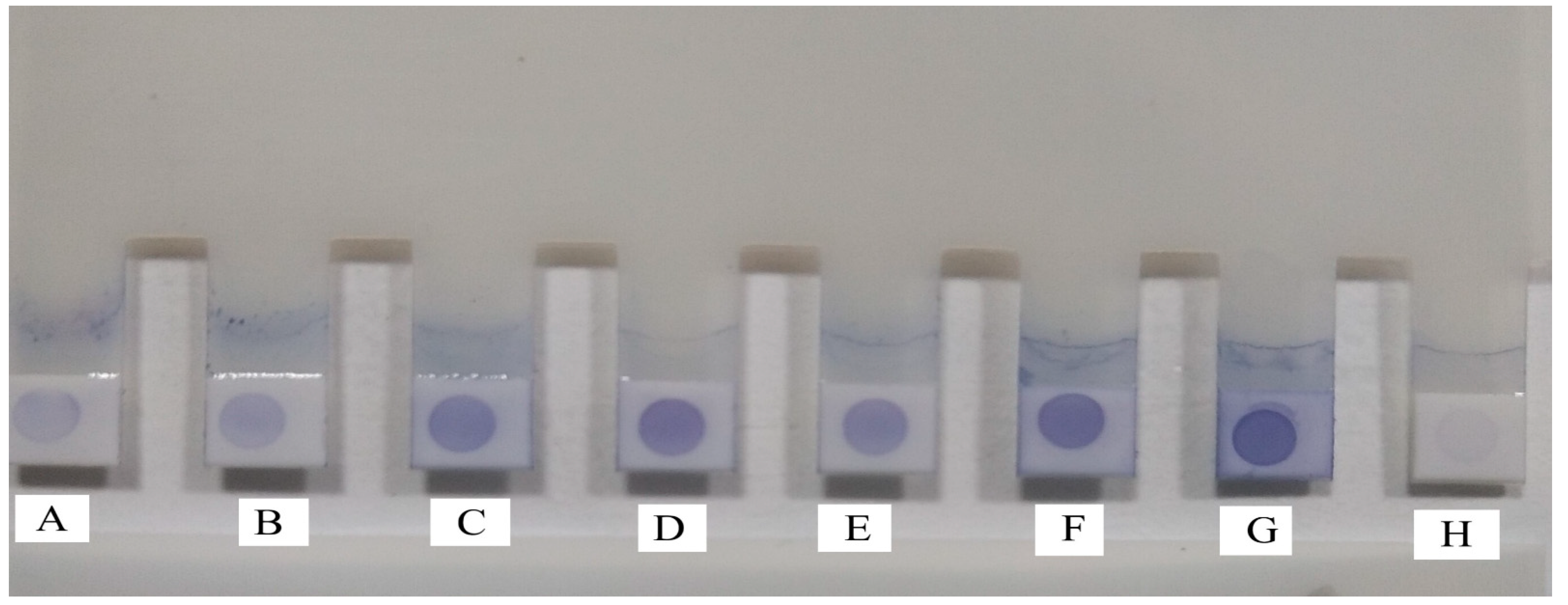
3.4. Latex Agglutination Test and Correlation between MAT Titre and LAT Score
3.5. Recombinant LigA/BCon1-5-Based IgM Dot ELISA Dipstick Test and Latex Agglutination Test Compared with MAT as Gold Standard
3.6. Bayesian Latent Class Modelling for MAT, Recombinant LigA/BCon1-5-Based IgM Dot-ELISA Dipstick Test and Latex Agglutination Test
4. Discussion
5. Conclusions and Further Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Bharti, A.R.; Nally, J.E.; Ricardi, J.N.; Matthaias, M.A.; Diaz, M.M.; Lovett, M.A.; Levett, P.N.; Gilman, R.H.; Willig, M.R.; Gutozzo, E.; et al. Leptospirosis: A zoonotic disease of global importance. Lancet Infect. Dis. 2003, 3, 757–771. [Google Scholar] [CrossRef]
- Sellors, P.; Watson, R.F.; Bate, R.; Bentham, G.L.; Haigh, K. Clinical Features and Severity of Leptospirosis Cases Reported in the Hawke’s Bay Region of New Zealand. J. Trop. Med. 2021, 2021, 5567081. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Leptospirosis Laboratory Manual. WCO India. 2007. Available online: https://apps.who.int/iris/handle/10665/205429 (accessed on 13 October 2021).
- Izurieta, R.; Galwankar, S.; Clem, A. Leptospirosis: The mysterious mimic. J. Emerg. Trauma Shock 2008, 1, 21–33. [Google Scholar]
- World Health Organization. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. Available online: https://www.who.int/publications/i/item/human-leptospirosis-guidance-for-diagnosis-surveillance-and-control (accessed on 13 October 2021).
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef]
- Torgerson, P.R.; Hagan, J.E.; Costa, F.; Calcagno, J.; Kane, M.; Martinez-Silveira, M.S.; Goris, M.G.A.; Stein, C.; Ko, A.I.; Abela-Ridder, B. Global Burden of Leptospirosis: Estimated in Terms of Disability Adjusted Life Years. PLoS Negl. Trop. Dis. 2015, 9, e0004122. [Google Scholar] [CrossRef] [PubMed]
- Day, N.; Calderwood, S.B.; Hall, K.K. Leptospirosis: Epidemiology, Microbiology, Clinical Manifestations, and Diagnosis. Available online: https://www.uptodate.com/contents/leptospirosis-epidemiology-microbiology-clinical-manifestations-and-diagnosis#:~:text=EPIDEMIOLOGY%20Leptospirosis%20is%20a%20widespread,no%20reliable%20global%20incidence%20figures (accessed on 24 May 2022).
- Reis, R.B.; Ribeiro, G.S.; Felzemburgh, R.D.; Santana, F.S.; Mohr, S.; Melendez, A.X.T.O.; Queiroz, A.; Santos, A.C.; Ravines, R.R.; Tassinari, W.S.; et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Negl. Trop. Dis. 2008, 2, e228. [Google Scholar] [CrossRef]
- Khalil, H.; Santana, R.; de Oliveira, D.; Palma, F.; Lustosa, R.; Eyre, M.T.; Carvalho-Pereira, T.; Reis, M.G.; Ko, A.I.; Diggle, P.J.; et al. Poverty, sanitation, and Leptospira transmission pathways in residents from four Brazilian slums. PLoS Negl. Trop. Dis. 2021, 15, e0009256. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Niloofa, R.; Fernando, N.; de Silva, N.L.; Karunanayake, L.; Wickramasinghe, H.; Dikmadugoda, N.; Premawansa, G.; Wickramasinghe, R.; de Silva, H.J.; Premawansa, S.; et al. Diagnosis of leptospirosis: Comparison between microscopic agglutination test, IgM-ELISA and IgM rapid immunochromatography test. PLoS ONE 2015, 10, e0129236. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Saleem, M.H.; McDonough, P.; McDonough, S.P.; Divers, T.J.; Chang, Y.F. Development of an Enzyme-Linked Immunosorbent Assay Using a Recombinant LigA Fragment Comprising Repeat Domains 4 to 7.5 as an Antigen for Diagnosis of Equine Leptospirosis. Clin. Vaccine Immunol. 2013, 20, 1143–1149. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rajapakse, S.; Rodrigo, C.; Handunnetti, S.M.; Fernando, S.D. Current immunological and molecular tools for leptospirosis: Diagnostics, vaccine design, and biomarkers for predicting severity. Ann. Clin. Microbiol. Antimicrob. 2015, 14, 2. [Google Scholar] [CrossRef]
- Cerqueira, G.M.; McBride, A.J.A.; Queiroz, A.; Pinto, L.S.; Silva, E.F.; Hartskeerl, R.A.; Reis, M.G.; Ko, A.I.; Dellagostin, O.A. Monitoring Leptospira strain collections: The need for quality control. Am. J. Trop. Med. Hyg. 2010, 82, 83–87. [Google Scholar] [CrossRef]
- Budihal, S.V.; Perwez, K. Leptospirosis diagnosis: Competancy of various laboratory tests. J. Clin. Diagn. Res. 2014, 8, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, T.; Subathra, M.; Phil, M.; Ramadass, P.; Ramaswamy, V. Rapid serodiagnosis of leptospirosis by latex agglutination test and flow-through assay. Indian J. Med. Microbiol. 2008, 26, 45–49. [Google Scholar] [CrossRef]
- Amutha, R.; Vasan, P. Evaluation of ELISA and latex agglutination assay with recombinant LipL32 protein for rapid serodiagnosis of bovine and human leptospirosis. Life Sci. Arch. 2017, 3, 940–946. [Google Scholar]
- Thongsukkaeng, K.; Boonyom, R. Development and evaluation of latex agglutination test coating with recombinant antigen, LipL32 for serodiagnosis of human leptospirosis. J. Genet. Eng. Biotechnol. 2018, 16, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Alamuri, A.; Vinod Kumar, K.; Varghese, B.; Palkhade, R.; Mahadeviah, S.N.; Chaudhari, S.; Roy, P.; Balamurugan, V. Evaluation of recombinant leptospiral surface antigen (Lsa27) lipoprotein for serodiagnosis of human leptospirosis by latex agglutination test. Indian J. Med. Microbiol. 2021, 39, 212–217. [Google Scholar] [CrossRef]
- Tansuphasiri, U.; Deepradit, S.; Phulsuksombati, D.; Tangkanakul, W. A test strip IgM dot-ELISA assay using leptospiral antigen of endemic strains for serodiagnosis of acute leptospirosis. J. Med. Assoc. Thail. 2005, 88, 391–398. [Google Scholar]
- Shekatkar, S.; Harish, B.N.; Parija, S.C. IgM Dot-ELISA Assay using prevalent Leptospira strain for diagnosis of leptospirosis. Int. J. Collab. Res. Intern. Med. Public Health 2010, 2, 338–346. [Google Scholar]
- Behera, S.K.; Sabarinath, T.; Mishra, P.K.K.; Deneke, Y.; Kumar, A.; ChandraSekar, S.; SenthilKumar, K.; Verma, M.; Ganesh, B.; Gurav, A.; et al. Immunoinformatic Study of Recombinant LigA/BCon1-5 Antigen and Evaluation of Its Diagnostic Potential in Primary and Secondary Binding Tests for Serodiagnosis of Porcine Leptospirosis. Pathogens 2021, 10, 1082. [Google Scholar] [CrossRef]
- Croda, J.; Ramos, J.G.; Matsunaga, J.; Queiroz, A.; Homma, A.; Riley, L.W.; Haake, D.A.; Reis, M.G.; Ko, A.I. Leptospira immunoglobulin-like proteins as a serodiagnostic marker for acute leptospirosis. J. Clin. Microbiol. 2007, 45, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Ptak, C.P.; Hsieh, C.L.; Lin, Y.P.; Maltsev, A.S.; Raman, R.; Sharma, Y.; Oswald, R.E.; Chang, Y.F. NMR Solution Structure of the Terminal Immunoglobulin-like Domain from the Leptospira Host-Interacting Outer Membrane Protein, LigB. Biochemistry 2014, 53, 5249–5260. [Google Scholar] [CrossRef] [PubMed]
- Limmathurotsakul, D.; Turner, E.L.; Wuthiekanun, V.; Thaipadungpanit, J.; Suputtamongkol, Y.; Chierakul, W.; Smythe, L.D.; Day, N.P.J.; Cooper, B.; Peacock, S.J. Fool’s gold: Why imperfect reference tests are undermining the evaluation of novel diagnostics: A reevaluation of 5 diagnostic tests for leptospirosis. Clin. Infect. Dis. 2012, 55, 322–331. [Google Scholar] [CrossRef]
- Schlichting, D.; Nöckler, K.; Bahn, P.; Luge, E.; Greiner, M.; Müller-Graf, C.; Mayer-Scholl, A. Estimation of the sensitivity and specificity of a Leptospira spp. in-house ELISA through Bayesian modelling. Int. J. Med. Microbiol. 2015, 305, 756–761. [Google Scholar] [CrossRef]
- World Health Organization. Report of the Second Meeting of the Leptospirosis Burden Epidemiology Reference Group. 2011. Available online: https://apps.who.int/iris/handle/10665/44588 (accessed on 13 October 2021).
- USDA (United States Department of Agriculture). National Veterinary Services Laboratories. Microtitre technique for detection of Leptospira antibodies. Proc. Annu. Meet. US Anim. Health Assoc. 1987, 91, 65–73. [Google Scholar]
- Deneke, Y.; Sabarinath, T.; Gogia, N.; Lalsiamthara, J.; Viswas, K.N.; Chaudhuri, P. Evaluation of recombinant LigB antigen-based indirect ELISA and latex agglutination test for the serodiagnosis of bovine leptospirosis in India. Mol. Cell. Probes 2014, 28, 141–146. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Dey, S.; Madhan Mohan, C.; Ramadass, P.; Nachimuthu, K. Recombinant antigen-based Latex Agglutination Test for Rapid Serodiagnosis of leptospirosis. Vet. Res. Commun. 2007, 31, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Smits, H.L.; van der Hoorn, M.A.; Goris, M.G.; Gussenhoven, G.C.; Yersin, C.; Sasaki, D.M. Simple latex agglutination assay for rapid serodiagnosis of human leptospirosis. J. Clin. Microbiol. 2000, 38, 1272–1275. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Wannapinij, P.; White, L.; Day, N.P.; Cooper, B.S.; Peacock, S.J.; Limmathurotsakul, D. Using a web-based application to define the accuracy of diagnostic tests when the gold standard is imperfect. PLoS ONE 2013, 8, e79489. [Google Scholar] [CrossRef]
- Faine, S.B.; Adler, B.; Bolin, C.; Perolat, P. Leptospira and Leptospirosis, 2nd ed.; MediSci: Melburne, Australia, 1999. [Google Scholar]
- Suputtamongkol, Y.; Pongtavornpinyo, W.; Lubell, Y.; Suttinont, C.; Hoontrakul, S.; Phimda, K.; Losuwanaluk, K.; Suwancharoen, D.; Silpasakorn, S.; Chierakul, W.; et al. Strategies for Diagnosis and Treatment of Suspected Leptospirosis: A Cost-Benefit Analysis. PLoS Negl. Trop. Dis. 2010, 4, e610. [Google Scholar] [CrossRef]
- Boey, K.; Shiokawa, K.; Rajeev, S. Leptospira infection in rats: A literature review of global prevalence and distribution. PLoS Negl. Trop. Dis. 2019, 13, e0007499. [Google Scholar] [CrossRef]
- Hull-Jackson, C.; Glass, M.B.; Ari, M.D.; Bragg, S.L.; Branch, S.L.; Whittington, C.U.; Edwards, C.N.; Levett, P.N. Evaluation of a commercial latex agglutination assay for serological diagnosis of leptospirosis. J. Clin. Microbiol. 2006, 44, 1853–1855. [Google Scholar] [CrossRef]
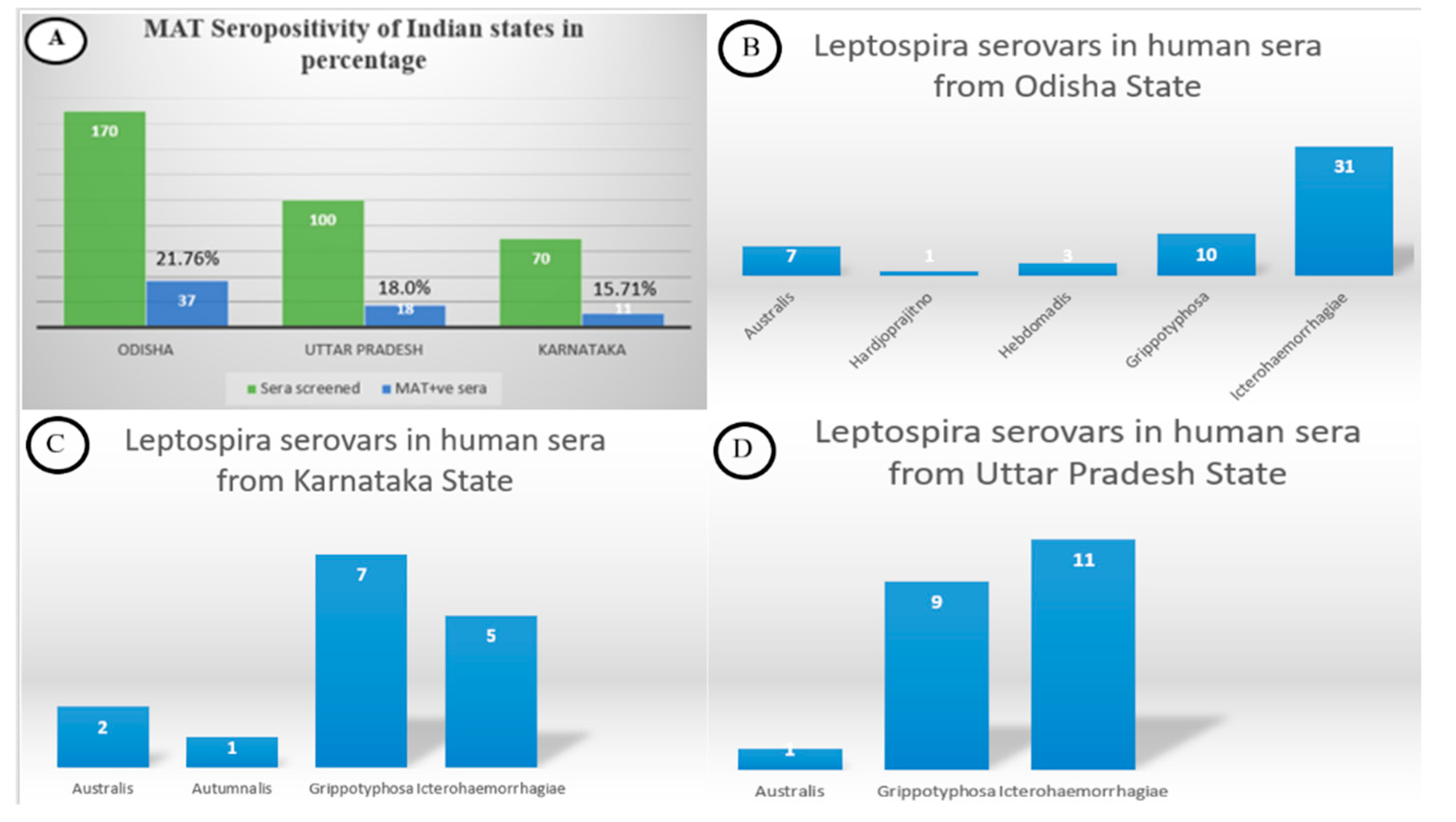
| Genomo- Species | Serogroup | Serovar | Strain | Positive (N) * | Prevalence (%) | 95% LCI | 95% UCI |
|---|---|---|---|---|---|---|---|
| L. interrogans | Icterohaemorrhagiae | Icterohaemorrhagiae | RGA | 47 | 13.82 | 10.55 | 17.89 |
| Australis | Australis | Ballico | 10 | 2.94 | 1.60 | 5.33 | |
| Hebdomadis | Hebdomadis | Hebdomadis | 03 | 0.88 | 0.30 | 2.56 | |
| Autumnalis | Autumnalis | Akiyami A | 01 | 0.29 | 0.05 | 1.64 | |
| Sejroe | Hardjo | Hardjoprajitno | 01 | 0.29 | 0.05 | 1.64 | |
| L. kirshneri | Grippotyphosa | Grippotyphosa | Moskova V | 26 | 7.65 | 5.27 | 10.97 |
| Parameters | MAT Considered as Gold Standard Test (%) | Bayesian Latent Class Model (%) |
|---|---|---|
| Prevalence | 15.0 (11.5–19.3) | 18.0 (14.2–22.3) |
| Acute MAT | ||
| Sensitivity | 100 | 83.3 (72.8–91.3) |
| Specificity | 100 | 99.9 (99.1–100) |
| PPV | 100 | 99.5 (94.9–100) |
| NPV | 100 | 96.5 (93.9–98.2) |
| rLigA/BCon1-5-based IgM Dot ELISA Dipstick Test | ||
| Sensitivity | 100 (91.3–100) | 99.6 (96.0–100) |
| Specificity | 95.5 (92.2–97.5) | 98.9 (97.2–99.8) |
| PPV | 79.7 (67.4–88.3) | 95.2 (88.4–98.9) |
| NPV | 100 (98.3–100) | 99.9 (99.1–100) |
| rLigA/BCon1-5-based LAT | ||
| Sensitivity | 100 (91.3–100) | 99.5 (95.2–100) |
| Specificity | 96.5 (93.5–98.2) | 99.9 (99.1–100) |
| PPV | 83.6 (71.5–91.4) | 99.6 (96.0–100) |
| NPV | 100 (98.3–100) | 99.9 (98.9–100) |
| Parameters | MAT Considered as Gold Standard Test (%) | Bayesian Latent Class Model (%) |
|---|---|---|
| Prevalence | 19.4 (15.4–24.1) | 18.9 (14.9–23.2) |
| Acute MAT/Paired MAT | ||
| Sensitivity | 100 | 98.2 (93.0–99.8) |
| Specificity | 100 | 98.9 (97.2–99.7) |
| PPV | 100 | 95.2 (88.4–98.8) |
| NPV | 100 | 99.6 (98.3–100) |
| rLigA/BCon1-5-based LAT | ||
| Sensitivity | 90.9 (80.6–96.3) | 94.9 (87.8–98.6) |
| Specificity | 99.6 (97.7–100) | 99.9 (99.1–100) |
| PPV | 98.4 (90.0–99.9) | 99.6 (95.9–100) |
| NPV | 97.8 (95.1–99.1) | 98.8 (97.1–99.7) |
| rLigA/BCon1-5-based IgM Dot ELISA Dipstick Test | ||
| Sensitivity | 95.5 (86.4–98.8) | 99.6 (96.0–100) |
| Specificity | 99.6 (97.7–100) | 99.9 (99.1–100) |
| PPV | 98.4 (90.5–99.9) | 99.6 (96.2–100) |
| NPV | 98.9 (96.6–99.7) | 99.9 (99.1–100) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behera, S.K.; Sabarinath, T.; Ganesh, B.; Mishra, P.K.K.; Niloofa, R.; Senthilkumar, K.; Verma, M.R.; Hota, A.; Chandrasekar, S.; Deneke, Y.; et al. Diagnosis of Human Leptospirosis: Comparison of Microscopic Agglutination Test with Recombinant LigA/B Antigen-Based In-House IgM Dot ELISA Dipstick Test and Latex Agglutination Test Using Bayesian Latent Class Model and MAT as Gold Standard. Diagnostics 2022, 12, 1455. https://doi.org/10.3390/diagnostics12061455
Behera SK, Sabarinath T, Ganesh B, Mishra PKK, Niloofa R, Senthilkumar K, Verma MR, Hota A, Chandrasekar S, Deneke Y, et al. Diagnosis of Human Leptospirosis: Comparison of Microscopic Agglutination Test with Recombinant LigA/B Antigen-Based In-House IgM Dot ELISA Dipstick Test and Latex Agglutination Test Using Bayesian Latent Class Model and MAT as Gold Standard. Diagnostics. 2022; 12(6):1455. https://doi.org/10.3390/diagnostics12061455
Chicago/Turabian StyleBehera, Sujit Kumar, Thankappan Sabarinath, Balasubramanian Ganesh, Prasanta Kumar K. Mishra, Roshan Niloofa, Kuppusamy Senthilkumar, Med Ram Verma, Abhishek Hota, Shanmugam Chandrasekar, Yosef Deneke, and et al. 2022. "Diagnosis of Human Leptospirosis: Comparison of Microscopic Agglutination Test with Recombinant LigA/B Antigen-Based In-House IgM Dot ELISA Dipstick Test and Latex Agglutination Test Using Bayesian Latent Class Model and MAT as Gold Standard" Diagnostics 12, no. 6: 1455. https://doi.org/10.3390/diagnostics12061455
APA StyleBehera, S. K., Sabarinath, T., Ganesh, B., Mishra, P. K. K., Niloofa, R., Senthilkumar, K., Verma, M. R., Hota, A., Chandrasekar, S., Deneke, Y., Kumar, A., Nagarajan, M., Das, D., Khatua, S., Sahu, R., & Ali, S. A. (2022). Diagnosis of Human Leptospirosis: Comparison of Microscopic Agglutination Test with Recombinant LigA/B Antigen-Based In-House IgM Dot ELISA Dipstick Test and Latex Agglutination Test Using Bayesian Latent Class Model and MAT as Gold Standard. Diagnostics, 12(6), 1455. https://doi.org/10.3390/diagnostics12061455








